Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Measurements and Analysis
2.3. Statistical Analysis
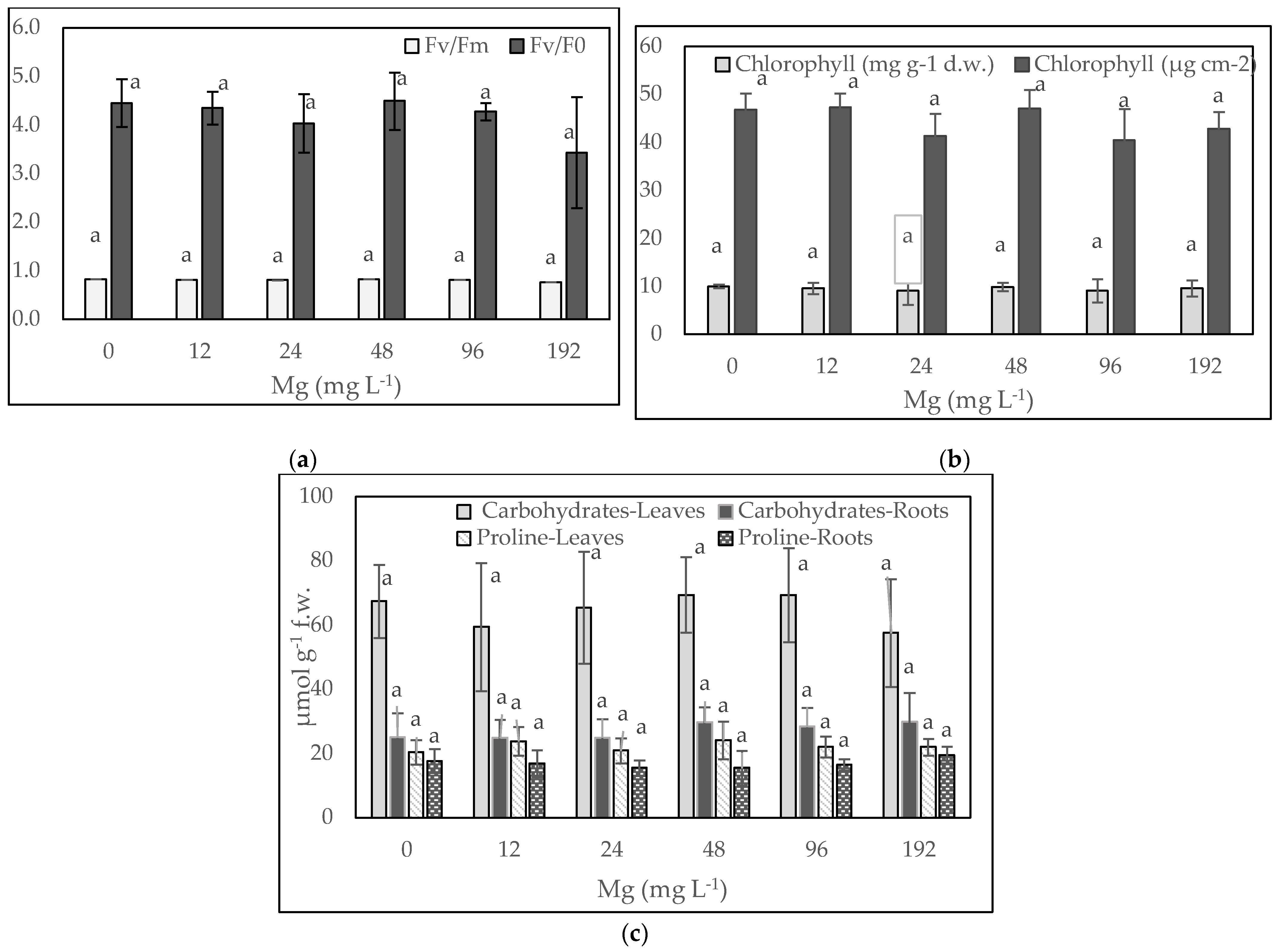
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 1–672. [Google Scholar]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015; pp. 1–761. [Google Scholar]
- Spiegel-Roy, P.; Goldschmidt, E.E. The Biology of Citrus; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Sun, O.J.; Payn, T.W. Magnesium nutrition and photosynthesis in Pinus radiata: Clonal variation and influence of potassium. Tree Physiol. 1999, 19, 535–540. [Google Scholar] [CrossRef]
- Tůma, J.; Skalický, M.; Tůmová, L.; Bláhová, P.; Rosůlková, M. Potassium, magnesium and calcium content in individual parts of Phaseolus vulgaris L. plant as related to potassium and magnesium nutrition. Plant Soil Environ. 2004, 50, 18–26. [Google Scholar]
- Lasa, B.; Frechilla, S.; Aleu, M.; Gonzalez-Moro, B.; Lamsfus, C.; Aparicio-Tejo, P.M. Effects of low and high levels of magnesium on the response of sunflower plants grown with ammonium and nitrate. Plant Soil 2000, 225, 167–174. [Google Scholar] [CrossRef]
- D’Egidio, S.; Galieni, A.; Stagnari, F.; Pagnani, G.; Pisante, M. Yield, quality and physiological traits of red beet under different magnesium nutrition and light intensity levels. Agronomy 2019, 9, 379. [Google Scholar] [CrossRef]
- Geng, G.; Cakmak, I.; Ren, T.; Lu, Z.; Lu, J. Effect of magnesium fertilization on seed yield, seed quality, carbon assimilation and nutrient uptake of rapeseed plants. Field Crops Res. 2021, 264, 108082. [Google Scholar] [CrossRef]
- Yang, G.H.; Yang, L.T.; Jiang, H.X.; Li, Y.; Wang, P.; Chen, L.S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Delfs-Fritz, W. Citrus: Cultivation and Fertilization; (No. DEL 634.3 (BR 522.6)); Ruhr-Stickstoff AG: Bochum, Germany, 1970. [Google Scholar]
- Billard, V.; Maillard, A.; Coquet, L.; Jouenne, T.; Cruz, F.; Garcia-Mina, J.M.; Yvin, J.-C.; Qurry, A.; Etienne, P. Mg deficiency affects leaf Mg remobilization and the proteome in Brassica napus. Plant Physiol. Biochem. 2016, 107, 337–343. [Google Scholar] [CrossRef]
- Huang, J.H.; Xu, J.; Ye, X.; Luo, T.Y.; Ren, L.H.; Fan, G.C.; Qi, Y.-P.; Qiang, L.; Ferrarezi, R.S.; Chen, L.S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees 2019, 33, 171–182. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants: Development, Visual and Analytical Diagnosis; Gustav Fischer: New York, NY, USA, 1992. [Google Scholar]
- Ling, L.L.; Peng, L.Z.; Cao, L.; Jiang, C.L.; Chun, C.P.; Zhang, G.Y.; Wang, Z.X. Effect of magnesium deficiency on photosynthesis characteristic of Beibei 447 Jinchen orange. J. Fruit Sci. 2009, 26, 275–280. [Google Scholar]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Edmeades, D.C. The magnesium requirements of pastures in New Zealand: A review. N. Z. J. Agric. Res. 2004, 47, 363–380. [Google Scholar] [CrossRef]
- Morton, A.R.; Trolove, S.N.; Kerckhoffs, L.H.J. Magnesium deficiency in citrus grown in the Gisborne district of New Zealand. N. Z. J. Crop Hortic. Sci. 2008, 36, 199–213. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.Q.; Lin, F.; Ten, Y.; Lin, J.; Zhu, D.H.; Guo, P.; Weng, Y.-B.; Chen, L.S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015, 15, 615–628. [Google Scholar] [CrossRef]
- Ma, C.L.; Qi, Y.P.; Liang, W.W.; Yang, L.T.; Lu, Y.B.; Guo, P.; Ye, X.; Chen, L.S. MicroRNA regulatory mechanisms on Citrus sinensis leaves to magnesium-deficiency. Front. Plant Sci. 2016, 7, 201. [Google Scholar] [CrossRef]
- Liang, W.W.; Huang, J.H.; Li, C.P.; Yang, L.T.; Ye, X.; Lin, D.; Chen, L.S. MicroRNA-mediated responses to long-term magnesium-deficiency in Citrus sinensis roots revealed by Illumina sequencing. BMC Genom. 2017, 18, 657. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef]
- Aitken, R.L.; Dickson, T.; Hailes, K.J.; Moody, P.W. Response of field-grown maize to applied magnesium in acidic soils in north-eastern Australia. Aust. J. Agric. Res. 1999, 50, 191–198. [Google Scholar] [CrossRef]
- Ling, L.L.; Peng, L.Z.; Wang, N.Q.; Xing, F.; Jiang, C.L.; Cao, L.; Chun, C.P. Influence of magnesium deficiency on chlorophyll fluorescence characteristic in leaves of Newhall navel orange. Acta Ecol. Sin. 2013, 33, 71–78. [Google Scholar]
- Xiao, J.X.; Hu, C.Y.; Chen, Y.Y.; Yang, B.; Hua, J. Effects of low magnesium and an arbuscular mycorrhizal fungus on the growth, magnesium distribution and photosynthesis of two citrus cultivars. Sci. Hortic. 2014, 177, 14–20. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Hippler, F.W.; Ferreira, G.A.; Azevedo, R.A.; Quaggio, J.A.; Mattos, D. The possible role of extra magnesium and nitrogen supply to alleviate stress caused by high irradiation and temperature in lemon trees. Plant Soil 2020, 457, 57–70. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In Circular—California Agricultural Experiment Station, 2nd ed.; CAS: Columbus, OH, USA, 1950; p. 347. [Google Scholar]
- Papadakis, I.E.; Dimassi, K.N.; Therios, I.N. Response of two citrus genotypes to six boron concentrations: Concentration and distribution of nutrients, total absorption, and nutrient use efficiency. Aust. J. Agric. Res. 2003, 54, 571–580. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Chemical and Microbiological Properties; SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Wintermans, J.F.G.M.; De Mots, A. Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Bioch. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Khan, A.A.; McNeilly, T.; Collins, J.C. Accumulation of amino acids, proline and carbohydrates in response to aluminum and manganese stress in maize. J. Plant Nutr. 2000, 23, 1303–1314. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. Absorption of magnesium and its influence on the uptake of phosphorus, potassium, and calcium by intact groundnut plants. Plant Soil 1974, 40, 313–320. [Google Scholar] [CrossRef]
- Moss, G.I.; Higgins, M.L. Magnesium influences on the fruit quality of sweet orange (Citrus sinensis L. Osbeck). Plant Soil 1974, 41, 103–112. [Google Scholar]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Mühling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef]
- de Lima Días, K.G.; Guimarães, P.T.G.; Furtini Neto, A.E.; Silveira, H.R.O.D.; Lacerda, J.J.D.J. Effect of magnesium on gas exchange and photosynthetic efficiency of coffee plants grown under different light levels. Agriculture 2017, 7, 85. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Faust, F.; Schubert, S. Protein synthesis is the most sensitive process when potassium is substituted by sodium in the nutrition of sugar beet (Beta vulgaris). Plant Physiol. Biochem. 2016, 107, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar]
- Tian, X.Y.; He, D.D.; Bai, S.; Zeng, W.Z.; Wang, Z.; Wang, M.; Wu, L.Q.; Chen, Z.C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrovam, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Henderson, J.N.; Liles, K.; Hilton, M.T.; Wachter, R.M. Regulation of ribulose-1, 5-bisphosphate carboxylase/oxygenase (rubisco) activase: Product inhibition, cooperativity, and magnesium activation. J. Biol. Chem. 2015, 290, 24222–24236. [Google Scholar] [CrossRef]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]
| Parameter | Mg Concentration of the Nutrient Solution (mg L−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 48 | 96 | 192 | ||
| Mg (% d.w. *) | Leaves | 0.26 ± 0.03 d 1 | 0.30 ± 0.05 cd | 0.33 ± 0.03 bc | 0.36 ± 0.02 b | 0.37 ± 0.04 ab | 0.40 ± 0.03 a |
| Scion’s stems | 0.19 ± 0.02 d | 0.21 ± 0.02 bc | 0.21 ± 0.01 bc | 0.23 ± 0.02 b | 0.22 ± 0.02 bc | 0.33 ± 0.06 a | |
| Rootstock’s stem | 0.09 ± 0.01 b | 0.09 ± 0.01 b | 0.09 ± 0.01 b | 0.10 ± 0.01 ab | 0.10 ± 0.01 ab | 0.11 ± 0.01 a | |
| Roots | 0.28 ± 0.03 bc | 0.27 ± 0.03 c | 0.30 ± 0.02 bc | 0.30 ± 0.02 bc | 0.31 ± 0.03 ab | 0.34 ± 0.04 a | |
| Ca (% d.w.) | Leaves | 2.67 ± 0.14 a | 2.77 ± 0.13 a | 2.66 ± 0.09 a | 2.44 ± 0.14 b | 2.46 ± 0.15 b | 2.19 ± 0.08 c |
| Scion’s stems | 1.52 ± 0.19 ab | 1.65 ± 0.19 a | 1.62 ± 0.25 a | 1.37 ± 0.09 bc | 1.50 ± 0.13 ab | 1.27 ± 0.17 c | |
| Rootstock’s stem | 0.89 ± 0.07 b | 0.93 ± 0.04 b | 0.93 ± 0.08 b | 0.97 ± 0.12 b | 0.96 ± 0.06 b | 1.06 ± 0.04 a | |
| Roots | 1.25 ± 0.09 a | 1.25 ± 0.16 a | 1.22 ± 0.09 a | 1.25 ± 0.09 a | 1.23 ± 0.09 a | 1.00 ± 0.09 b | |
| K (% d.w.) | Leaves | 2.22 ± 0.19 a | 2.25 ± 0.12 a | 2.21 ± 0.10 a | 2.25 ± 0.18 a | 2.31 ± 0.10 a | 2.30 ± 0.08 a |
| Scion’s stems | 1.58 ± 0.14 a | 1.55 ± 0.18 a | 1.50 ± 0.12 a | 1.57 ± 0.13 a | 1.48 ± 0.36 a | 1.65 ± 0.25 a | |
| Rootstock’s stem | 0.47 ± 0.05 a | 0.39 ± 0.06 a | 0.44 ± 0.04 a | 0.44 ± 0.04 a | 0.43 ± 0.10 a | 0.46 ± 0.06 a | |
| Roots | 1.31 ± 0.07 b | 1.27 ± 0.24 b | 1.27 ± 0.12 b | 1.47 ± 0.14 ab | 1.47 ± 0.19 ab | 1.60 ± 0.15 a | |
| P (% d.w.) | Leaves | 0.24 ± 0.03 a | 0.24 ± 0.02 a | 0.23 ± 0.02 a | 0.23 ± 0.02 a | 0.24 ± 0.05 a | 0.24 ± 0.04 a |
| Scion’s stems | 0.26 ± 0.02 a | 0.27 ± 0.02 a | 0.23 ± 0.03 a | 0.25 ± 0.02 a | 0.20 ± 0.10 a | 0.25 ± 0.10 a | |
| Rootstock’s stem | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a | |
| Roots | 0.09 ± 0.01 a | 0.10 ± 0.01 a | 0.11 ± 0.02 a | 0.10 ± 0.01 a | 0.09 ± 0.01 a | 0.11 ± 0.02 a | |
| Fe (mg kg−1 d.w.) | Leaves | 61.05 ± 5.48 bc | 62.00 ± 2.26 bc | 59.17 ± 2.73 c | 62.83 ± 3.86 bc | 65.50 ± 5.53 ab | 69.92 ± 6.46 a |
| Scion’s stems | 45.42 ± 1.02 a | 44.42 ± 3.83 a | 42.27 ± 10.72 a | 42.03 ± 4.57 a | 34.54 ± 17.02 a | 43.00 ± 2.61 a | |
| Rootstock’s stem | 32.17 ± 2.36 a | 35.55 ± 5.86 a | 34.83 ± 4.17 a | 35.33 ± 3.27 a | 36.75 ± 3.36 a | 39.58 ± 3.48 a | |
| Roots | 1040 ± 71 ab | 1158 ± 237 a | 980 ± 114 abc | 1005 ± 152 ab | 947 ± 126 bc | 804 ± 137 c | |
| Mn (mg kg−1 d.w.) | Leaves | 14.33 ± 1.25 a | 9.25 ± 1.29 bc | 9.00 ± 1.10 c | 10.92 ± 1.16 b | 9.67 ± 2.09 bc | 9.67 ± 0.88 bc |
| Scion’s stems | 3.92 ± 0.92 a | 4.08 ± 0.97 a | 3.58 ± 0.49 a | 3.89 ± 0.71 a | 3.32 ± 1.82 a | 4.33 ± 0.75 a | |
| Rootstock’s stem | 4.00 ± 0.45 a | 3.25 ± 0.52 a | 2.75 ± 0.76 b | 3.17 ± 0.41 bc | 3.00 ± 0.63 bc | 2.33 ± 0.41 c | |
| Roots | 43.67 ± 8.48 a | 45.17 ± 7.00 b | 35.00 ± 6.81 abc | 33.50 ± 4.04 b | 34.50 ± 3.67 bc | 27.17 ± 4.12 c | |
| Zn (mg kg−1 d.w.) | Leaves | 9.08 ± 0.58 a | 9.50 ± 0.95 a | 9.83 ± 0.68 a | 10.67 ± 1.47 a | 9.25 ± 0.61 a | 9.50 ± 1.14 a |
| Scion’s stems | 8.86 ± 1.82 a | 10.12 ± 2.21 a | 9.08 ± 2.76 a | 10.78 ± 2.03 a | 9.12 ± 4.66 a | 9.08 ± 2.22 a | |
| Rootstock’s stem | 4.25 ± 0.27 c | 5.08 ± 0.86 abc | 5.83 ± 1.17 a | 5.58 ± 0.66 a | 5.33 ± 0.61 ab | 4.58 ± 0.49 bc | |
| Roots | 11.00 ± 0.89 b | 13.67 ± 1.97 a | 14.67 ± 1.37 a | 16.33 ± 3.56 a | 15.33 ± 1.21 a | 14.33 ± 2.94 a | |
| Total plant weight | Fresh weight (g) | 91.84 ± 8.53 a | 91.52 ± 16.76 a | 87.01 ± 10.72 a | 93.81 ± 10.26 a | 84.29 ± 12.16 a | 83.00 ± 14.01 a |
| Dry weight (g) | 34.46 ± 3.19 a | 35.12 ± 7.29 a | 32.73 ± 3.18 a | 35.80 ± 4.71 a | 32.72 ± 4.59 a | 30.86 ± 5.81 a | |
| Parameters | Macronutrients | Micronutrients | ||||||
|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Mn | Zn | Fe | ||
| Nutrient concentrations of various plant parts | Leaves | 0.095 | 0.248 | −0.808 *** | 0.722 *** | −0.299 | −0.029 | 0.605 *** |
| Scion’s stems | −0.138 | 0.124 | −0.497 ** | 0.802 *** | 0.098 | −0.058 | −0.137 | |
| Rootstock’s stem | −0.280 | 0.142 | 0.588 *** | 0.579 *** | −0.547 ** | −0.118 | 0.487 ** | |
| Roots | 0.213 | 0.584 *** | −0.612 *** | 0.634 *** | −0.633 *** | −0.226 | −0.544 ** | |
| Total element absorption | −0.599 *** | −0.371 * | −0.495 ** | 0.511 ** | −0.700 *** | −0.175 | −0.717 *** | |
| Element use efficiency | 0.239 | −0.029 | 0.177 | −0.677 *** | 0.478 ** | −0.151 | 0.464 ** | |
| Parameter | Mg Concentration of the Nutrient Solution (mg L−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 48 | 96 | 192 | ||
| Mg | Total amount per plant (mg) | 51.11 ± 4.78 c 1 | 57.95 ± 8.79 bc | 56.74 ± 5.05 bc | 62.90 ± 4.13 ab | 60.36 ± 3.62 abc | 67.84 ± 14.21 a |
| Use efficiency (mg d.w./mg Mg) | 678.76 ± 85.06 a | 603.15 ± 58.88 ab | 577.54 ± 38.50 b | 570.14 ± 71.95 b | 543.60 ± 79.17 b | 459.11 ± 56.08 c | |
| Ca | Total amount per plant (mg) | 452.23 ± 29.74 a | 424.06 ± 66.37 a | 413.49 ± 40.40 a | 415.99 ± 40.79 a | 402.01 ± 52.79 a | 365.95 ± 30.06 a |
| Use efficiency (mg d.w./mg Ca) | 76.21 ± 5.01 a | 82.71 ± 11.31 a | 79.15 ± 1.35 a | 86.55 ± 12.23 a | 81.56 ± 6.09 a | 84.11 ± 12.42 a | |
| K | Total amount per plant (mg) | 331.83 ± 15.79 a | 318.01 ± 35.32 ab | 298.51 ± 18.83 b | 337.67 ± 29.20 a | 311.50 ± 19.79 ab | 290.84 ± 25.46 b |
| Use efficiency (mg d.w./mg K) | 103.76 ± 6.65 a | 109.56 ± 12.57 a | 109.84 ± 10.70 a | 106.05 ± 9.92 a | 105.77 ± 19.14 a | 106.35 ± 19.87 a | |
| P | Total amount per plant (mg) | 38.38 ± 3.39 a | 36.53 ± 5.04 ab | 35.08 ± 3.46 ab | 36.84 ± 2.12 ab | 33.17 ± 3.34 bc | 30.61 ± 1.71 c |
| Use efficiency (mg d.w./mg P) | 902.10 ± 93.76 a | 955.95 ± 107.57 a | 937.03 ± 90.52 a | 975.18 ± 146.43 a | 990.64 ± 138.35 a | 1009.85 ± 194.9 a | |
| Mn | Total amount per plant (μg) | 4290 ± 290 a | 3878 ± 350 b | 3129 ± 443 cd | 3298 ± 238 c | 2846 ± 372 de | 2705 ± 273 e |
| Use efficiency (mg d.w./μg Mn) | 8.08 ± 1.09 c | 9.01 ± 1.41 bc | 10.63 ± 1.73 ab | 10.91 ± 1.71 ab | 11.58 ± 1.54 a | 11.51 ± 2.37 a | |
| Zn | Total amount per plant (μg) | 2199 ± 91 ab | 2344 ± 228 abc | 2404 ± 80 bc | 2735 ± 227 d | 2485 ± 58 c | 2168 ± 227 a |
| Use efficiency (mg d.w./μg Zn) | 15.65 ± 0.92 a | 14.95 ± 2.70 a | 13.66 ± 1.76 a | 13.22 ± 2.48 a | 13.15 ± 1.58 a | 14.25 ± 2.15 a | |
| Fe | Total amount per plant (μg) | 71,591 ± 4295 cd | 72,105 ± 7047 d | 62,928 ± 11,334 bc | 63,332 ± 2952 bc | 56,337 ± 4348 ab | 50,543 ± 8353 a |
| Use efficiency (mg d.w./μg Fe) | 0.48 ± 0.05 a | 0.50 ± 0.13 a | 0.53 ± 0.09 a | 0.56 ± 0.05 a | 0.58 ± 0.06 a | 0.62 ± 0.13 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, I.E.; Antonopoulou, C.; Sotiropoulos, T.; Chatzissavvidis, C.; Therios, I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants. Appl. Sci. 2023, 13, 7995. https://doi.org/10.3390/app13147995
Papadakis IE, Antonopoulou C, Sotiropoulos T, Chatzissavvidis C, Therios I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants. Applied Sciences. 2023; 13(14):7995. https://doi.org/10.3390/app13147995
Chicago/Turabian StylePapadakis, Ioannis E., Chrysovalantou Antonopoulou, Thomas Sotiropoulos, Christos Chatzissavvidis, and Ioannis Therios. 2023. "Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants" Applied Sciences 13, no. 14: 7995. https://doi.org/10.3390/app13147995
APA StylePapadakis, I. E., Antonopoulou, C., Sotiropoulos, T., Chatzissavvidis, C., & Therios, I. (2023). Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants. Applied Sciences, 13(14), 7995. https://doi.org/10.3390/app13147995








