Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application
Abstract
1. Introduction
2. Experimental
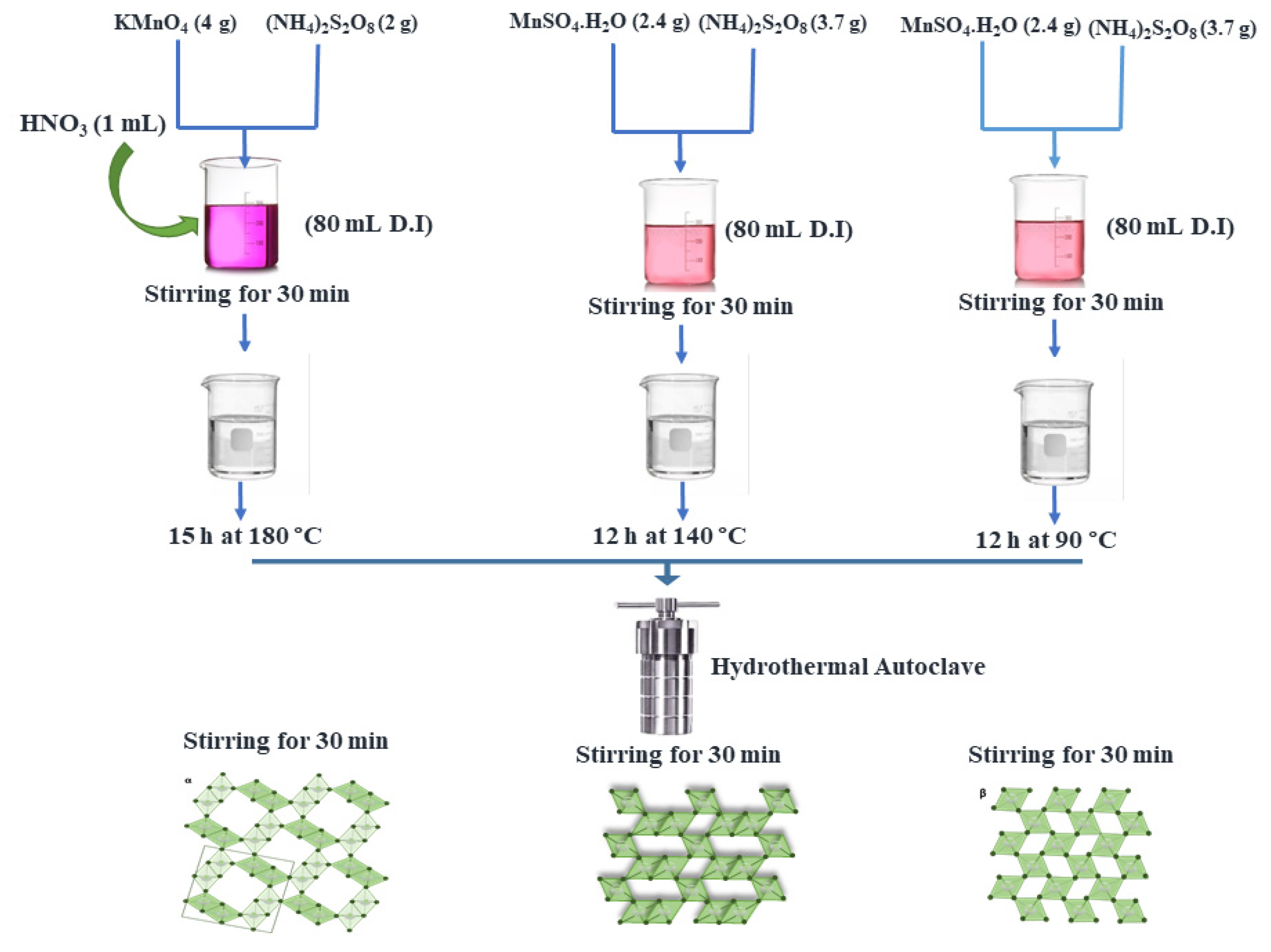
2.1. Material Preparation
2.2. Electrode Preparation and Electrochemical Measurements for Supercapacitor
3. Results and Discussion
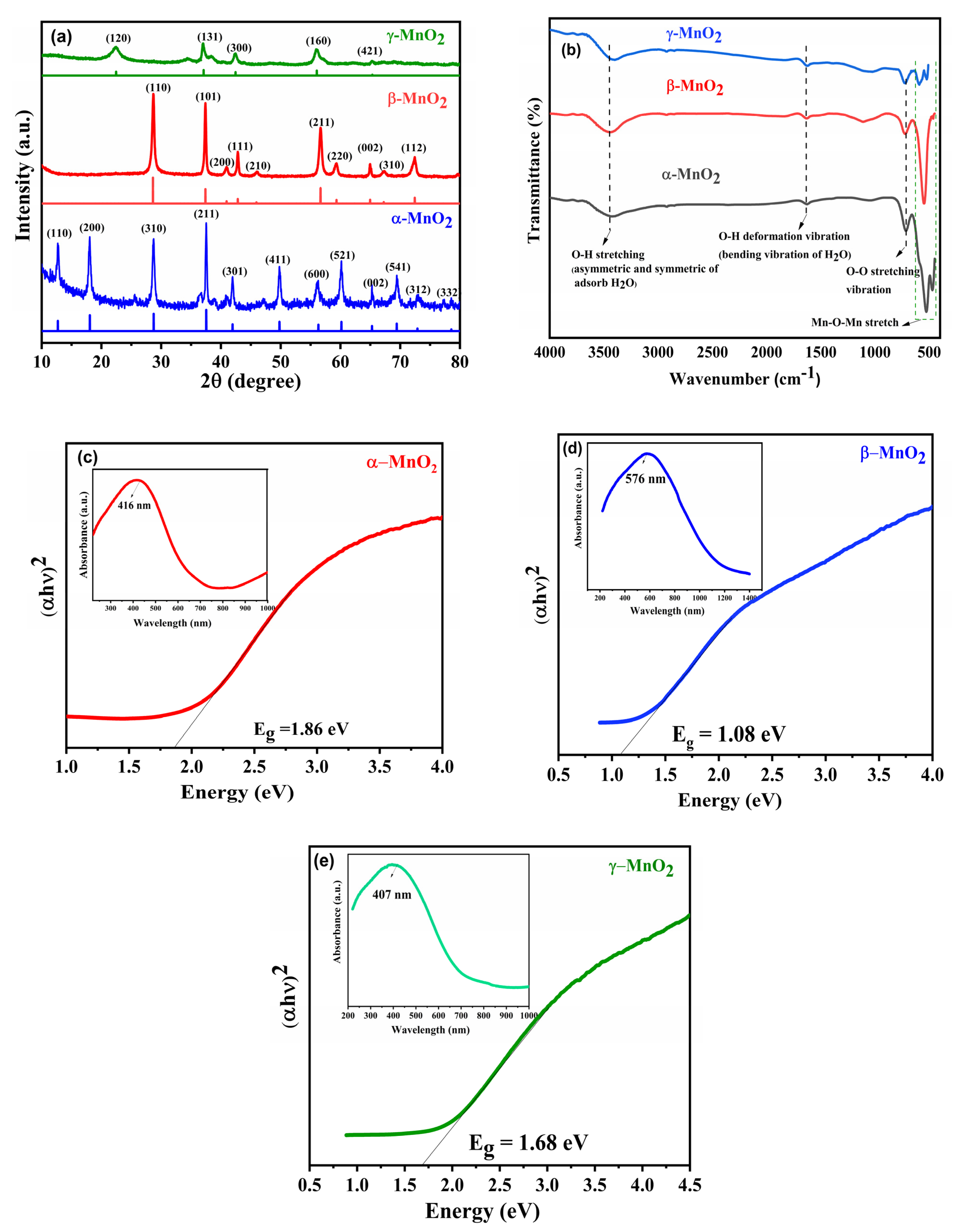
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. UV–Vis Analysis
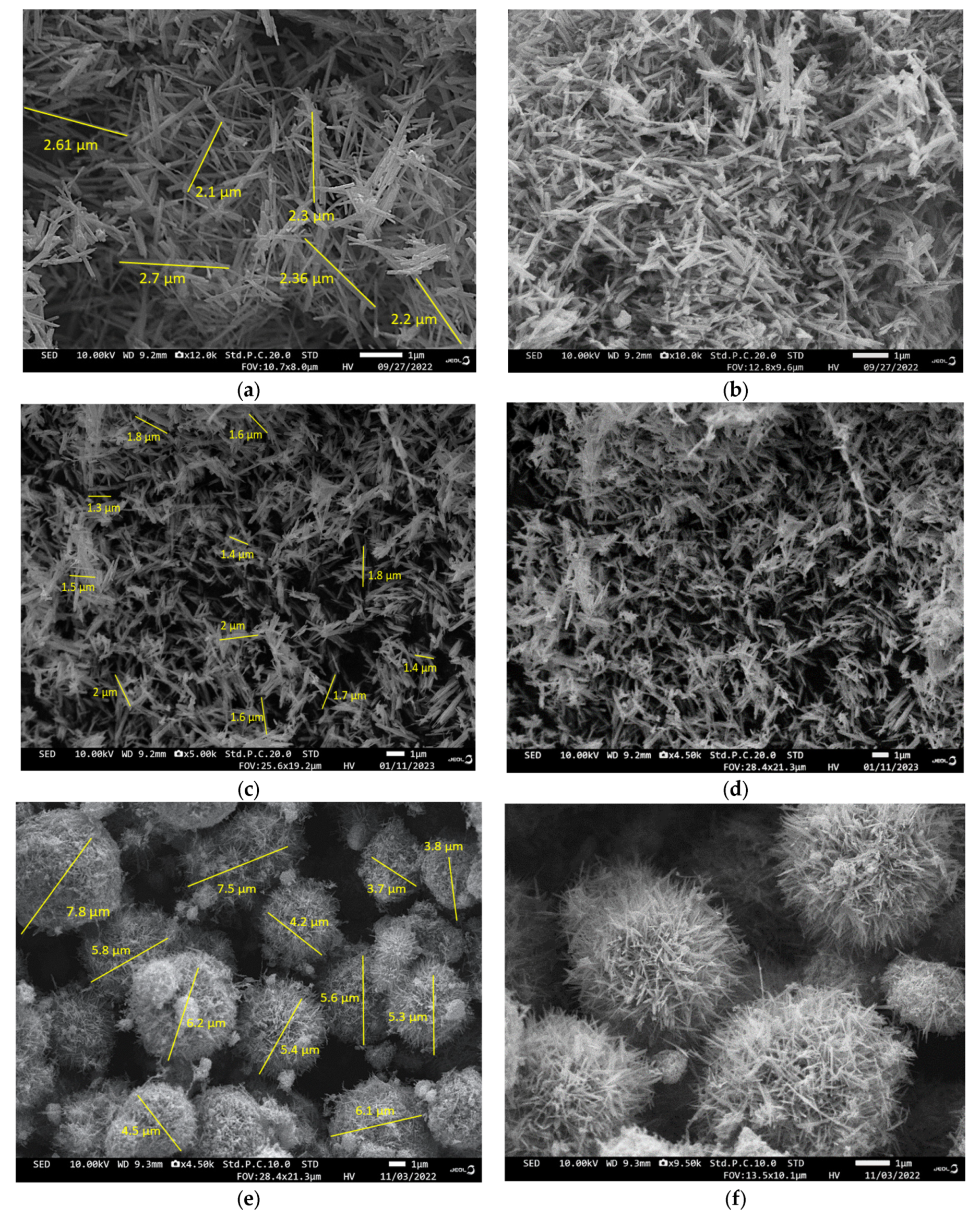
3.4. FE-SEM Analysis
3.5. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musil, M.; Choi, B.; Tsutsumi, A. Morphology and Electrochemical Properties of α-, β-, γ-, and δ-MnO2 Synthesized by Redox Method. J. Electrochem. Soc. 2015, 162, A2058. [Google Scholar] [CrossRef]
- Su, X.; Liang, Z.; He, Q.; Guo, Y.; Luo, G.; Han, S.; Yu, L. Advanced three-dimensional hierarchical porous α-MnO2 nanowires network toward enhanced supercapacitive performance. Nanotechnology 2022. [Google Scholar] [CrossRef] [PubMed]
- Modi, K.H.; Pataniya, P.M.; Siraj, S.; Sahatiya, P.; Patel, V.; Sumesh, C.K. Synergistic effect from Ni2+ ions with SnS for all solid-state type symmetric supercapacitor. J. Energy Storage 2023, 63, 107040. [Google Scholar] [CrossRef]
- Rani, P.; Dahiya, R.; Bulla, M.; Devi, R.; Jeet, K.; Jatrana, A.; Kumar, V. Hydrothermal-assisted green synthesis of reduced graphene oxide nanosheets (rGO) using lemon (Citrus Limon) peel extract. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Jatrana, A.; Agrawal, A.; Singh, P. Development of biochar-based functional materials for electrochemical supercapacitor applications. ECS Trans. 2022, 107, 7979. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Sisodiya, A.K.; Mishra, A.K.; Jatrana, A.; Singh, P. Development of Activated Carbon by Bio Waste Material for Application in Supercapacitor Electrodes. Mater. Lett. 2023, 335, 133830. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Dabhi, S.; Patel, V.; Sumesh, C.K. Liquid phase exfoliated ReS2 nanocrystals on paper based electrodes for hydrogen evolution and supercapacitor applications. Surf. Interfaces 2022, 34, 102318. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Devi, R.; Sisodia, A.K.; Jatrana, A.; Singh, R.B.; Mishra, A.K. Sustainable and Scalable Approach for Enhancing the Electrochemical Performance of Molybdenum Disulfide (MoS2). Adv. Mater. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Chand, P.; Joshi, A.; Lal, S.; Singh, V. Effect of hydrothermal temperature on structural, optical and electrochemical properties of α-MnO2 nanostructures for supercapacitor application. Chem. Phys. Lett. 2021, 777, 138742. [Google Scholar]
- Zhu, S.; Li, L.; Liu, J.; Wang, H.; Wang, T.; Zhang, Y.; Dong, F. Structural directed growth of ultrathin parallel birnessite on β-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 2018, 12, 1033–1042. [Google Scholar] [CrossRef]
- Sivakumar, S.; Prabu, L.N. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 2021, 7, 52–55. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Source 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Toufiq, A.M.; Wang, F.; Javed, Q.U.A.; Li, Q.; Li, Y. Hydrothermal synthesis of MnO2 nanowires: Structural characterizations, optical and magnetic properties. Appl. Phys. A 2014, 116, 1127–1132. [Google Scholar] [CrossRef]
- Chen, W.M.; Qie, L.; Shao, Q.G.; Yuan, L.X.; Zhang, W.X.; Huang, Y.H. Controllable synthesis of hollow bipyramid β-MnO2 and its high electrochemical performance for lithium storage. ACS Appl. Mater. Interfaces 2012, 4, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, G.; Lan, B.; Zheng, X.; Sun, M.; Ye, F.; Cheng, X. Crystallization design of MnO2 via acid towards better oxygen reduction activity. CrystEngComm 2016, 18, 6895–6902. [Google Scholar] [CrossRef]
- Taranu, B.O.; Novaconi, S.D.; Ivanovici, M.; Gonçalves, J.N.; Rus, F.S. α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications. Appl. Sci. 2022, 12, 6821. [Google Scholar] [CrossRef]
- Gangwar, D.; Rath, C. Structural, optical and magnetic properties of α-and β-MnO2 nanorods. Appl. Surf. Sci. 2021, 557, 149693. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Sato, N. Electrochemistry at Metal and Semiconductor Electrodes; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Khan, Y.; Durrani, S.K.; Mehmood, M.; Khan, M.R. Mild hydrothermal synthesis of γ-MnO2 nanostructures and their phase transformation to α-MnO2 nanowires. J. Mater. Res. 2011, 26, 2268–2275. [Google Scholar] [CrossRef]
- Rajagopal, R.; Ryu, K.S. Synthesis of MnO2 nanostructures with MnS-deposits for high performance supercapacitor electrodes. New J. Chem. 2019, 43, 12987–13000. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, D.; Zhao, J.; Zhang, H. Snowflake-like core-shell α-MnO2@ δ-MnO2 for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 251, 344–354. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Li, S.; Zhu, H.; Ge, Y.; Wang, S.; Liu, Y. Electrochemical synthesis of MnO2 porous nanowires for flexible all-solid-state supercapacitor. New J. Chem. 2017, 41, 3750–3757. [Google Scholar] [CrossRef]
- Singu, B.S.; Hong, S.E.; Yoon, K.R. Ultra-thin and ultra-long α-MnO2 nanowires for pseudocapacitor material. J. Solid State Electrochem. 2017, 21, 3215–3220. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Kumar, Y.; Chandra, R. An efficient α-MnO2 nanorods forests electrode for electrochemical capacitors with neutral aqueous electrolytes. Electrochim. Acta 2016, 220, 712–720. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, C.; Ye, J.; Zhao, D.; Chen, Y.; Zhang, J.M.; Huang, Z. Bi2S3/rGO nanocomposites with covalent heterojunctions as a high-performance aqueous zinc ion battery material. Ceram. Int. 2023, 49, 22160–22169. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Isaikina, O.Y.; Novotortsev, R.Y.; Stolbov, D.N.; Xia, H.; Savilov, S.V. Application of MnO2/MWCNT composite in supercapacitors. Mater. Today Proc. 2022, 60, 1008–1011. [Google Scholar] [CrossRef]
- Aquino, C.L.E.; Gorospe, A.E.; Rezaga, B.F.Y.; Avila, R.A.M.; Macugay, J.K.; Tercero, J.U.; Balela, M.D.L. Effect of ammonium persulfate on the growth of MnO2 nanostructures prepared via hydrothermal synthesis for supercapacitor applications. Mater. Today Proc. 2020, 33, 1945–1948. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Consequences of electrodeposition parameters on the microstructure and electrochemical behavior of electrolytic manganese dioxide (EMD) for supercapacitor. Ceram. Int. 2022, 48, 19913–19924. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical properties of sodium manganese oxide/nickel foam supercapacitor electrode material. Inorg. Nano-Met. Chem. 2022, 52, 548–555. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Erdemir, F.; Tuzcu, E.; Bilgin, S.; Alver, Ü.; Çanakçı, A. Influence of fluorine doping of zinc oxide on its electrochemical performance in supercapacitors. Mater. Chem. Phys. 2021, 259, 124033. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Sharma, S.; Sharma, A. Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Appl. Sci. 2023, 13, 7907. https://doi.org/10.3390/app13137907
Devi R, Kumar V, Kumar S, Bulla M, Sharma S, Sharma A. Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Applied Sciences. 2023; 13(13):7907. https://doi.org/10.3390/app13137907
Chicago/Turabian StyleDevi, Raman, Vinay Kumar, Sunil Kumar, Mamta Bulla, Shruti Sharma, and Ashutosh Sharma. 2023. "Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application" Applied Sciences 13, no. 13: 7907. https://doi.org/10.3390/app13137907
APA StyleDevi, R., Kumar, V., Kumar, S., Bulla, M., Sharma, S., & Sharma, A. (2023). Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Applied Sciences, 13(13), 7907. https://doi.org/10.3390/app13137907





