Whole Rock, Mineral Chemistry during Skarn Mineralization-Case Study from Tongshan Cu-Mo Skarn Profile
Abstract
1. Introduction
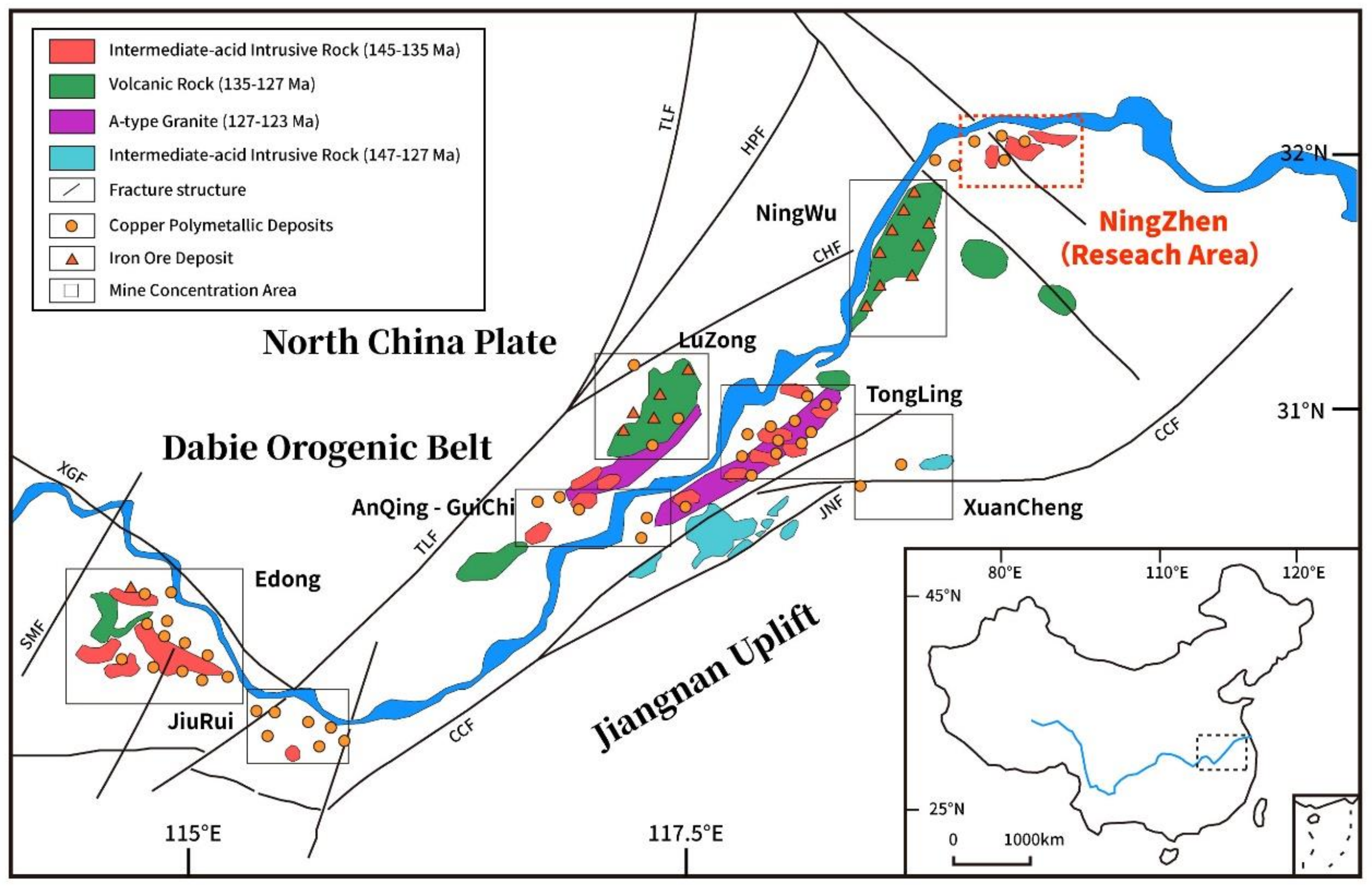
2. Geological Background and Sampling
2.1. Geological Background
2.2. Geology of Tongshan Skarn Cu-Mo Deposit
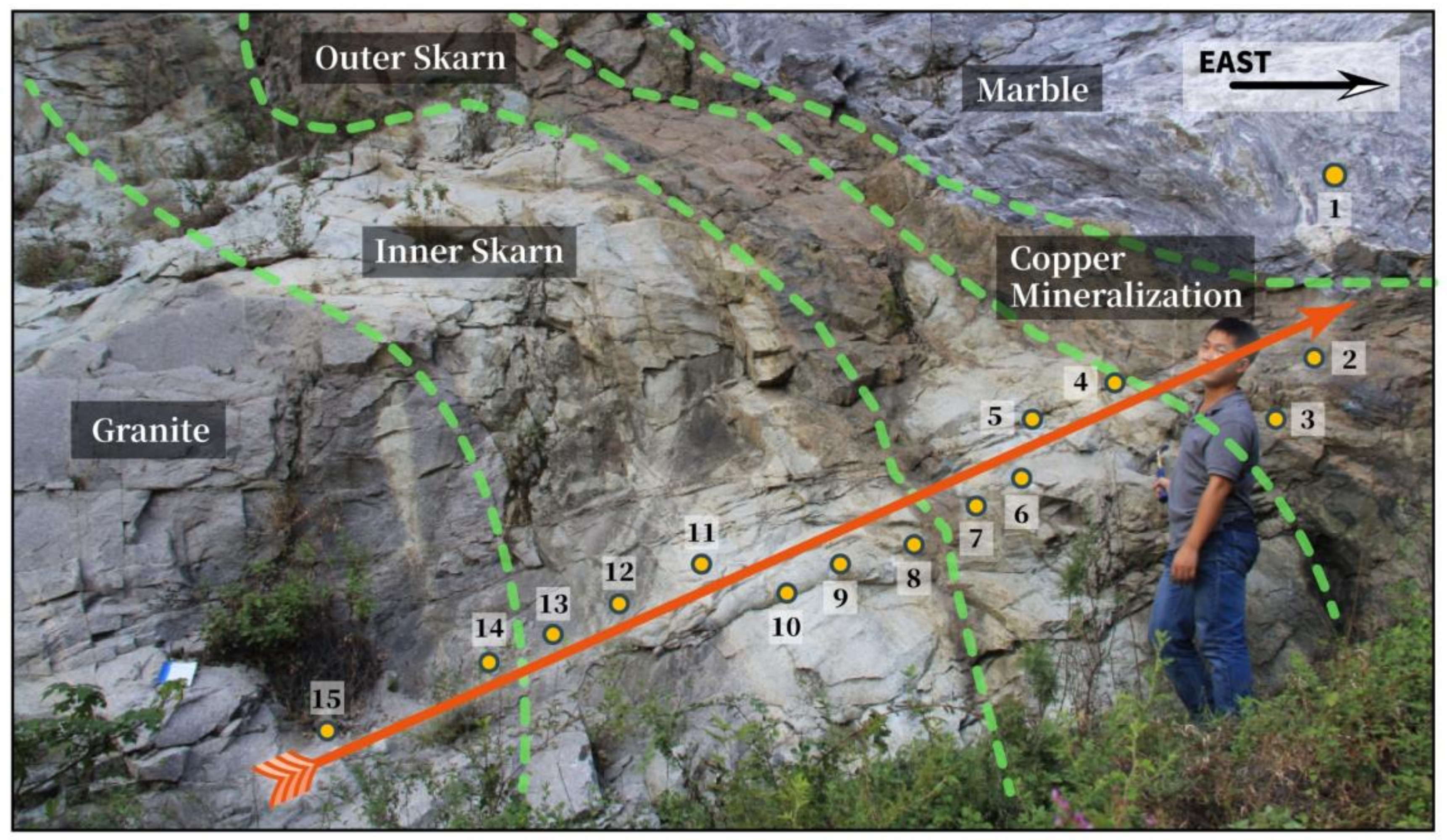
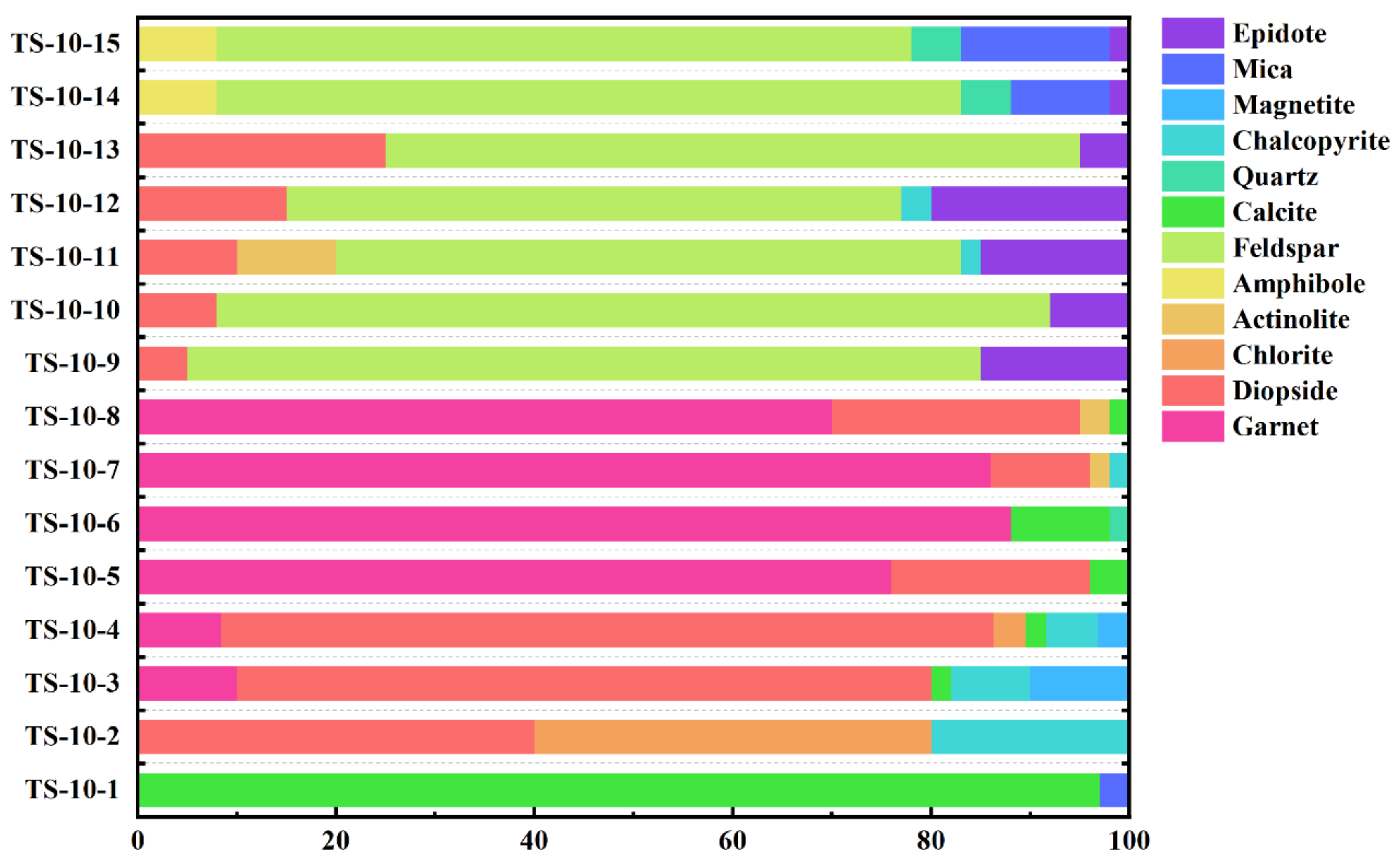
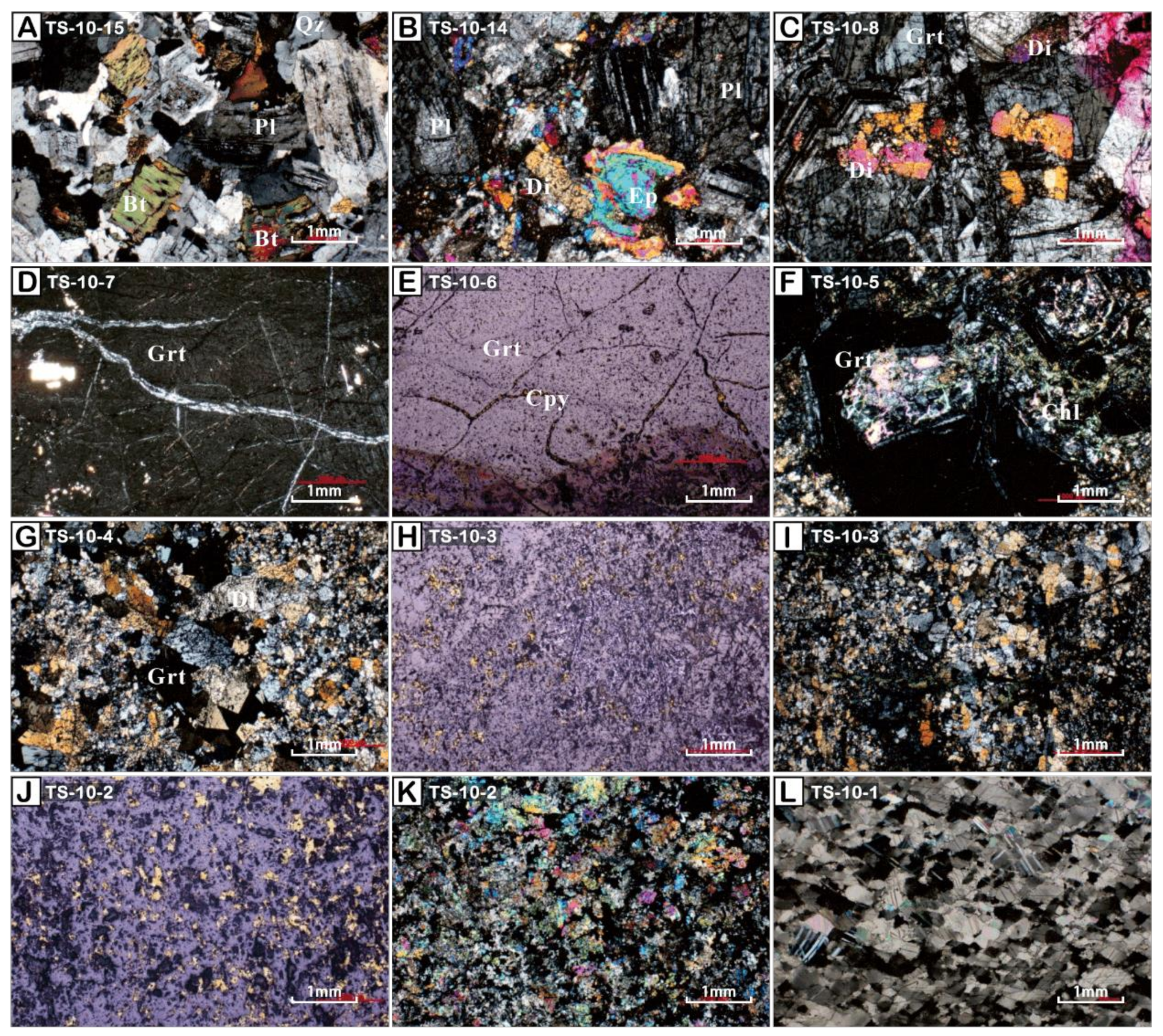
2.3. Sample Petrological Characteristics
2.4. Garnet in Skarn Profiles
3. Analytical Methods
3.1. Whole-Rock Major and Trace Element Analysis
3.2. In-Situ Trace Element Analysis by LA-ICP-MS
3.3. Mapping Analysis
4. Results
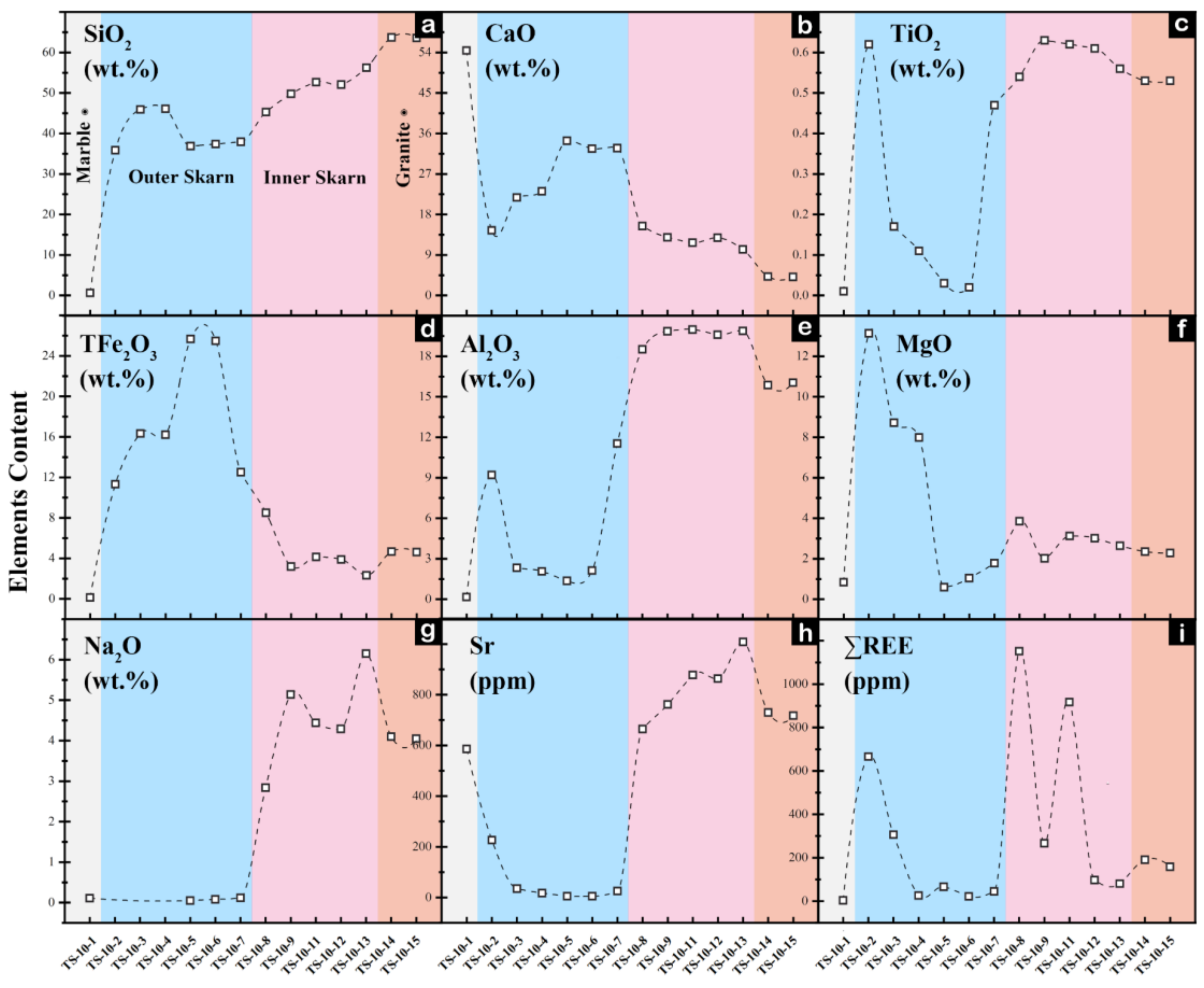
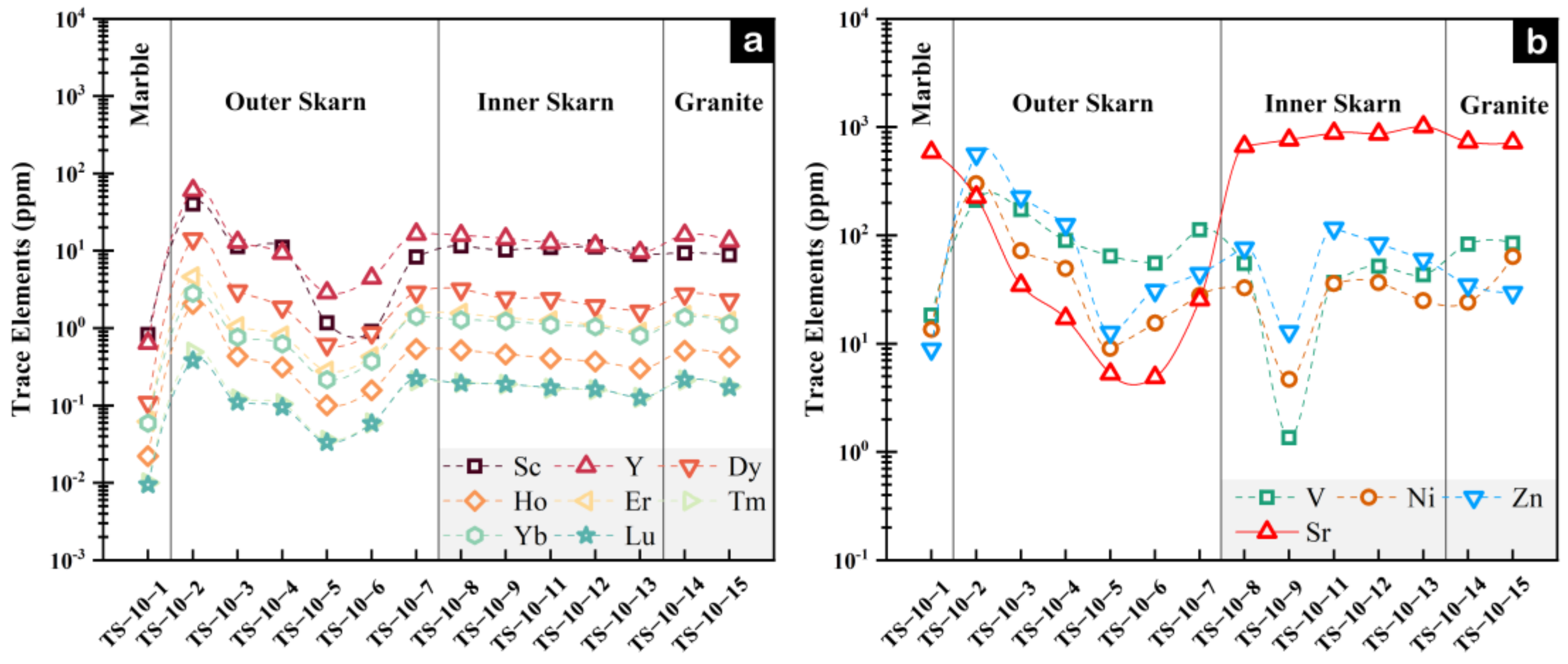
4.1. Whole-Rock Geochemical Characteristics
4.2. Geochemical Characteristic of Garnet
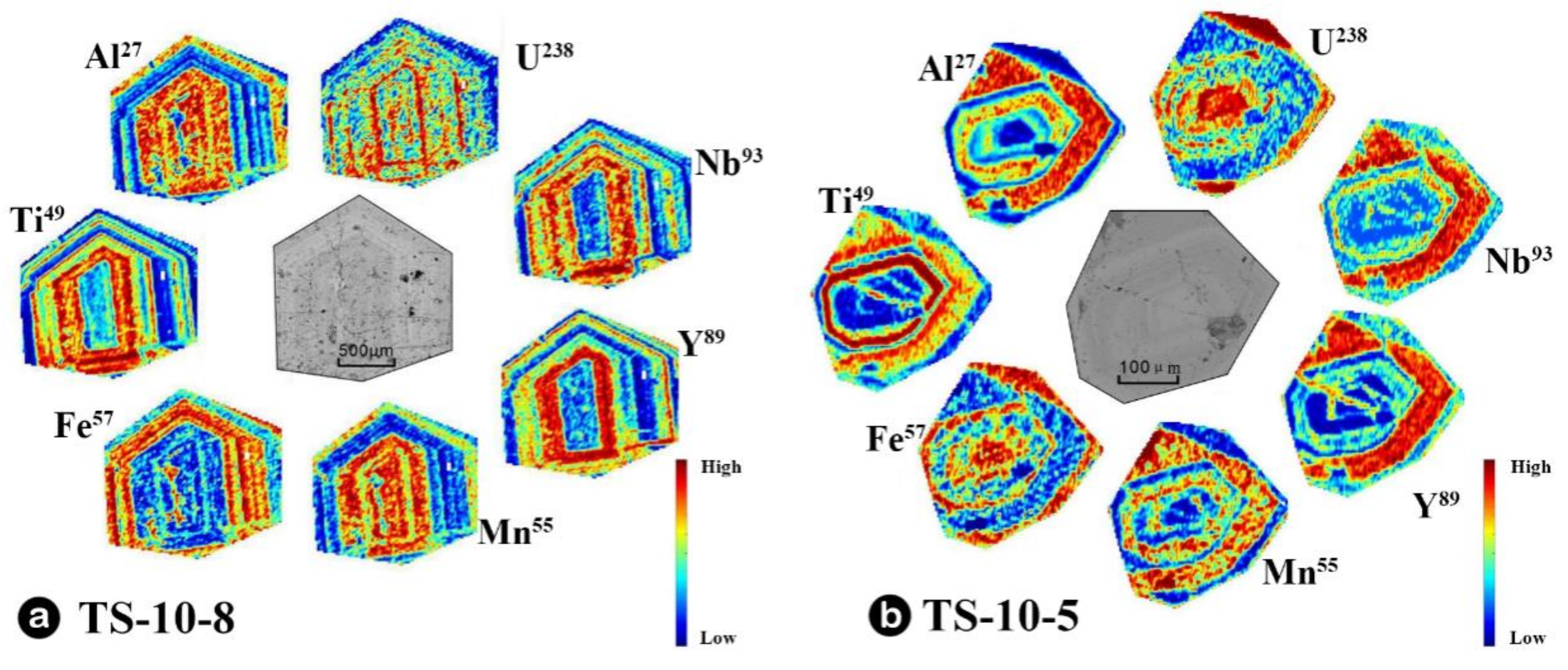
4.3. Mapping of Garnets
5. Discussion
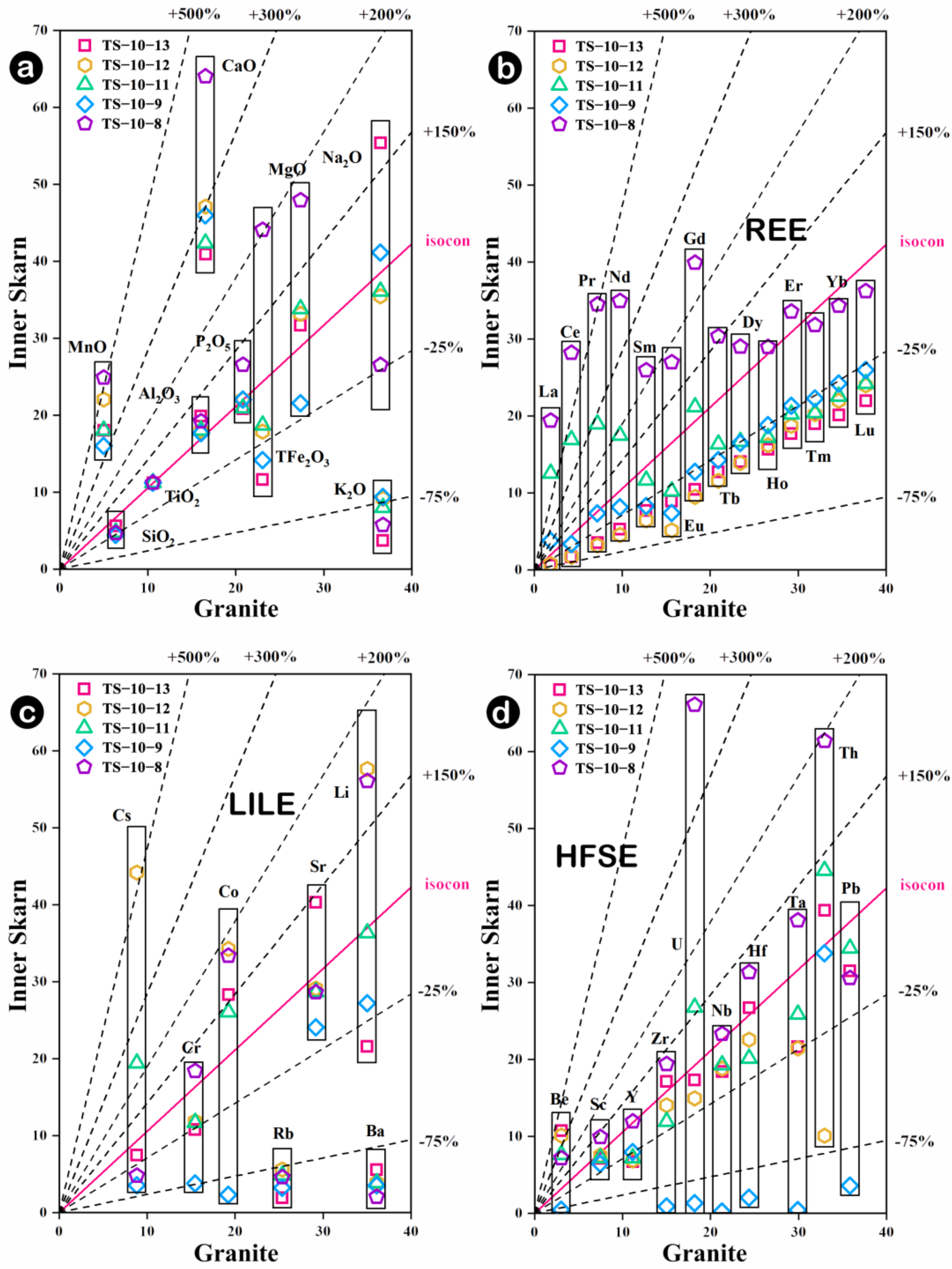
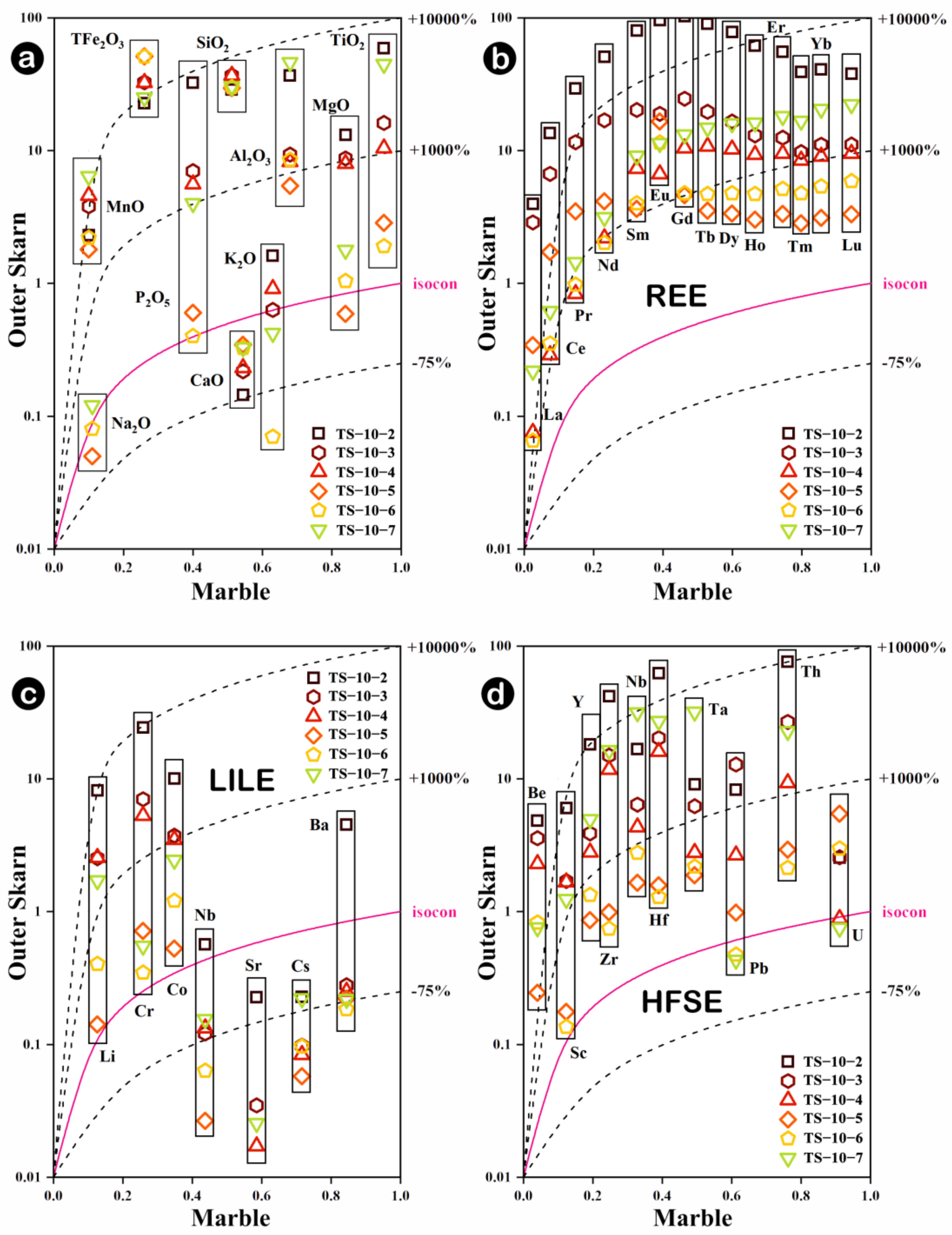
5.1. Mechanisms of Whole-Rock Element Migration
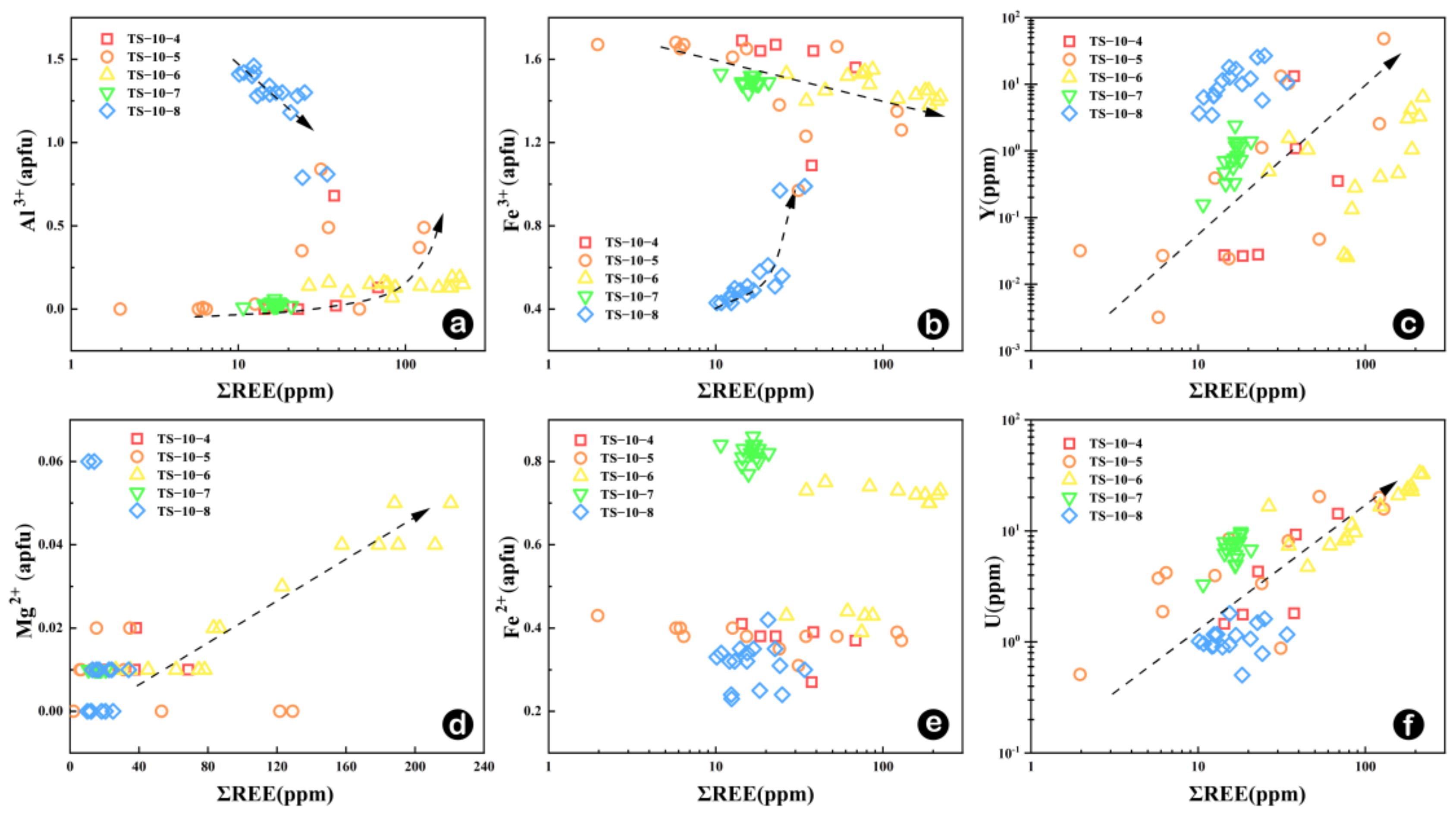
5.2. Geochemical Characteristics of Garnets in Skarn
5.2.1. Mechanism of Rare Earth Element into Garnet
5.2.2. The Y/Ho Ratio and Its Water-Rock Ratio Indication
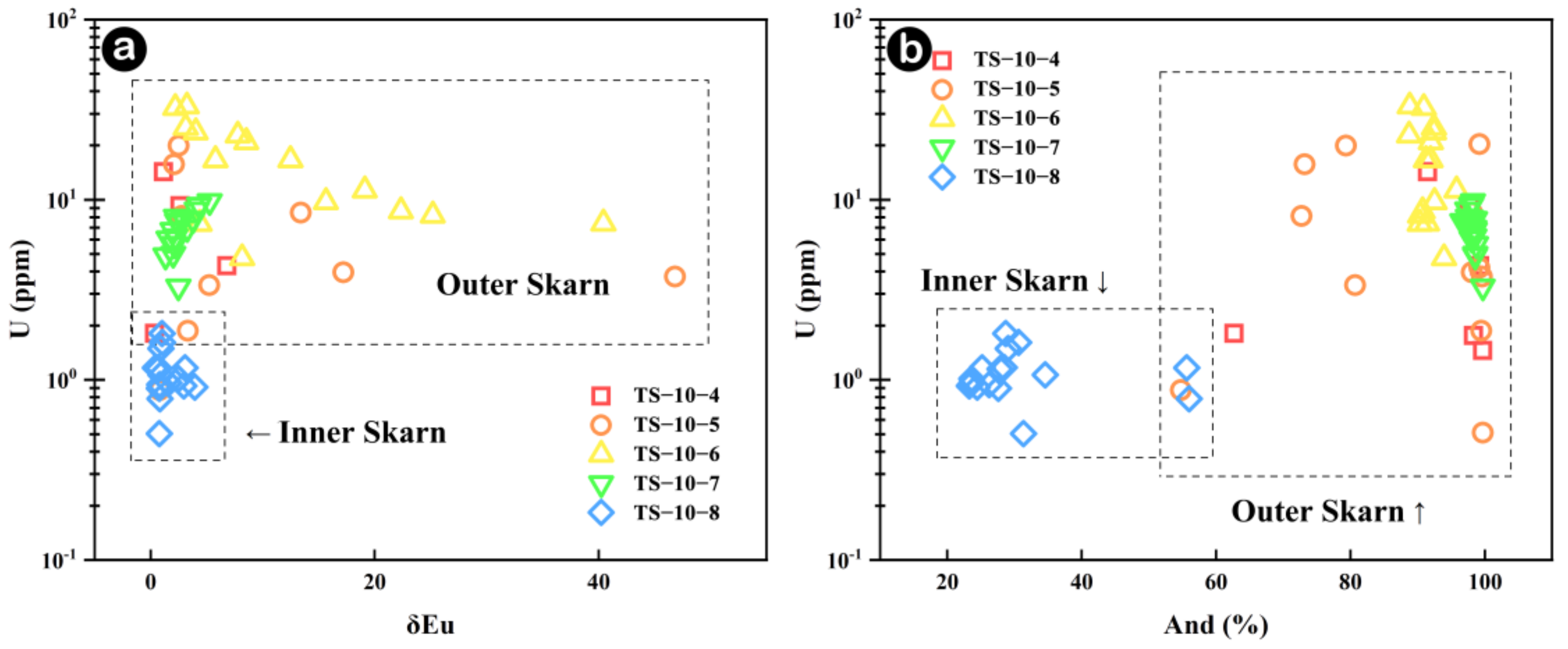
5.2.3. Fluid Behavior Processes Recorded by Garnet
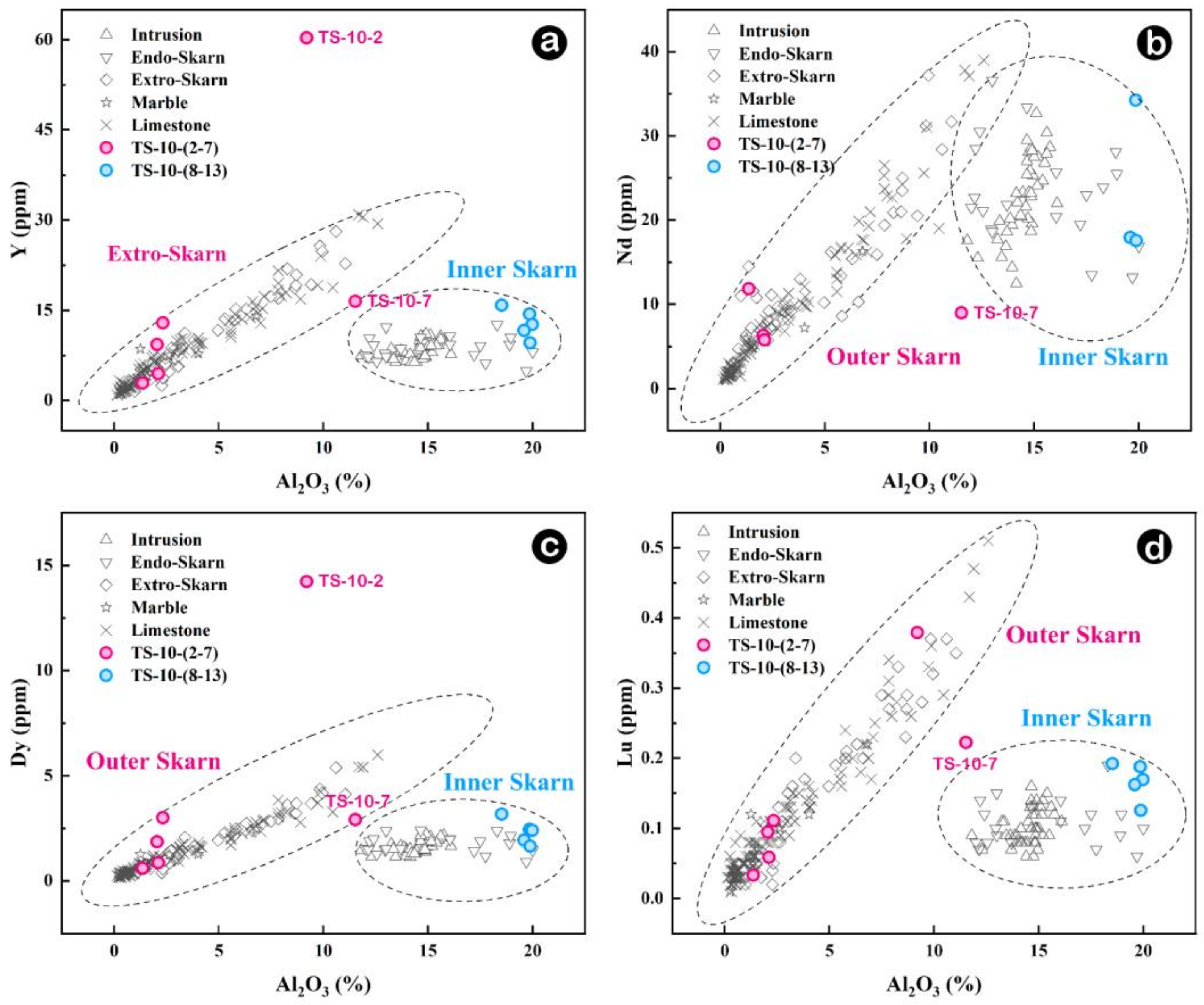
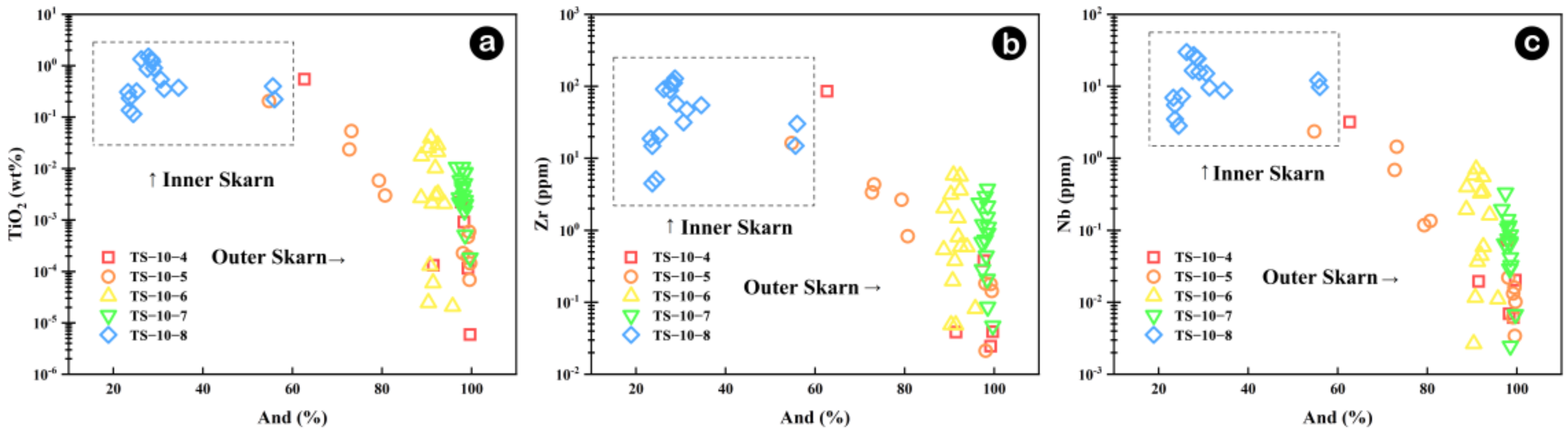
5.3. The Definition of the Inner and Outer Boundaries of Skarn
6. Conclusions
- (1)
- During the skarn formation process, inner skarn is enriched in Ca and Mn, accompanied by the loss of K, Rb and Ba. Compared to the marble zone, the outer skarn shows a large-scale enrichment of elements and certain dilutions of Ca, Sr and Cs. The element contents fluctuate more significantly at the contact zone between the rock body and the surrounding rocks, and the migration and transformation of Th and U are most significant.
- (2)
- Garnet REEs are coupled with Fe in the inner skarn but with Al in the outer skarn following the YAG-type substitution mechanism. Garnets from the outer skarn show a significant variation in the Y/Ho ratio, indicating a large-scale water-rock interaction process in the external outer skarn zone. The U content in garnets may be greatly influenced by fO2 and fluid activity, while the Eu anomaly is not only controlled by fO2 changes in the system but is mainly restricted by various factors such as water-rock ratio, crystal structure and fO2, especially the change in the crystal structure, which is the main controlling factor.
- (3)
- Whole-rock in Na, Al, Ti, and Sr elements between inner and outer skarn, particularly in chondrite-normalized diagrams of trace elements Lu, Dy and Y versus Al2O3 can effectively distinguish inner and outer skarn, except for samples located at the boundary. Fe, In, Al, Mn, Ni, Ti, Zr and Nb contents of garnets can also tell the boundary between inner and outer skarn. However, garnets in skarn are not fully developed, and multi-stage garnet features often occur, which may bring some interference. Whole-rock and mineral geochemistry each have their own advantages and disadvantages. It is more effective to determine the contact boundary of a rock body by combining the two methods for comprehensive analysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Einaudi, M.T.; Meinert, L.D.; Newberry, R.J.; Skinner, B.J. Skarn Deposits. In Seventy-Fifth Anniversary Volume; Economic Geology Publishing Company: Littleton, CO, USA, 1981. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S.; Hedenquist, J.W.; Thompson, J.F.H.; Goldfarb, R.J.; Richards, J.P. World Skarn Deposits. In One Hundredth Anniversary Volume; Economic Geology Publishing Company: Littleton, CO, USA, 2005. [Google Scholar]
- Zhao, Y.; Lin, W.; Bi, C.; Li, D.; Jiang, C. Skarn Deposits of China; Geology Publishing House: Beijing, China, 1990; Volume 354. [Google Scholar]
- Zhou, T. Isotope geochemistry of copper mineralization in Yueshan, Anhui. Miner. Depos. 1996, 15, 341–350. [Google Scholar]
- Lu, H.-Z.; Liu, Y.; Wang, C.; Xu, Y.; Li, H. Mineralization and fluid inclusion study of the Shizhuyuan W-Sn-Bi-Mo-F skarn deposit, Hunan Province, China. Econ. Geol. 2003, 98, 955–974. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, G.; Wang, J.; Li, W.; Yu, B. The relationship between the evaporate and skarn-type iron deposit: A case study of the Jinshandian iron skarn deposit in Hubei. Acta Geol. Sin. 2013, 87, 1419–1428. [Google Scholar]
- Wilkinson, J.J. Triggers for the formation of porphyry ore deposits in magmatic arcs. Nat. Geosci. 2013, 6, 917–925. [Google Scholar] [CrossRef]
- Mrozek, S.A.; Chang, Z.; Spandler, C.; Windle, S.; Raraz, C.; Paz, A. Classifying skarns and quantifying metasomatism at the Antamina deposit, Peru: Insights from whole-rock geochemistry. Econ. Geol. 2020, 115, 177–188. [Google Scholar] [CrossRef]
- Chang, Z.; Meinert, L.D. The Empire Cu-Zn mine, Idaho: Exploration implications of unusual skarn features related to high fluorine activity. Econ. Geol. 2008, 103, 909–938. [Google Scholar] [CrossRef]
- Zang, Z.; Liu, W.; Dong, L. Geology, Geochemistry and Re-Os Age of the Qiaoxiahala Deposit in NW China: Evidence of an Overprinted Fe (-Cu/Au) Deposit. J. Earth Sci. 2021, 32, 208–218. [Google Scholar] [CrossRef]
- Matthews, S.; Marquillas, R.; Kemp, A.; Grange, F.; Gardeweg, M. Active skarn formation beneath Lascar Volcano, northern Chile: A petrographic and geochemical study of xenoliths in eruption products. J. Metamorph. Geol. 1996, 14, 509–530. [Google Scholar] [CrossRef]
- Sun, W.; Niu, Y.; Ma, Y.; Liu, Y.; Zhang, G.; Hu, Z.; Zhang, Z.; Chen, S.; Li, J.; Wang, X. Petrogenesis of the Chagangnuoer deposit, NW China: A general model for submarine volcanic-hosted skarn iron deposits. Sci. Bull. 2015, 60, 363–379. [Google Scholar] [CrossRef]
- Yano, R.I. Trace Element Distribution in Chalcopyrite-Bearing Porphyry and Skarn Deposits; University of Nevada: Reno, NV, USA, 2012. [Google Scholar]
- Gaspar, M.; Knaack, C.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA-ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, Z.; Cheng, Z.; Santosh, M. Factors controlling the crystal morphology and chemistry of garnet in skarn deposits: A case study from the Cuihongshan polymetallic deposit, Lesser Xing’an Range, NE China. Am. Mineral. 2019, 104, 1455–1468. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.-f.; White, N.C.; Zhang, L.-J.; Fan, Y.; Wang, F.-Y.; Chen, X.-F. The formation and trace elements of garnet in the skarn zone from the Xinqiao Cu-S-Fe-Au deposit, Tongling ore district, Anhui Province, Eastern China. Lithos 2018, 302, 467–479. [Google Scholar] [CrossRef]
- Quelhas, P.; Mata, J.; Dias, Á.A. Magmatic Evolution of Garnet-Bearing Highly Fractionated Granitic Rocks from Macao, Southeast China: Implications for Granite-Related Mineralization Processes. J. Earth Sci. 2021, 32, 1454–1471. [Google Scholar] [CrossRef]
- Wang, F.; Ge, C.; Ning, L.; Nie, L.; Zhong, G.; White, N.C. A new approach to LA-ICP-MS mapping and application in geology. Acta Petrol. Sin. 2017, 33, 3422–3436. [Google Scholar]
- Zhu, X.; Wang, Z.; Chen, H. Advances in Isotope Geochronology and Isotope Geochemistry: A Preface. J. Earth Sci. 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Z.; Feng, L.; Wang, Z.; Liu, Y.; Feng, Y.; Liu, H. Accurate determination of Zr isotopic ratio in zircons by femtosecond laser ablation MC-ICP-MS with “Wet” plasma technique. J. Earth Sci. 2022, 33, 67–75. [Google Scholar] [CrossRef]
- Menzer, G. Die Kristallstruckture von granat. Z. Kristallogr. 1926, 63, 157–158. [Google Scholar]
- McIntire, W. Trace element partition coefficients—A review of theory and applications to geology. Geochim. Cosmochim. Acta 1963, 27, 1209–1264. [Google Scholar] [CrossRef]
- Jamtveit, B.; Hervig, R.L. Constraints on transport and kinetics in hydrothermal systems from zoned garnet crystals. Science 1994, 263, 505–508. [Google Scholar] [CrossRef]
- Smith, M.; Henderson, P.; Jeffries, T.; Long, J.; Williams, C. The rare earth elements and uranium in garnets from the Beinn an Dubhaich Aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef]
- Lessing, P.; Standish, R.P. Zoned garnet from Crested Butte, Colorado. Am. Mineral. J. Earth Planet. Mater. 1973, 58, 840–842. [Google Scholar]
- Zhai, D.-G.; Liu, J.-J.; Zhang, H.-Y.; Wang, J.-P.; Su, L.; Yang, X.-A.; Wu, S.-H. Origin of oscillatory zoned garnets from the Xieertala Fe–Zn skarn deposit, northern China: In situ LA–ICP-MS evidence. Lithos 2014, 190, 279–291. [Google Scholar] [CrossRef]
- He, Z.; Li, S.; Li, Y.; Gao, J. Multi-Directional and Multiphase Tectonic Modification, and Hydrocarbon Differential Enrichment in the Middle-Upper Yangtze Region. J. Earth Sci. 2022, 33, 1246–1259. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.-A.; Li, S.; Akhtar, S.; He, Y. Zircon U–Pb ages, Hf–O isotopes and trace elements of Mesozoic high Sr/Y porphyries from Ningzhen, eastern China: Constraints on their petrogenesis, tectonic implications and Cu mineralization. Lithos 2014, 200, 299–316. [Google Scholar] [CrossRef]
- Zhou, T. Advances on petrogenesis and metallogeny study of the mineralization belt of the Middle and Lower Reaches of the Yangtze River area. Acta Petrol. Sin. 2008, 24, 1666–1678. [Google Scholar]
- Liu, S.-A.; Li, S.; He, Y.; Huang, F. Geochemical contrasts between early Cretaceous ore-bearing and ore-barren high-Mg adakites in central-eastern China: Implications for petrogenesis and Cu–Au mineralization. Geochim. Cosmochim. Acta 2010, 74, 7160–7178. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Lehmann, B.; Yu, J.; Du, A.; Mei, Y.; Li, Y.; Zang, W.; Stein, H.J.; Zhou, T. Molybdenite Re–Os and albite 40Ar/39Ar dating of Cu–Au–Mo and magnetite porphyry systems in the Yangtze River valley and metallogenic implications. Ore Geol. Rev. 2006, 29, 307–324. [Google Scholar] [CrossRef]
- Wang, L.; Ji, K.; Chen, D. Re-Os isotope ages of molybdenite from the Anjishan copper deposit and the Tongshan copper-molybdenum deposit and their implications. Acta Petrol. Mineral. 1997, 16, 154–159. [Google Scholar]
- Zhang, J.; Liang, X.; Wang, F.; Wang, H.; Fan, Y.; Ba, T.; Meng, X. CorelKit: An Extensible CorelDraw VBA Program for Geoscience Drawing. J. Earth Sci. 2023, 34, 735–757. [Google Scholar] [CrossRef]
- Rudnick, R.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry; Rudnick, R.L., Ed.; The Crust; Elsevier: Pergamon, Turkey, 2003; Volume 3. [Google Scholar]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Hu, Z.; Gao, C.; Zong, K.; Wang, D. Continental and oceanic crust recycling-induced melt–peridotite interactions in the Trans-North China Orogen: U–Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Petrol. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- Shen, J.; Qin, L.; Fang, Z.; Zhang, Y.; Liu, J.; Liu, W.; Wang, F.; Xiao, Y.; Yu, H.; Wei, S. High-temperature inter-mineral Cr isotope fractionation: A comparison of ionic model predictions and experimental investigations of mantle xenoliths from the North China Craton. Earth Planet. Sci. Lett. 2018, 499, 278–290. [Google Scholar] [CrossRef]
- Guo, S.; Ye, K.; Chen, Y.; Liu, J.-B. A normalization solution to mass transfer illustration of multiple progressively altered samples using the isocon diagram. Econ. Geol. 2009, 104, 881–886. [Google Scholar] [CrossRef]
- Grant, J.A. The isocon diagram; a simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Guo, S.; Ye, K.; Chen, Y.; Liu, J.; Zhang, L. Introduction of mass-balance calculation method for component transfer during the opening of a geological system. Acta Petrol. Sin. 2013, 29, 1486–1498. [Google Scholar]
- Gresens, R.L. Composition-volume relationships of metasomatism. Chem. Geol. 1967, 2, 47–65. [Google Scholar] [CrossRef]
- Ague, J.J. Mass transfer during Barrovian metamorphism of pelites, south-central Connecticut; I, Evidence for changes in composition and volume. Am. J. Sci. 1994, 294, 989–1057. [Google Scholar] [CrossRef]
- Ague, J.J. Crustal mass transfer and index mineral growth in Barrow’s garnet zone, northeast Scotland. Geology 1997, 25, 73–76. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth Parts A/B/C 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Enami, M.; Bolin, C.; Yoshida, T.; Kawabe, I. A mechanism for Na incorporation in garnet: An example from garnet in orthogneiss from the Su-Lu terrane, eastern China. Am. Mineral. 1995, 80, 475–482. [Google Scholar] [CrossRef]
- Carlson, W.D. Rates and mechanism of Y, REE, and Cr diffusion in garnet. Am. Mineral. 2012, 97, 1598–1618. [Google Scholar] [CrossRef]
- Grew, E.S.; Marsh, J.H.; Yates, M.G.; Lazic, B.; Armbruster, T.; Locock, A.; Bell, S.W.; Dyar, M.D.; Bernhardt, H., Jr.; Medenbach, O. Menzerite-(Y), a new species,{(Y, REE)(Ca, Fe2+) 2}[(Mg, Fe2+)(Fe3+, Al)](Si3) O12, from a felsic granulite, Parry Sound, Ontario, and a new garnet end-member,{Y2Ca}[Mg2](Si3) O12. Can. Mineral. 2010, 48, 1171–1193. [Google Scholar] [CrossRef]
- Jaffe, H.W. The role of yttrium and other minor elements in the garnet group. Am. Mineral. J. Earth Planet. Mater. 1951, 36, 133–155. [Google Scholar]
- Quartieri, S.; Antonioli, G.; Geiger, C.; Artioli, G.; Lottici, P. XAFS characterization of the structural site of Yb in synthetic pyrope and grossular garnets. Phys. Chem. Miner. 1999, 26, 251–256. [Google Scholar] [CrossRef]
- Quartieri, S.; Chaboy, J.; Antonioli, G.; Geiger, C. XAFS characterization of the structural site of Yb in synthetic pyrope and grossular garnets. II: XANES full multiple scattering calculations at the Yb LI-and L III-edges. Phys. Chem. Miner. 1999, 27, 88–94. [Google Scholar] [CrossRef]
- Dziggel, A.; Wulff, K.; Kolb, J.; Meyer, F.M.; Lahaye, Y. Significance of oscillatory and bell-shaped growth zoning in hydrothermal garnet: Evidence from the Navachab gold deposit, Namibia. Chem. Geol. 2009, 262, 262–276. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Liang, X. Garnets of Grossular—Andraditend Series: Their Characteristics and Metasomatic Mechanism. Acta Petrol. Mineral. 1994, 13, 342–352. [Google Scholar]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W., Jr. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- Van Westrenen, W.; Allan, N.; Blundy, J.; Purton, J.; Wood, B. Atomistic simulation of trace element incorporation into garnets—Comparison with experimental garnet-melt partitioning data. Geochim. Cosmochim. Acta 2000, 64, 1629–1639. [Google Scholar] [CrossRef]
- Paz, A.; Raraz, C.; Paredes, D.; Mendoza, C.; Windle, S. Caracterización Litogeoquímica de las Formaciones Jumasha-Celendin Metalotectos Relacionados a la Mineralización de Cu-Zn Cercano al Yacimiento de Antamina. In Proceedings of the 9th International Congress of Prospectors and Explorers (ProEXPLO), Lima, Peru, 18–20 May 2015; Extended Abstract. p. 43. [Google Scholar]


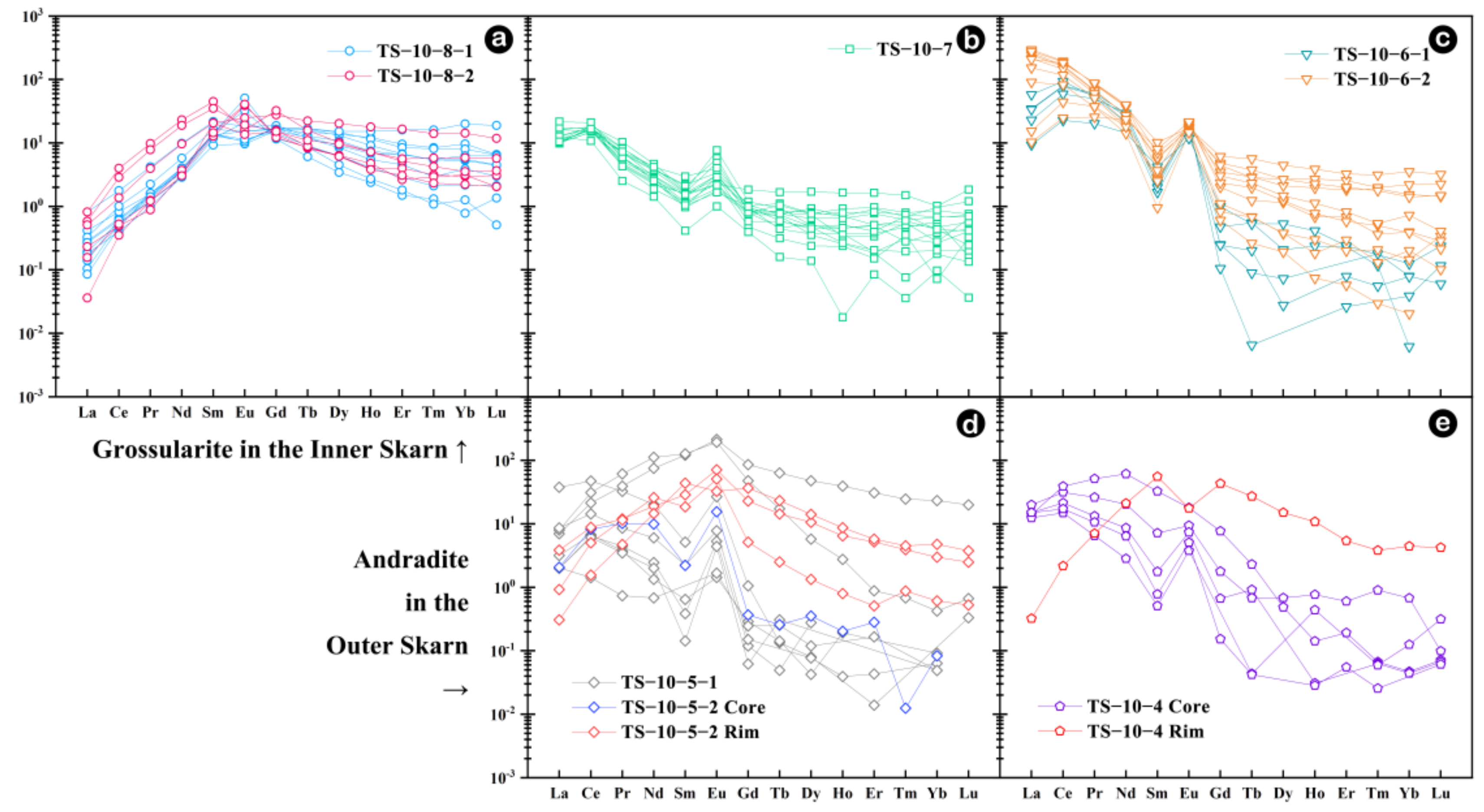


| Sample | Belt | Min (∑REE) | Max (∑REE) | Avg (∑REE) |
|---|---|---|---|---|
| TS10-8 | Inner Skarn | 10.14 | 34.02 | 17.33 |
| TS10-7 | Outer Skarn | 10.71 | 20.71 | 16.29 |
| TS10-6 | 26.48 | 220.73 | 117.40 | |
| TS10-5 | 1.96 | 129.13 | 36.84 | |
| TS10-4 | 14.35 | 68.50 | 33.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, R.; Wang, F.; Zhang, W. Whole Rock, Mineral Chemistry during Skarn Mineralization-Case Study from Tongshan Cu-Mo Skarn Profile. Appl. Sci. 2023, 13, 8118. https://doi.org/10.3390/app13148118
Bi R, Wang F, Zhang W. Whole Rock, Mineral Chemistry during Skarn Mineralization-Case Study from Tongshan Cu-Mo Skarn Profile. Applied Sciences. 2023; 13(14):8118. https://doi.org/10.3390/app13148118
Chicago/Turabian StyleBi, Ran, Fangyue Wang, and Wenqi Zhang. 2023. "Whole Rock, Mineral Chemistry during Skarn Mineralization-Case Study from Tongshan Cu-Mo Skarn Profile" Applied Sciences 13, no. 14: 8118. https://doi.org/10.3390/app13148118
APA StyleBi, R., Wang, F., & Zhang, W. (2023). Whole Rock, Mineral Chemistry during Skarn Mineralization-Case Study from Tongshan Cu-Mo Skarn Profile. Applied Sciences, 13(14), 8118. https://doi.org/10.3390/app13148118






