Parafoveal and Perifoveal Accommodation Response to Defocus Changes Induced by a Tunable Lens
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
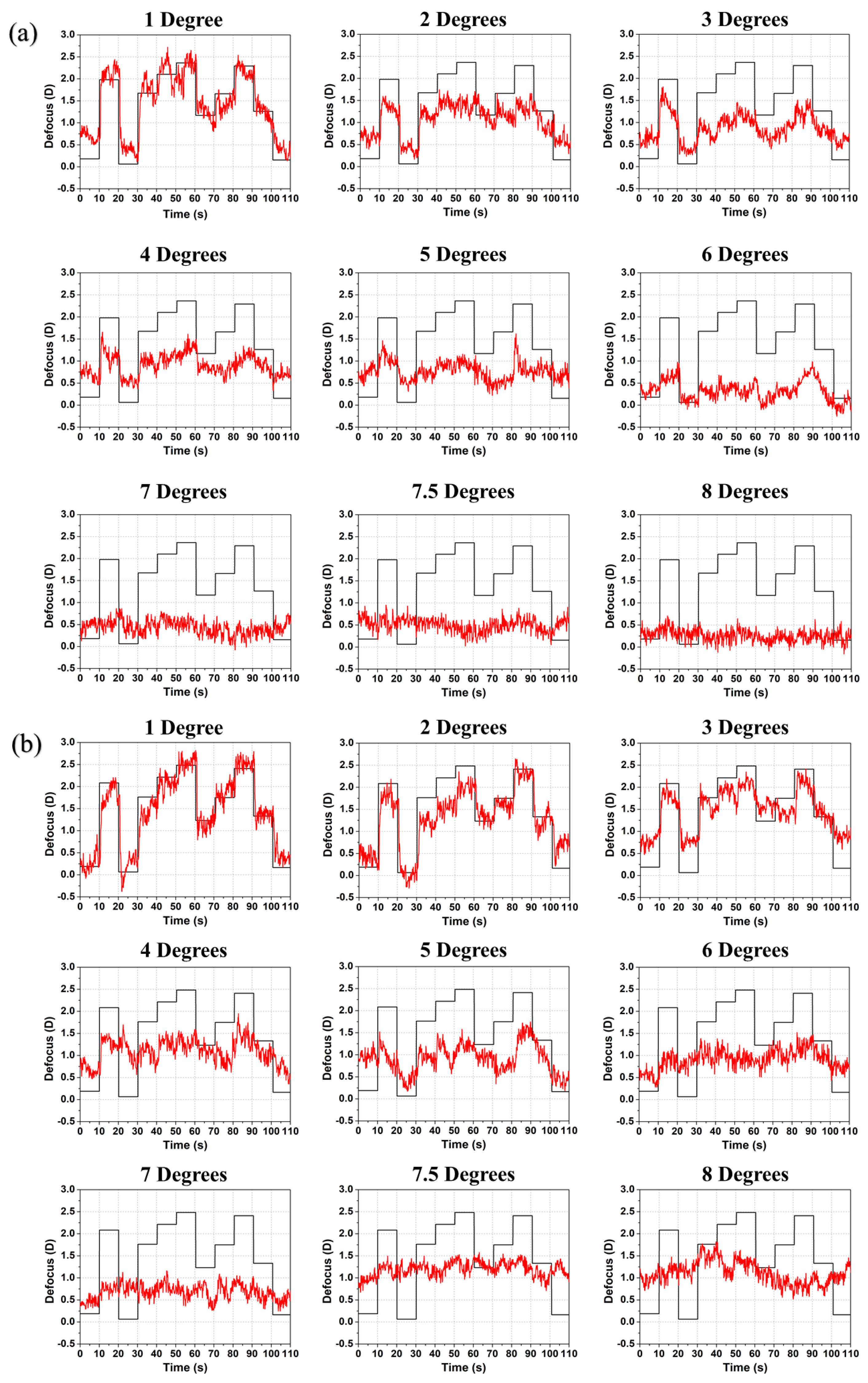
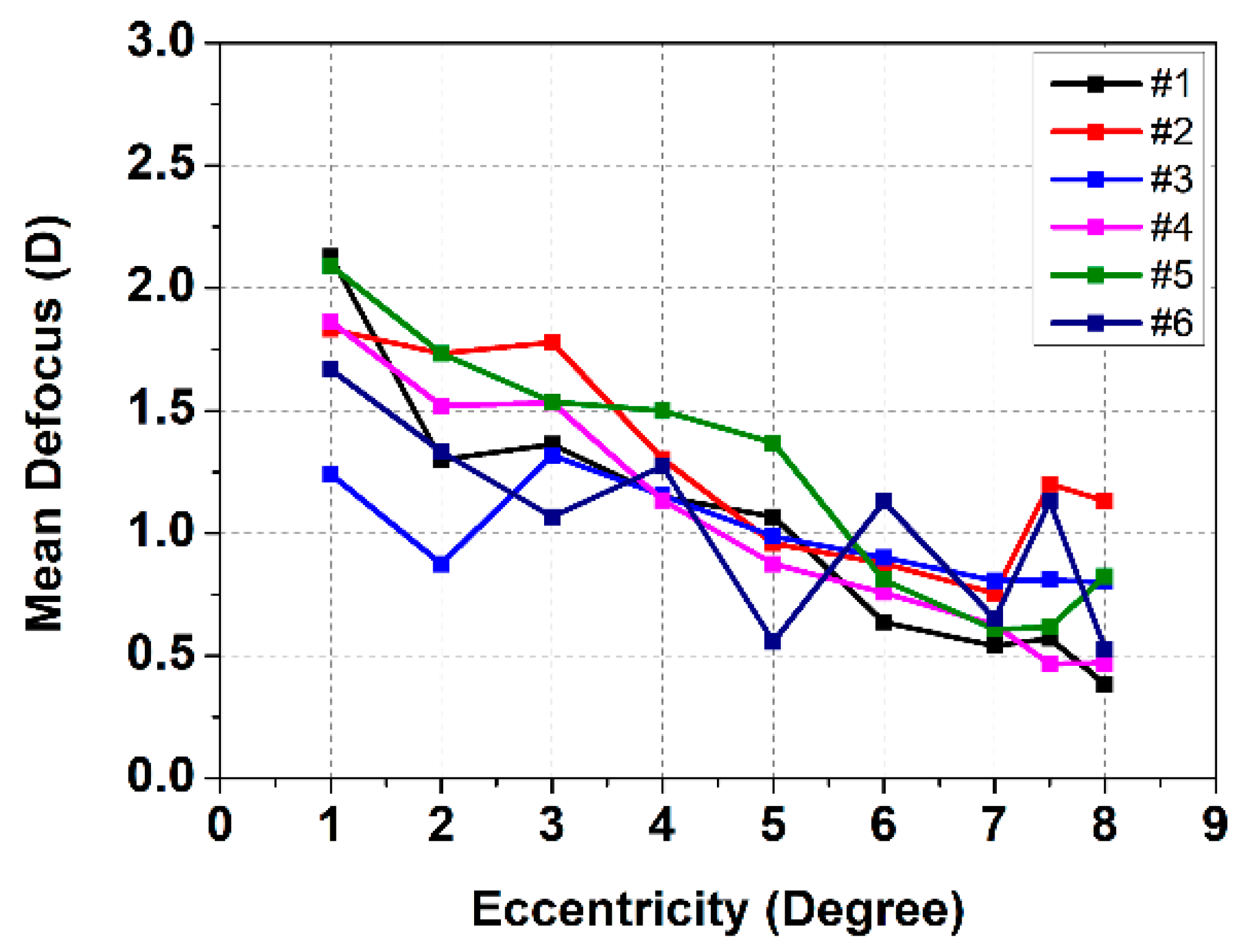
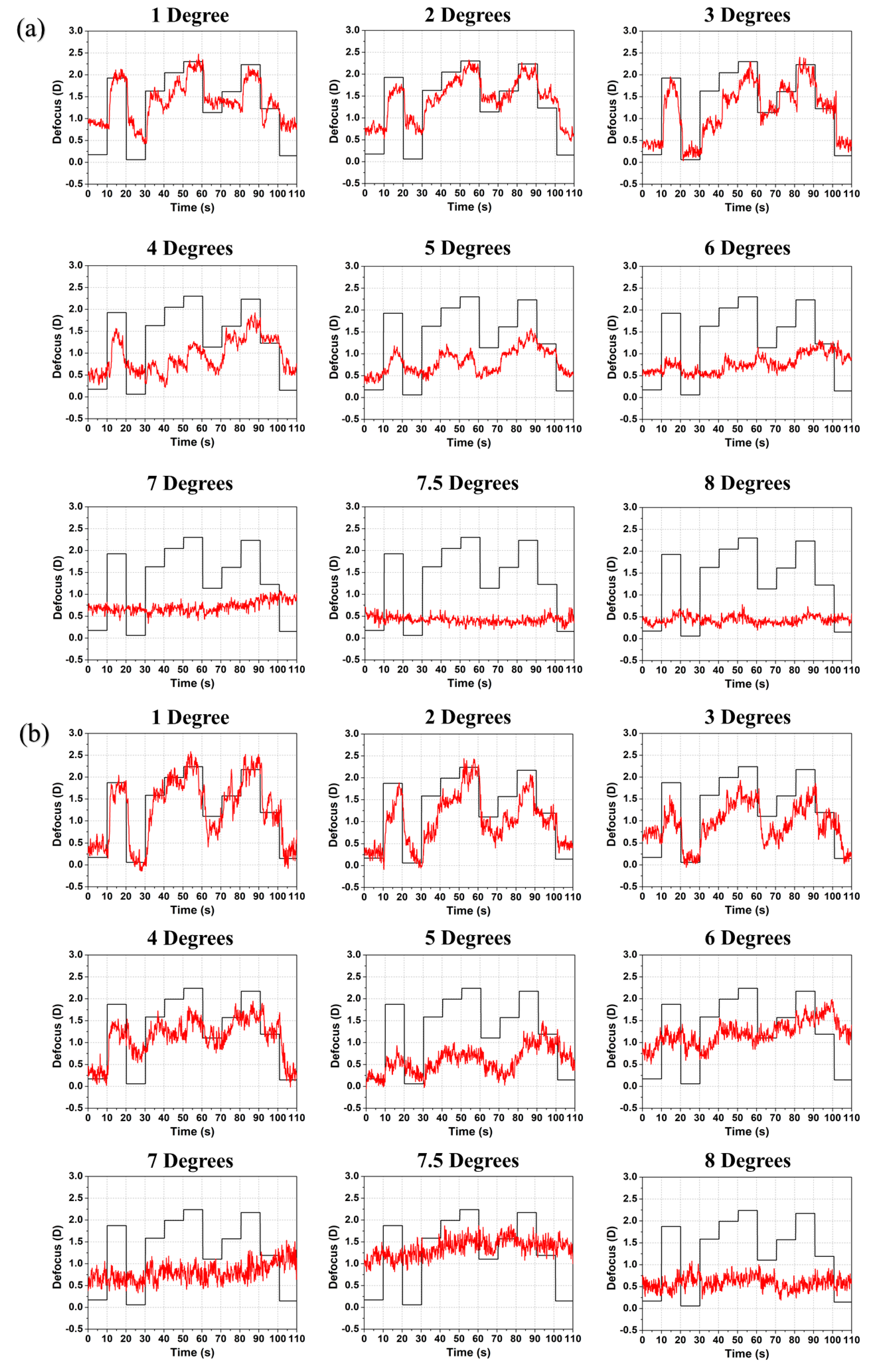
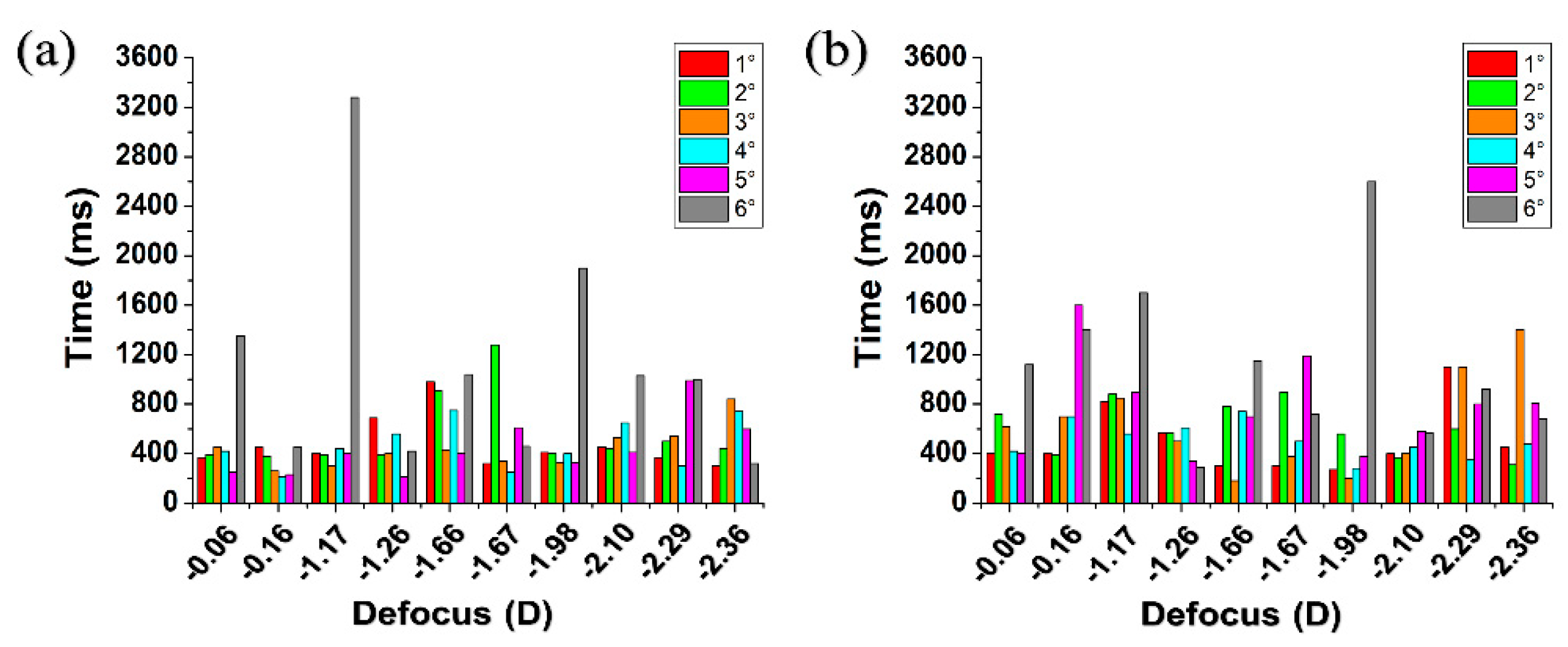
3. Experimental Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamasaki, D.; Ong, J.; Marg, E. The amplitude of accommodation in presbyopia. Am. J. Optom. Arch. Am. Acad. Optom. 1956, 33, 3–14. [Google Scholar] [CrossRef]
- Mordi, J.A.; Ciuffreda, K.J. Static aspects of accommodation: Age and presbyopia. Vis. Res. 1998, 38, 1643–1653. [Google Scholar] [CrossRef]
- Jackson, E. Amplitude of accommodation at different periods of life. Calif. State J. Med. 1907, 5, 163–166. [Google Scholar]
- Taylor, J.; Charman, W.N.; O’Donnell, C.; Radhakrishnan, H. Effect of target spatial frequency on accommodative response in myopes and emmetropes. J. Vis. 2009, 9, 16. [Google Scholar] [CrossRef]
- Liu, T.; Sreenivasan, V.; Thibos, L.N. Uniformity of accommodation across the visual field. J. Vis. 2016, 16, 6. [Google Scholar] [CrossRef]
- Campbell, F.W. The minimum quantity of light required to elicit the accommodation reflex in man. J. Physiol. 1954, 123, 357–366. [Google Scholar] [CrossRef]
- Fincham, E.F. The accommodation reflex and its stimulus. Br. J. Ophthalmol. 1951, 35, 381–393. [Google Scholar] [CrossRef]
- Semmlow, J.L.; Tinor, T. Accommodative convergence response to off-foveal retinal images. J. Opt. Soc. Am. 1978, 68, 1497–1501. [Google Scholar] [CrossRef]
- Gu, Y.; Legge, G.E. Accommodation to stimuli in peripheral vision. J. Opt. Soc. Am. A 1987, 4, 1681–1687. [Google Scholar] [CrossRef]
- Hartwig, A.; Charman, W.N.; Radhakrishnan, H. Accommodative response to peripheral stimuli in myopes and emmetropes. Ophthalmic Physiol. Opt. 2011, 31, 91–99. [Google Scholar] [CrossRef]
- Hennessy, R.T.; Leibowitz, H.W. The effect of a peripheral stimulus on accommodation. Percept. Psychophys. 1971, 10, 129–132. [Google Scholar] [CrossRef]
- Labhishetty, V.; Cholewiak, S.A.; Banks, M.S. Contributions of foveal and non-foveal retina to the human eye’s focusing response. J. Vis. 2019, 19, 18. [Google Scholar] [CrossRef]
- Timberlake, G.T.; Mainster, M.A.; Peli, E.; Augliere, R.A.; Essock, E.A.; Arend, L.E. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1137–1147. [Google Scholar]
- Chung, S.T.L. Enhancing visual performance for people with central vision loss. Optom. Vis. Sci. 2010, 87, 276–284. [Google Scholar] [CrossRef]
- García, M.G.; Ohlendorf, A.; Schaeffel, F.; Wahl, S. Dioptric defocus maps across the visual field for different indoor environments. Biomed. Opt. Express 2017, 9, 347–359. [Google Scholar] [CrossRef]
- Sprague, W.W.; Cooper, E.A.; Reissier, S.; Yellapragada, B.; Banks, M.S. The natural statistics of blur. J. Vis. 2016, 16, 23. [Google Scholar] [CrossRef]
- Öner, V.; Bulut, A.; Oruç, Y.; Özgür, G. Influence of indoor and outdoor activities on progression of myopia during puberty. Int. Ophthalmol. 2016, 36, 121–125. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Sharmin, N.; Vohnsen, B. Monocular accommodation response to random defocus changes induced by a tuneable lens. Vis. Res. 2019, 165, 45–53. [Google Scholar] [CrossRef]
- Cornsweet, T.N.; Crane, H.D. Training the visual accommodation system. Vis. Res. 1973, 13, 713–715. [Google Scholar] [CrossRef]
- Lopez-Gil, N.; Peixoto-de-Matos, S.C.; Thibos, L.N.; González-Méijome, J.M. Shedding light on night myopia. J. Vis. 2012, 12, 4. [Google Scholar] [CrossRef]
- Eckmiller, M.S. Defective cone photoreceptor cytoskeleton, alignment, feedback, and energetics can lead to energy depletion in macular degeneration. Prog. Retin. Eye Res. 2004, 23, 495–522. [Google Scholar] [CrossRef]
- Vohnsen, B. Directional sensitivity of the retina: A layered scattering model of outer-segment photoreceptor pigments. Biomed. Opt. Express 2014, 5, 1569–1587. [Google Scholar] [CrossRef]
- Atchison, D.A. Optics of the Human Eye, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2023; p. 161. [Google Scholar]
- Charman, W.N. The eye in focus: Accommodation and presbyopia. Clin. Exp. Optom. 2008, 91, 207–225. [Google Scholar] [CrossRef]
- Tucker, J.; Charman, W.N. Reaction and response times for accommodation. Am. J. Optom. Physiol. Opt. 1979, 56, 490–503. [Google Scholar] [CrossRef]
- Campbell, F.W.; Westheimer, G.; Robson, J.G. Significance of fluctuations of accommodation. J. Opt. Soc. Am. 1958, 48, 669. [Google Scholar] [CrossRef]
- Diez, P.S.; Ohlendorf, A.; Schaeffel, F.; Wahl, S. Effect of spatial filtering on accommodation. Vis. Res. 2019, 164, 62–68. [Google Scholar] [CrossRef]
- Charman, W.N.; Heron, G. Microfluctuations in accommodation: An update on their characteristics and possible role. Ophthalmic Physiol. Opt. 2015, 35, 476–499. [Google Scholar] [CrossRef]
- Williams, M.M.; Tresilian, J.R.; Strang, N.C.; Kochhar, P.; Wann, J.P. Improving vision: Neural compensation for optical defocus. Proc. Biol. Sci. 1998, 265, 71–77. [Google Scholar] [CrossRef]
- Cufflin, M.P.; Mallen, E.A.H. Blur adaptation: Clinical and refractive considerations. Clin. Exp. Optom. 2020, 103, 104–111. [Google Scholar] [CrossRef]
- Pesudovs, K.; Brennan, N. Decreased uncorrected vision after a period of distance fixation with spectacle wear. Optom. Vis. Sci. 1993, 70, 528–531. [Google Scholar] [CrossRef]
- Carmichael Martins, A.; Vohnsen, B. Directional light-capture efficiency of the foveal and parafoveal photoreceptors at different luminance levels: An experimental and analytical study. Biomed. Opt. Express 2019, 10, 3760–3772. [Google Scholar] [CrossRef]
- Bernal-Molina, P.; Esteve-Taboada, J.J.; Ferrer-Blasco, T.; Montés-Micó, R. Influence of contrast polarity on the accommodative response. J. Optom. 2019, 12, 38–43. [Google Scholar] [CrossRef]
- Ward, P.A. The effect of stimulus contrast on the accommodation response. Ophthal. Physiol. Opt. 1987, 7, 9–15. [Google Scholar] [CrossRef]
- Bakaraju, R.C.; Yeotikar, N.S.; Rao, V.S. Accommodative lag versus different stimuli. J. Mod. Opt. 2007, 54, 1299–1305. [Google Scholar] [CrossRef]
- Buehren, T.; Collins, M.J. Accommodation stimulus-response function and retinal image quality. Vis. Res. 2006, 46, 1633–1645. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.Y.; Hyun, H.G.; Moon, B.Y. Effects of variation of illumination on visual function factors. J. Korean Ophthalmic Opt. Soc. 2015, 20, 195–200. [Google Scholar] [CrossRef][Green Version]
- Watson, A.B. A formula for human retinal ganglion cell receptive field density as a function of visual field location. J. Vis. 2014, 14, 15. [Google Scholar] [CrossRef]
- Lundström, L.; Mira-Agudelo, A.; Artal, P. Peripheral optical errors and their change with accommodation differ between emmetropic and myopic eyes. J. Vis. 2009, 9, 17. [Google Scholar] [CrossRef]
- Vohnsen, B.; Carmichael, A.; Sharmin, N.; Qaysi, S.; Valente, D. Volumetric integration model of the Stiles-Crawford effect of the first kind and its experimental verification. J. Vis. 2017, 17, 18. [Google Scholar] [CrossRef]
- Vohnsen, B. Geometrical scaling of the developing eye and photoreceptors and a possible relation to emmetropization and myopia. Vis. Res. 2019, 189, 46–53. [Google Scholar] [CrossRef]
- Abbott, M.L.; Schmid, K.L.; Strang, N.C. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol. Opt. 1998, 18, 13–22. [Google Scholar] [CrossRef]
- Swiatczak, B.; Schaeffel, F. Myopia: Why the retina stops inhibiting eye growth. Sci. Rep. 2022, 12, 21704. [Google Scholar] [CrossRef]
- Rozema, J.; Dankert, S.; Iribarren, R. Emmetropization and non-myopic eye growth. Surv. Ophthalmol. 2023, 68, 759–783. [Google Scholar] [CrossRef]


| Subject | Age (Years) | Sphere (D) | Cylinder (D) | Ac. Range (D) |
|---|---|---|---|---|
| #1 | 29 | −0.50 | 0.00 | 3.40 |
| #2 | 28 | −0.75 | 0.00 | 3.53 |
| #3 | 34 | −0.75 | 0.00 | 2.42 |
| #4 | 24 | −4.75 | 0.00 | 3.35 |
| #5 | 23 | −5.75 | 0.75 (Axis 30°) | 3.55 |
| #6 | 25 | −6.00 | −2.25 (Axis 15°) | 3.28 |
| Subject | 1° | 2° | 3° | 4° | 5° | 6° | 7° | 7.5° | 8° |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 0.388 | 0.628 | 0.802 | 0.805 | 0.956 | 1.259 | 1.247 | 1.222 | 0.925 |
| #2 | 0.378 | 0.482 | 0.504 | 0.816 | 0.883 | 0.936 | 1.116 | 0.855 | 0.935 |
| #3 | 0.415 | 0.435 | 0.676 | 0.444 | 0.418 | 0.425 | 0.497 | 0.469 | 0.499 |
| #4 | 0.510 | 0.476 | 0.481 | 0.797 | 0.845 | 0.967 | 1.039 | 1.224 | 1.199 |
| #5 | 0.476 | 0.499 | 0.719 | 0.671 | 0.842 | 1.112 | 1.294 | 1.258 | 1.185 |
| #6 | 0.401 | 0.544 | 0.634 | 0.564 | 0.997 | 0.760 | 0.954 | 0.752 | 1.059 |
| Average | 0.428 | 0.510 | 0.636 | 0.683 | 0.823 | 0.910 | 1.024 | 0.963 | 0.967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmin, N.; Papadogiannis, P.; Romashchenko, D.; Lundström, L.; Vohnsen, B. Parafoveal and Perifoveal Accommodation Response to Defocus Changes Induced by a Tunable Lens. Appl. Sci. 2023, 13, 8645. https://doi.org/10.3390/app13158645
Sharmin N, Papadogiannis P, Romashchenko D, Lundström L, Vohnsen B. Parafoveal and Perifoveal Accommodation Response to Defocus Changes Induced by a Tunable Lens. Applied Sciences. 2023; 13(15):8645. https://doi.org/10.3390/app13158645
Chicago/Turabian StyleSharmin, Najnin, Petros Papadogiannis, Dmitry Romashchenko, Linda Lundström, and Brian Vohnsen. 2023. "Parafoveal and Perifoveal Accommodation Response to Defocus Changes Induced by a Tunable Lens" Applied Sciences 13, no. 15: 8645. https://doi.org/10.3390/app13158645
APA StyleSharmin, N., Papadogiannis, P., Romashchenko, D., Lundström, L., & Vohnsen, B. (2023). Parafoveal and Perifoveal Accommodation Response to Defocus Changes Induced by a Tunable Lens. Applied Sciences, 13(15), 8645. https://doi.org/10.3390/app13158645







