Abstract
Bioactive glasses have developed into a variety of items that are used in order to treat a wide range of medical problems. Apart from being mostly applied in the healing processes of hard tissues, bioactive glasses are becoming very popular materials in soft tissues healing. Bioactive glasses have exhibited the ability to accelerate skin regeneration by enhancing angiogenesis and collagen deposition in the proliferation stage, as well as positive effects on all the other important stages of wound healing. They can adhere to hard tissues such as bone and aid in the regeneration of those tissues by forming a calcium–phosphate-like layer on their surfaces. The formation of this apatite layer results in a linkage between the hard tissue and the glass, which further leads to bone healing. This short review summarizes the dynamic process of wound healing along with the basic concepts of bioactive glasses applied in this domain. We aimed to explore constructs which aid different phases of wound healing. Moreover, several research studies dedicated to bioactive glass thin films are briefly discussed.
1. Introduction
Either of natural or synthetic origin, biomaterials are used to repair damaged tissues or to restore biological functions in different branches of the regenerative medicine domain [1].
In the 50 years since the first report of silicate-based BG by Hench et al. in 1971, the key features of BGs (bioactivity, osteoconduction, and osteostimulation) as bioactive systems suitable for hard tissue repair have been well established by numerous scientific works [2,3,4,5,6]. Bioactive glasses (BGs) are silicate-based inorganic materials, typically composed of Na2O, SiO2, CaO, and P2O5, which bring unique features to many biomedical applications. With the fabrication of 45S5 BG (consisting of the following ingredients: 45 SiO2, 24.5Na2O, 24.5CaO, and 6P2O5 (wt%)), a large number of BGs based on silicate, phosphate, and borate have been conceived [7].
In simulated body fluid (SBF), a layer of carbonated hydroxyapatite (CHA), a phase chemically and physically comparable to the mineral segment of the bone, forms an interface that connects with tissues.
Nowadays, the applicability of BG translated from their use in hard tissue to soft tissue regeneration and wound healing. These multifunctional materials have the capacity to release therapeutic ions in the wound media that target many stages of the wound healing cascade. Glass networks can incorporate and release different ions, such as Ag, Ca, Ce, Co, Ga, Mg, Se, Sr, and Zn [8].
High potency hemostatic and antibacterial qualities, as well as many biological effects on cell processes, are all characteristics of BGs and their dissolution products (e.g., Si, Ca, P), which make them useful in wound healing applications [9,10]. BGs have recently been employed to treat wounds and have demonstrated enormous promise in the field of cutaneous wound healing [9,10].
Numerous research studies have investigated how BGs affect cells involved in tissue healing. Accordingly, BGs not only encourage fibroblast migration but also up-regulate growth factor release and extracellular matrix synthesis [11]. Additionally, it has been shown that BGs can polarize macrophages toward the M2 phenotype, which modifies the inflammatory responses that occur during wound healing [12,13]. In order to increase neovascularization, BGs can also activate human umbilical vein endothelial cells (HUVECs’) gap junction and encourage the expression of angiogenic genes in those cells [14]. In addition, it is assumed that BG may be able to modify the barrier functional behaviors of keratinocytes because the soluble ions released can activate diverse signal transduction pathways in cells [15].
The number of papers mentioning BGs has increased significantly since their debut. The glass structure, dissolution kinetics, biology, and composites, thin films and coatings, scaffolds, etc., have all been the subject of substantial research. A Scopus and Web of Science search of articles with “bioactive glass” in the title, beginning in 1971, yields more than 5070 results (Figure 1a). The same search of articles by title was applied to Web of Science and the number of results was 4582. Of the total number of articles, more than 200 were review papers. Refining the article search using different keywords gives us an insight into which areas BGs receive more attention. Articles which refer to “bone” received the most results, with over 1200 publications. Articles which reference “bone regeneration” (∼620 articles) represent almost half of articles referencing “bone”. In contrast, when refining the articles for “wound healing”, the results showed more than 150 articles. The refining for “tissue regeneration” gave us 409 articles. Figure 1b provides an insight into the top keywords associated with BGs in the scientific literature and shows the literature refinement for some subcategories which we considered to be useful for our present manuscript.
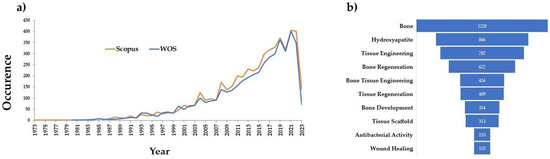
Figure 1.
(a) Publications since the invention of bioactive glass by Larry Hench. (b) Article refinement in Scopus showing the distribution of articles referring to given subcategories.
In this short review, we aim to discuss the fundamental concepts of BGs as well as the dynamic process of healing damaged tissues. We present how BGs actively contribute to the regeneration of both soft and hard tissues during healing. Lastly, studies that have used BGs in applications for wound healing are briefly reviewed, and potential developments in this area are discussed.
2. BGs in the Physiological Media of the Human Body
Leaching, dissolution, and precipitation are the three main processes which take place when BGs are submerged in an aqueous solution. Leaching happens when alkali or alkaline earth elements are released, typically by cation exchange with H+ or H3O+. Ion exchange happens because these cations do not make up the glass network and merely alter it by creating non-bridging oxygen connections. In the case of silicate BGs, –Si–O–Si–O–Si bonds are broken during network dissolution by the action of hydroxyl (OH–) ions. Then, the silica is locally released into the solution. After this step, a deposit of silica-rich gel is formed as an outcome of the hydrated silica (SiOH) which forms on the glass. A calcium–phosphate-rich (CaP) layer is shaped on the surface of the glass during the precipitation step by the release of calcium and phosphate ions from the glass as well as those from the solution. The bioactivity of BG and the nucleation and subsequent production of an apatite layer are attributed to the phosphate species’ solubility [16]. This property has prompted a recent major surge in studies dedicated to the use of BGs in soft tissue repair, along with research results that have strongly implied the significance of controllably released ions in soft tissues and their biological functions [17,18]. Through their dissolution function and release products, BGs can stimulate and improve cellular activity in tissue regenerative processes (released ions, induced biomineral precipitation) [7]. In particular, depending on the kind and concentration of the released ions, several biological processes such as osteogenesis and angiogenesis, as well as antimicrobial and anti-inflammatory effects, can be induced [18]. In addition to the dissolving products, the morphology, surface topography, and chemistry of BGs can regulate some cellular activities, which can further impact tissue regeneration and therapeutic results [19].
Despite the fact that silicate-based BGs such as 45S5 and 13-93 (composition: 53SiO2–20CaO–6Na2O–12K2O–5MgO–4P2O5 in wt%) have been successful in many therapeutic applications, they also seem to have limits in terms of degradation time. First, CaP conversion is incomplete [20]. Additionally, applications for wound healing need faster solubility. In in vivo experiments, it was observed that 45S5 slowly converts to HA, while 13-93 at an even slower rate. The potential for 45S5 to crystallize after heat treatments is another drawback, making it challenging to produce nanocrystalline 45S5-based 3D scaffolds and fibers [21].
Due to the limitations of silicate-based BGs, other types of BGs have been developed. One of these materials is phosphate-based BGs, which offer a wide solubility range which may be predicted and controlled by varying the glass composition [22]. Compared to silicate-based BGs, the phosphate ones promote faster solubility speeds [23]. Their dissolution rates are ascribed to the hydrolysis of soluble P-O-P bonds and ion leaching of cationic modifiers. These processes can be regulated by modifying the structure and composition (e.g., the quantity of P2O5) [24]. Phosphate-based BGs can entirely dissolve in aqueous environments, permitting their contents to be liberated as ionic components. The majority of these constituents, PO43, Ca2+, Mg2+, Na+, and Fe3+, are already present in significant amounts in the human body in the form of ionic species [25].
For cutting-edge biomedical applications, phosphate-based BGs (PBG) may be used alone or in a biodegradable matrix due to their unique material features. For instance, PBG could be used as a therapeutic platform by including different dopants within its structure, with a view to stimulating or increasing particular biological activities after release [26,27].
Recently, borate BGs have entered into the class of most-appreciated materials to be used for the repair of bone defects. Borate BGs are produced by replacing network silica ions with boron ions in the glass network [21]. By dissolution–precipitation mechanisms, similar to those for the case of silicate glasses, borate BGs are transformed into HA without the production of a layer rich in silica [28]. 13-93B3 borate glass, for instance, degrades faster than silicate glasses and transforms almost completely into HA [29]. Applications for borate BGs include the hard tissue regeneration, the treatment of wounds, and the engineering of nerve tissue [30].
Aside from the fact that BGs have been extensively investigated and used in the fields of bone repair and regeneration, there has been little research on the use of BGs to repair or regenerate soft tissues.
3. BGs in Tissue Healing
3.1. BGs in Soft Tissues Healing
3.1.1. Soft Tissues Healing
Under normal medical circumstances, the body’s natural regeneration process involves four overlapping stages: hemostasis, inflammation, proliferation, and tissue remodeling [31,32]. The epidermis, specifically the stratum corneum and tight junctions (TJs) between keratinocytes, is where the skin’s barrier function is located [33].
The stratum corneum, or “corneocytes”, is the epidermis’ outermost layer and is composed of terminally developed enucleated keratinocytes. To help against dehydration, poisons, and germs, the enucleated keratinocytes of the stratum corneum assemble into a brick-and-mortar construction within a lipid-rich extracellular matrix. The treatment of oral and cutaneous ulcers, as well as surgical, traumatic, and chronic wounds, are just a few of the many therapeutic applications that fall under the umbrella of wound healing. Moreover, TJs, which are made of cytosolic plaque proteins called zonula occludens and transmembrane proteins, extend from adjacent keratinocytes to create paired strands that shut the paracellular channel, preventing the flow of chemicals via the intracellular space [34]. Vasoconstriction, platelet aggregation, and blood coagulation stop bleeding quickly in a process known as hemostasis. An up-to-four-day period of inflammation follows, during which immune cells are enlisted to fight infection and promote capillary formation [35].
The body’s natural attempt to control bleeding is called hemostasis and it is achieved by vascular constriction, platelet plug formation, and coagulation. Intrinsic and extrinsic routes start the intricate enzymatic process of coagulation. These come together to form a stable blood clot along a shared channel [36]. Hemostatic dressings (hemostats) can assist in achieving hemostasis during surgical operations. Numerous commercially available biomaterial-based hemostats are being created to improve hemostasis, such as AristaTM (Bard Davol Inc., Rhode Island, RI, USA).
The inflammatory phase is crucial to wound healing since it greatly influences how things progress. The inflammatory phase occurs at the very beginning of wound healing and typically lasts from one hour to seven days following the injury. Different cell types, including monocyte, neutrophil, and macrophage, are involved in the inflammation phase and have the ability to secrete a variety of cytokines and chemokines [37].
Inflammatory cytokines are crucial for the immune system’s response because they control immune cell infiltration, regulate the immunological response, and contribute to angiogenesis. Macrophages often have two different phenotypes of activation: (i) M1 (classically activated) and (ii) M2 (alternatively activated) [38]. While M2 macrophages are engaged in debris scavenging, tissue remodeling, and the resolution of inflammation, M1 macrophages have the ability to kill microbes and can emit large quantities of pro-inflammatory cytokines [38]. Macrophages play an important role in the vascularization by generating important angiogenic factors: vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β).
Additionally, macrophages have the ability to secrete a range of chemokines, cytokines, and proteases that can draw endothelial cells, fibroblasts, and keratinocytes to wound sites, where they can then induce angiogenesis, collagen production, and the development of granulation tissue [38]. Activated macrophages communicate with mending cells such as fibroblasts and endothelial cells in a paracrine manner, which is why the healing of wounds depends not only on the activity of macrophages but also on these connections [38].
Several processes, such as re-epithelialization, angiogenesis, fibroblast production, and the creation of extracellular matrix (ECM) components, which result in the formation of granulation tissue and wound contraction, occur during the proliferative phase [39].
Remodeling, the last phase of wound healing, lasts for several months and is characterized by a continually low rate of collagen formation, deconstruction, and reorganization [40].
Nevertheless, in patients with weakened immune systems, a variety of factors play a role in the slow healing, which leads to chronic wounds, such as ulcers in diabetic patients, who are constantly at danger of inflammation and frequently fatal infections [41].
In order to promote healing, it is necessary to speed up blood coagulation, prevent infection, and promote vascularization because these three factors are crucial for determining whether healing is successful.
Ionically-doped BGs can speed up these processes since many different metallic oxides can be added to the glass network to change its chemical and physical characteristics. Their subsequent ionic release as a result of glass dissolution in biological settings can provide hemostatic attributes (e.g., Ca2+ [42]) and antimicrobial properties (e.g., Ag+ [43] and Cu2+ [44]).
There are many interesting review articles which cover the physiology of wound healing [32,37,45].
3.1.2. BGs Formulations Applied in Soft Tissues Healing
BGs’ capacity to promote angiogenesis may offer a potent substitute for the pricey growth factors currently used to encourage the neovascularization of damaged tissues. BGs have recently been employed to treat wounds and have demonstrated enormous promise in the field of wound healing; certain BG formulations can be applied to help increase the healing rate by aiding the stages involved in wound healing (Figure 2). A list of the BG compositions, synthesis routes, and the results of the studies are provided in Table 1.
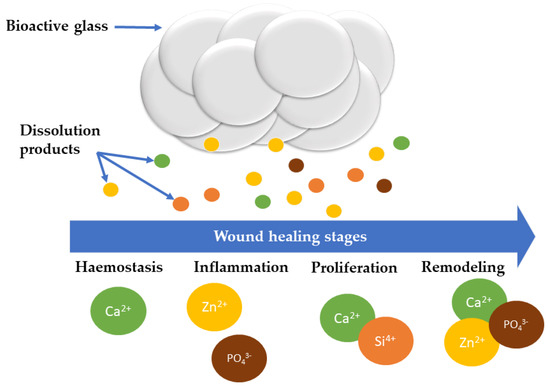
Figure 2.
Schematic representation of various BG dissolution products and their involvement in different stages of wound healing.

Table 1.
Different BG compositions considered for wound healing.
Numerous research works have investigated how BGs affect and mitigate cells involved in wound healing. According to studies, BGs not only encourage fibroblast migration but also up-regulate growth factor secretion and ECM synthesis [11], together with genes expression linked to wound healing. Examples include CD44 antigen hematopoietic form precursor, fibronectin receptor beta subunit, fibroblast growth factor receptor1 precursor (N-sam), VEGF precursor, and vascular cell adhesion protein 1 precursor (V-CAM 1) [46].
In the proliferation phase, fibroblasts and endothelial cells multiply and migrate. Fibroblasts secrete angiogenic growth factors such as basic fibroblast growth factor (bFGF) (powerful mitogen and chemoattractant for fibroblasts and endothelial cells) and VEGF in the same stage [39]. A new ECM is produced by fibroblasts, in addition to secreting angiogenic growth factors, in order to boost cell development and create a granulation tissue bed for macrophages and new capillaries [39]. Additionally, fibroblasts can develop into myofibroblasts in the latter stages, which are in charge of excessive ECM deposition and wound contraction [39]. Consequently, fibroblasts and the growth substances they release are crucial [39].
BGs can modify inflammatory responses which occur during wound healing by polarizing macrophages toward the M2 phenotype [13].
In order to increase neo-vascularization, BGs can also stimulate the gap junction of human umbilical vein endothelial cells (HUVECs) for 7 days in in vitro settings and induce the production of angiogenic genes in these cells [47]. In vitro, the culture media containing BG 1/64, BG 1/128, and BG 1/256 significantly elevated bFGF, bFGFR, VEGF, and KDR gene expression in HUVECs at 1 day in culture, when compared to endothelial cell medium. The expression of these growth factors and receptors was unaffected by both the highest concentration, BG 1/32, and the lowest one. The stimulatory effects of BG ion extracts on growth factors and receptors were still present after 7 days of culture even if their expression, particularly that of VEGF and KDR, had diminished [48].
To investigate the effects on keratinocytes, ionic extracts from 58S BGs were employed as a model. To examine the BGs effects on barrier restoration, diabetic rats with full-thickness cutaneous wounds were treated with them. Because soluble ions such as Si, Ca, and P stimulate numerous signal transduction pathways in various cells, improving re-epithelization and restoring the natural barrier function, BGs particles were able to alter the behaviors of keratinocytes that operate as barriers [14].
By lowering the inflammatory response and driving macrophages to convert their phenotype towards M2 extreme, 45S5 BG was able to stimulate the wound closure in rats after a complete thickness excision of skin. As a result, one of the mechanisms through which BG promotes wound healing may be through modulating inflammatory responses to wound healing and enhancing the paracrine interactions between macrophages and repairing cells [12].
By extending the window of opportunity for the injured to be brought to a competent medical facility, control of severe hemorrhages could reduce the fatality rate in extreme settings, such as the battlefield, natural disasters, and accident sites. In these situations, simple wound dressings are frequently insufficient to heal patients, and innovative biomaterial solutions have been developed. The intrinsic pathway, which is started by the activation of factor XII (FXII), is generally what causes BGs to positively affect coagulation. The activation of FXII by negatively charged surfaces, such as those given by BGs, can enhance the innate coagulation cascade and thus also hemostasis [49,56]. Mesoporous BGs have a large surface area because mesopores are present in their structure, which helps absorb the blood’s fluid component and draw clotting components close by [60]. As a result, the coagulation process starts. Mesopores (found inside the structure of the glass) and gaps between the glass particles produce capillary action, which aids in absorbing the blood’s fluid component [56]. The greater surface area of mesoporous BGs reveals more Si-OH groups, resulting in more negatively charged surfaces. Due to the presence of silanol groups, BGs exhibit negatively charged surfaces, which activate FXII. Furthermore, protein adsorption at the surfaces of the biomaterials starts biological processes; this process is reliant on the topography and chemistry of the surface [61]. In addition, due to the fact that Ca2 + ions can shorten the time for blood to clot, BGs alone can be utilized to manage severe hemorrhages [42]. More specifically, mesoporous BGs have a shorter clotting time than dense BGs, owing to the blood capillary adsorption inside the pores [50].
BGs with Trace/Doping Elements
Recent research has shown that adding trace elements such as Ti, Sr, Ta, Ga, and Ce to the matrix of mesoporous BGs and surface-modifying them with certain polymers can enhance their biological behavior.
A BG system, doped with Sr, encouraged early angiogenesis by controlling macrophage polarization to the M2 phenotypes. HUVEC’s ability to produce angiogenesis was improved by a macrophage/Sr-BGs culture in conditioned media, and in vivo research indicated that Sr-doped BGs promoted early vascularization [62].
In vivo studies have demonstrated that wound healing cascade works faster when hypoxia mimicking drugs are used [63]. Co is a well-known agent which mimics hypoxia because it activates the hypoxia-inducible factor (HIF) pathway by stabilizing HIF-1 at ambient oxygen levels. Prolyl hydroxylase domain enzymes hydroxylate HIF-1 in normal oxygen conditions, where it is constitutively produced and continuously destroyed by proteasomes [64]. In hypoxic conditions or in the presence of a substance which mimics hypoxia, such as Co, the stabilization of HIF-1 promotes the formation of the HIF complex, which is translocated to the nucleus, and causes an increase in the expression of the genes involved in the adaptation to hypoxia. Owing to the fact that BGs (having the composition of 50 mol% SiO2, 24 mol% Na2O, 24 mol% MgO, and 2 mol% CoO) and their electrospun composites and have been used to stimulate angiogenesis, these materials may be useful in applications for wound healing [65].
Comparison research showed that a mesoporous BG containing 1% Ga2O3 accelerates hemostasis in vitro more effectively than CeloxTM (CX) and ACS+, two commercially available hemostats, since it may considerably increase the intrinsic pathway’s activation, thrombus formation, and thrombin generation. Further evidence of the superior hemostatic activity was provided by the observation of larger platelet aggregates and a more widespread platelet pseudopodia in comparison to CX and ACS+. The polar silica framework and Ca2+ ions in the matrix of the 1%Ga-MBG, as well as its large specific surface area, were thought to be responsible for the platelet adherence and activity on its surface [59]. The results of this study demonstrated that the number of adherent platelets on the hemostatic materials surfaces increased in number with the incubation time of up to 60 min. After 60 min of incubation, the number of adhering platelets to the surfaces of 1%Ga-MBG and CX increased, whereas the number of adherent platelets to the surface of ACS+ was not that high. Although there was no discernible difference in the quantity of adherent platelets between the 1%Ga-MBG and CX, the platelets attached to the BG were more active than in the case of CX, as seen by the lengthy pseudopodia presence [59].
Ta-containing mesoporous BGs were recently reported [51]. When compared to no treatment, AristaTM, or mesoporous BGs without Ta, a substantial reduction in bleeding time (50% of average bleeding time) was seen in a mouse tail-cut model.
In another study, different compositions of Ta-containing mesoporous BGs powders were tested using a porcine fatal liver injury model [66]. A class IV bleeding state was induced in animal subjects. Within 10 min of application, the Ta-BGs were able to halt the bleeding, whereas the bleeding continued for up to 45 min without any intervention or in the presence of a commercial agent, AristaTM. The blood clots demonstrated that Ta-BGs presence had no effect on clot shape. Instead, the links between Ta-BGs powders and the blood clots fibrin threads suggested that the surfaces of the powders are engulfing the fibrin. A histopathological study of the liver tissue revealed that 5Ta was the only formulation that, when applied, reduced the amount of parenchymal bleeding and necrosis in the tissue. In worst-case scenario hemorrhage, when there was no adherent clot visible prior to the application of the powder, the same formulation was also able to create an adherent clot [66].
Another research was focused on developing and evaluating the hemostatic application and their antihemorrhagic effect of Ga-containing mesoporous BGs (Ga-MBGs), in comparison with a Ga-free MBG [52]. In a series of MBGs containing three different concentrations of Ga2O3 (1, 2, and 3 mol%), in vitro hemostatic activity, biocompatibility and antibacterial activity against E. coli and S. aureus were investigated. The findings showed that a key factor influencing hemostatic events was the ratio or concentration of Ga2O3 in MBG. The advantages of Ga2O3 were nullified at higher concentrations (3 mol%), but the 1% Ga-MBG increased intrinsic coagulation cascade, thrombus development, and platelet adhesion relative to MBG. As a result, the sample with the lowest Ga-MBG had better hemostatic effectiveness, high degradability, high cytocompatibility, and antibacterial activity against both strains [52].
BG Composites
Additionally, some BGs antibacterial properties can lower the risk of infection during the healing of chronic wounds. However, when used alone, BGs have drawbacks, including the inability to maintain moist conditions and bioadhesive strength for efficient wound treatment. Therefore, the combination of BGs with different alleviating substances is anticipated to be more successful than BGs used alone in applications for wound healing.
On this matter, Li et al. fabricated scaffolds made of BG, chitosan, and silk fibroin for the regeneration of severe burn wounds [53]. The three substances were applied as follows: chitosan was utilized to encourage the adsorption and enrichment of growth factors, silk fibroin to create a three-dimensional (3D) porous structure and mechanical support, and the BG to stimulate angiogenesis. Their findings demonstrated that BGs were essential for promoting the development and maturation of new blood vessels, which can greatly speed up tissue healing. Wang et al. created “easy-to-use” BGs/gelatin nanocomposite hydrogels, which were able to quickly regenerate cutaneous tissue and construct tissue structures in living organisms within 7 days [54]. Cu-BGN/eggshell nanocomposite membranes have revealed efficacy in wound healing [44]. Cu-BGN could promote proangiogenesis when added in the right quantities by increasing the secretion of proteins and gene expression [44]. Moreover, the addition of Cu-BGN reduced the development of bacteria. In addition, the in vivo tests demonstrated enhanced wound healing and the production of continuous and homogeneous epidermal layers. In another study, a borosilicate BG-incorporated sodium alginate (SA) wound dressing was synthesized [55]. The results show that the SA-borosilicate BG composite dressing had a good water absorption performance, which is of use for wound healing. In full-thickness skin defects in rats, the SA-borosilicate BG wound dressing demonstrated an excellent capability to treat wounds. On day 15, the SA-borosilicate BG dressing group-treated lesions were nearly healed. When the ratio of SA to BG in the sponge is 3:1, the wound healing effect was remarkably increased. In a recent work, BG was incorporated into alginate/carboxymethyl chitosan (SA/CMCS) hydrogel wound dressing. The incorporation of BGs led to better antibacterial, bioactivity and coagulation properties, as compared with hydrogels without BGs. By regulating the host inflammatory reactions, promoting angiogenesis, and improving collagen deposition at wound sites, the hydrogels could hasten the healing of skin wounds.
Co-doped borate BG/poly(lactic-co-glycolic acid) dressing supplied with vitamin E (0–3.0 wt%) was investigated by Hu et al. for its effectiveness in promoting wound healing [48]. The in vitro data made clear that the dressings’ ions promoted tubule development, migration, and VEGF secretion in HUVECs and fibroblasts. Furthermore, in vivo experiments revealed a considerable improvement in the epithelialization of wound closure and a significant increase in vessel sprouting and collagen remodeling. The use of this composite biomaterial as a wound dressing could actually be promising for accelerating the healing and reconstruction of full-thickness skin defects.
Due to its function in the movement of immune cells, the transport of proteins, and the preservation of tissue homeostasis, the lymphatic system is essential in the case of tissue regeneration. Techniques that promote lymphangiogenesis might be viewed as cutting-edge treatments for tissue regeneration and repair, particularly in the treatment of chronic wounds. In this respect, Xie et al. dedicated their work on the effects of Ce-containing mesoporous BG nanoparticles (Ce-MBGNs) on lymphangiogenesis, in vitro [67]. Their results showed that extracts of Ce-MBGNs (1, 5, or 10 wt/v% in DMEM) were non-cytotoxic and could promote the proliferation of lymphatic endothelial cells (LECs) in comparison to the blank control and the media containing VEGF-C [67].
PCL and PCL/BG composite fiber mats were synthesized by Sergi at al. [57]. To improve the biological responses to the fiber mats, BGs of various compositions were embedded into a PCL matrix. The presence of BG did not inhibit cell viability, which was higher (for 45S5 and BGMS10) or comparable (for BGMS_2Zn) with respect to neat PCL fibers. After the performed scratch test, the PCL/BG mats displayed a higher wound-healing rate as compared to neat PCL. The incorporation of BGs and, in particular, the amalgamation of BGMS10 achieved boosted cell viability while preserving the mechanical properties.
An effective dressing was designed by Chen at. al with the purpose of promoting revascularization and having an antibacterial effect [58]. The authors prepared a multifunctional injectable composite hydrogel by incorporating the Ce-containing BGs (Ce-BG) into gelatin methacryloyl (GelMA) hydrogel. The Ce-BG/GelMA hydrogels enhanced endothelial cell motility and tube formation by releasing Si ion and had good cytocompatibility. The 5 mol% CeO2-BG/GelMA hydrogel showed good antibacterial capabilities in in vitro antibacterial testing. The in vivo investigation showed that through promoting the growth of granulation tissue, collagen deposition, and angiogenesis, the 5/G hydrogel could dramatically enhance wound healing in diabetic rats.
In another study, PHAs were mixed with Co-doped 45S5 BG to induce antibacterial and angiogenic properties. Using the generated composite, scaffolds were created. It was discovered that the dissolution products produced from the BG-PHA scaffolds have a dose-dependent effect by performing indirect cell biology experiments utilizing stromal cells. By performing indirect cell biology tests using stromal cells, a dose-depending effect of the dissolution products released from the BG-PHA scaffolds was found. No harmful effect was seen at low concentrations. In contrast, greater concentrations resulted in a significant increase in VEGF release along with a small drop in cell survival. Cells grown on scaffolds made of plain PHA and 1%-CCM BG-PHA displayed their distinct phenotypic morphologies and adhered to the well plate. This suggests that the shape of ST2 cells grown in contact with the dissolution products of PHA scaffolds containing BG in concentrations below 10% is unaffected. However, cell growth and adherence were relatively poor when grown in 10%-CCM of all scaffolds [59].
3.2. BGs in Bone Regeneration
BGs are nowadays largely used in various fields of regenerative medicine, especially for the healing of hard tissues such as bones and teeth, as they can bind directly with bone [68]. This is possible due to their biocompatibility, bioactivity, degradability, cell viability, reduced cytotoxicity, antimicrobial activity, osteogenesis, and angiogenesis properties (Figure 3). The amount/concentration of silicate in BGs affects how biocompatible they are [69]. In order to find the most promising BGs to be used as potential bone replacements and for hard tissue regeneration and, more recently, in the treatment of infections, various aspects regarding their cytotoxicity, osteogenesis, and angiogenesis have been investigated. In particular, it was found that the ions present in BGs compositions mediate how the major proteins implicated in the process of forming new bone react [1]. The surface bioreactivity of BGs is responsible for the strong bonding with the surrounding tissue, which provides osteoconductive properties.
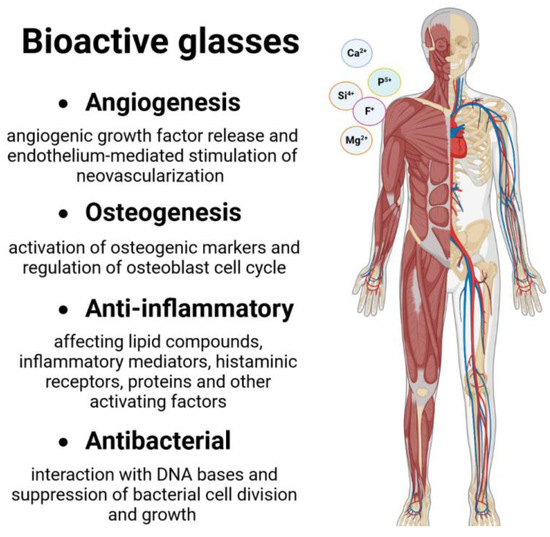
Figure 3.
General use of BGs in hard tissue healing.
A schematic figure of the surface reaction stages experienced by BGs in hard tissue healing is given in Figure 4.
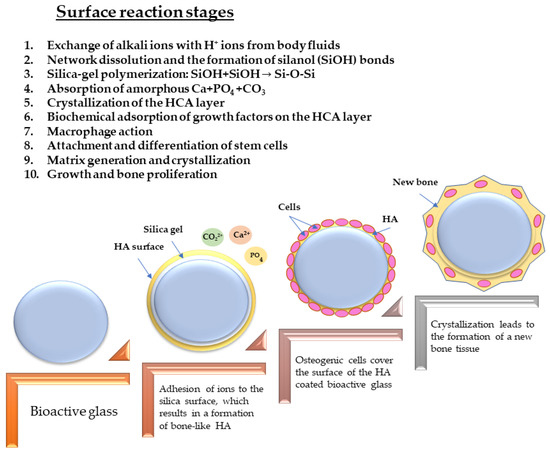
Figure 4.
Surface reaction stages of BGs in hard tissue healing.
Basically, after the implantation, the BG is exposed to body fluids, and different surface reactions occur which ensure the creation of a deposit of a calcium phosphate layer [70]. A discharge from the BG surface of sodium, silica, calcium, and phosphate ions elevates the local pH and osmotic pressure; subsequently, a layer of silica gel covers the glass surface; thereafter, amorphous calcium phosphates precipitate on it [71]. These amorphous aggregates appear to trigger osteoblasts necessary for the formation of new bone upon crystallization into a hydroxyapatite-like form [1]. The glass can be absorbed as a result of the ongoing processes that take place on its surface and layer. Additionally, these surface interactions give the glass the ability to promote angiogenesis. The mechanism outlined above is based on two distinct steps: (i) the degradation of the BGs; and (ii) the release of ions from the BG. These techniques can be translated into graft–bone bonding. Depending on their composition, the BGs can disintegrate at a rate that might take anywhere from a few hours to several months [1].
It has been thoroughly demonstrated that specific BG compositions generate surface hydroxy-carbonate apatite (HCA) when in contact with biological fluids, which enhances their attachment to mineralized tissues, with minimal inflammation and toxicity for the host [16]. The interfacial HCA layer forms at a pace that has a high structural and chemical similarity to that of the mineral phase of bone [72]. The bioactivity of BGs is appropriate for applications in bone tissue regeneration as the formation of HA represents the marker which characterizes material–bone strong bonding.
Unfortunately, BGs have poor mechanical strength and fracture toughness despite having excellent bioactive characteristics. Therefore, their use is often limited to non-load-bearing applications. Smart approaches, such as the development of novel production techniques (such as additive manufacturing), laser deposition for obtaining thin films, or the mixing with polymeric phases to make composites, provide a partial resolution of these issues. The next section will provide a picture of BG thin films for hard tissue healing.
BG Thin Films
The surface quality—in particular, the surface topography and the used material—influences bone healing and regeneration. Now, the majority of implantable materials are Ti alloys, stainless steel (SS), or Co-Cr alloy. The physiologically active surface that either promotes osteointegration or prevents infection is typically absent from these metals. Thus, efforts have been concentrated on creating different coatings to enhance the functionality of existing implants [73]. These coatings’ construction must meet a number of crucial requirements: first, the coating must be biocompatible and not cause any immune or foreign body reaction; second, it must be osseoconductive in order to encourage osteoblasts to adhere to, proliferate, and grow on the surface in order to form a bone–implant linkage [73].
As mentioned in the previous sections, when BGs are used to coat metallic implants, a hydroxyapatite-like phase can form at the site where the implant meets the host tissue, aiding in the implant’s better integration [74]. BGs of excellent bioactivity have been used by our group onto less biocompatible or bioinert materials (such as titanium or steel) in the form of coatings [75,76,77,78]. Our studies evidenced a transformation of BG into CHA in 28 days for BG57 (56.5% SiO2, 15% CaO, 11% Na2O, 8.5% MgO, 6% P2O5, and 3% K2O) or in 42 days in the case of BG61 (61.1 wt% SiO2, 10.3 wt% Na2O, 2.8 wt% K2O, 12.6 wt% CaO, 7.2 wt% MgO, and 6 wt% P2O5) after immersion in SBF [75,76,77,78].
One of the risks associated with the insertion of an implant into the human organism is the one represented by microbial infections. One effective way in fighting biofilm-associated infections is to prevent the adhesion and/or delay the growth of colonizing microorganisms via implant coating with bioactive films releasing antimicrobial drugs. Our group explored an innovative concept for implants coating based upon double layers of BG and antimicrobial plants extract/polymer deposited by Matrix Assisted Pulsed Laser Evaporation (MAPLE) onto different substrates. The BG dissolution resulted in the growth of a bioapatite layer with anti-corrosive protection of the substrate. Our layers proved bioactive action, antimicrobial effect, and strong shielding against metal ions release. Thin films of BG-PMMA-Doxycycline (Doxy) were deposited onto 316 L SS substrates by means of MAPLE technique [79]. When in contact with simulated body fluids (SBF), the composite films demonstrated the ability to stimulate the progress of biological-like HA on their surface, validating the film bioactivity. A prolonged release of active Doxy molecules occurs together with the BG dissolution in SBF, which is beneficial for preventing local infections. Four weeks after the sample’s immersion in SBF, apatite layers begin to form on the surface; the apatite layer together with the polymer provide excellent protection against deterioration and the release of dangerous metallic ions (Cr, Ni, Cu). The structures were highly biocompatible and resistant to microbial colonization, stronger against E. coli than the S. aureus, and induce a significant delay in the microbial biofilm initiation and further development [76]. The development of the CHA layer indicated the ability of BG+CIPRO/PMMA to bind to bone tissue. The coating successfully inhibited Gram-negative bacterial growth while having no detrimental effects on MC3T3-E1 osteoblasts, making it an excellent candidate for the creation of effective implant surfaces. The bioactivity of the BG+CIPRO/PMMA coating is encouraging, and it might have uses in tissue engineering and bone healing. When a quick bone–biomaterial interface is required in bone surgery, it provides more options for adapting to the bone metabolic activities [75].
The choice of antibiotic, application form, and administration time remains an issue in implantology. The need for new and competent antibiotics is in contrast to their toxicity when applied systemically. Plant products have the fewest side effects and are therefore largely explored for their capacity to prevent biofilm formation and/or eradicate it [80]. Long-time release of natural (Ayurvedic extracts) or synthetic antibiotics embedded in the structure will ensure the healing and vital antimicrobial protection at the implantation site [75,76,79]. Furthermore, we explored an innovative concept of thin films for implants based upon double layers of BG and antimicrobial Ayurvedic plants extract/polymer deposited by MAPLE onto SS (implant like) substrate.
Neem, Azadirachta indica, was chosen due to the extracts’ antibacterial activity against the most common pathogens causing osseous or dental infections, S. aureus and E. coli [78]. The primary accomplishment of this innovative idea is the antimicrobial action brought on by the use of a natural plant extract from Ayurveda medicine, which has a longer release and no negative consequences. The created layers had multiple uses and were shown to have bioactive and antibacterial properties, and a strong ability to block the leakage of metal ions into body fluids, from the SS substrates.
4. Concluding Remarks and Future Directions
More studies including innovative chemical compositions and morphologies of BGs, particularly mesoporous BGs, are necessary to achieve optimal rates of tissue regeneration and wound healing due to the growing need for improved and efficient materials to be used in tissue healing. Research in this field is likely to increase in future years, with BGs representing a valid alternative and an universal material as a consequence of their stimulating activity on the multitude of routes involved in tissue repair while providing a biomechanically and bioactively attractive delivery system. Moreover, the development of multifunctional materials (e.g., scaffolds, hydrogels, mats) based on BGs could enhance wound healing speed and reconstruct the skin tissue, alongside with their antibacterial and angiogenic functions. This can be of great significance to promote the repair, e.g., of diabetic wounds.
Improving the performance of bulk materials, such as metals and metal alloys from which different implantable devices are made, requires surface modification of the materials. Surface modification is mostly used in the biomedical sector to improve the material’s bioactivity, biocompatibility, and mechanical stability. For the three main purposes mentioned above in the human body, various types of BG coatings have been used. There are, however, some concerns related to the clinical use of BGs in hard tissues healing [81,82]. The first one is related to the glass dissolution products which may or may not have a positive effect on adult stem cells [83]. The second one is that sole BGs proved to be inadequate when utilized for the treatment of load-bearing bone defects. This drawback is connected with the low tensile strength and fracture toughness exhibited by BGs [84]. Last but not least, the market does not yet offer large-scale porous BGs; accordingly, the commercial success of BGs as scaffolds is reduced [81,85].
Author Contributions
Conceptualization, I.N.; software, C.R.; writing—original draft preparation, I.N. and C.R.; writing—review and editing, C.R.; visualization, I.N.; supervision, C.R.; project administration, I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Education and Research, under Romanian National Nucleu Program LAPLAS VII—contract no. 30N/2023. I.N. acknowledges the support by a grant of the Ministry of Research, Innovation and Digitization, CNCS—UEFISCDI, project no. PN-III-P2-2.1-PED-2021-3178 within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Romanian Ministry of Education and Research, under Romanian National Nucleu Program LAPLAS VII—contract no. 30N/2023. I.N. acknowledges the support by a grant of the Ministry of Research, Innovation and Digitization, CNCS—UEFISCDI, project no. PN-III-P2-2.1-PED-2021-3178 within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, T.; Zhang, Q.; Lei, Q.; Gao, S.; Xiao, K.; Yan, F.; Cai, L. A Composite of Cubic Calcium-Magnesium Sulfate and Bioglass for Bone Repair. Front. Bioeng. Biotechnol. 2022, 10, 898951. [Google Scholar] [CrossRef]
- Singh, S.; Patil, A.; Mali, S.; Jaiswal, H. Bioglass: A New Era in Modern Dentistry. Eur. J. Gen. Dent. 2022, 11, 1–6. [Google Scholar] [CrossRef]
- Salinas, A.J.; Esbrit, P. Mesoporous Bioglasses Enriched with Bioactive Agents for Bone Repair, with a Special Highlight of María Vallet-Regí’s Contribution. Pharmaceutics 2022, 14, 202. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, Y.; Liu, D.; Liu, Y.; Ke, Q.; Xu, H. A bioglass sustained-release scaffold with ECM-like structure for enhanced diabetic wound healing. Nanomedicine 2020, 15, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, P.; Dong, J.; Li, S.; Lv, P.; Liu, C. Scaffolds of bioactive glass (Bioglass®) combined with recombinant human bone morphogenetic protein -9 (rhBMP-9) for tooth extraction site preservation. Heliyon 2022, 8, e08796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Hassanzadeh Chinijani, T.; Malek Khachatourian, A.; Vinicius Lia Fook, M.; Baino, F.; Montazerian, M. A critical review of bioactive glasses and glass–ceramics in cancer therapy. Int. J. Appl. Glass Sci. 2023, 14, 69–87. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and Bioactive Glasses and Their Impact on Healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass Activated Skin Tissue Engineering Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chang, J.; Li, H. Bioglass promotes wound healing through modulating the paracrine effects between macrophages and repairing cells. J. Mater. Chem. B 2017, 5, 5240–5250. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Fu, X.; Tang, F.; Mo, Y.; Cheng, J.; Wang, H.; Chen, X. Dose-dependent modulation effects of bioactive glass particles on macrophages and diabetic wound healing. J. Mater. Chem. B 2019, 7, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, J.; Xie, W.; Mo, Y.; Ouyang, L.; Zhao, F.; Fu, X.; Chen, X. Bioactive glass promotes the barrier functional behaviors of keratinocytes and improves the Re-epithelialization in wound healing in diabetic rats. Bioact. Mater. 2021, 6, 3496–3506. [Google Scholar] [CrossRef] [PubMed]
- Fellenberg, J.; Losch, S.; Lehner, B.; Arango-Ospina, M.; Boccaccini, A.R.; Westhauser, F. Bioactive glass selectively promotes cytotoxicity towards giant cell tumor of bone derived neoplastic stromal cells and induces MAPK signalling dependent autophagy. Bioact. Mater. 2022, 15, 456–468. [Google Scholar] [CrossRef]
- Samudrala, R.K.; Patel, S.; Penugurthi, V.; Manavathi, B.; Azeem, A.P. In vitro studies of B2O3–SiO2–Na2O–CaO–ZnO bioactive glass system. J. Non-Cryst. Solids 2021, 574, 121164. [Google Scholar] [CrossRef]
- Bingel, L.; Groh, D.; Karpukhina, N.; Brauer, D.S. Influence of dissolution medium pH on ion release and apatite formation of Bioglass® 45S5. Mater. Lett. 2015, 143, 279–282. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses—Structure and Properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Yao, A.; Wang, D.; Huang, W.; Fu, Q.; Rahaman, M.N.; Day, D.E. In Vitro Bioactive Characteristics of Borate-Based Glasses with Controllable Degradation Behavior. J. Am. Ceram. Soc. 2007, 90, 303–306. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Bio Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Kennedy, A.R.; Macri-Pellizzeri, L.; Sottile, V.; Grant, D.M.; Scammell, B.E.; Ahmed, I. Porous calcium phosphate glass microspheres for orthobiologic applications. Acta Biomater. 2018, 72, 396–406. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef]
- Bitar, M.; Knowles, J.C.; Lewis, M.P.; Salih, V. Soluble phosphate glass fibres for repair of bone-ligament interface. J. Mater. Sci. Mater. Med. 2005, 16, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M.; Prasad, P.S.; Venkateswara Rao, P.; Govindan, N.P.; Singh, R.K.; Kim, H.-W.; Veeraiah, N. Titanium incorporated Zinc-Phosphate bioactive glasses for bone tissue repair and regeneration: Impact of Ti4+on physico-mechanical and in vitro bioactivity. Ceram. Int. 2019, 45 Pt B, 23715–23727. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Cui, X.; Huang, W.; Zhang, Y.; Huang, C.; Yu, Z.; Wang, L.; Liu, W.; Wang, T.; Zhou, J.; Wang, H.; et al. Evaluation of an injectable bioactive borate glass cement to heal bone defects in a rabbit femoral condyle model. Mater. Sci. Eng. C 2017, 73, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. Part A 2010, 95 Pt A, 164–171. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 588–600. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Adib Kadri, N.; Zeimaran, E.; Towler, M.R. Well-ordered mesoporous silica and bioactive glasses: Promise for improved hemostasis. Biomater. Sci. 2019, 7, 31–50. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Ostomel, T.A.; Shi, Q.; Tsung, C.-K.; Liang, H.; Stucky, G.D. Spherical Bioactive Glass with Enhanced Rates of Hydroxyapatite Deposition and Hemostatic Activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Iveson, S.; Catherall, P.; Hardman, M.J. A Novel Silver Bioactive Glass Elicits Antimicrobial Efficacy Against Pseudomonas aeruginosa and Staphylococcus aureus in an ex Vivo Skin Wound Biofilm Model. Front. Microbiol. 2018, 9, 1450. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01450 (accessed on 9 August 2022). [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound Healing Concepts in Clinical Practice of OMFS. J. Maxillofac. Oral Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Mao, C.; Zhang, J.; Li, Y.; Chen, X. Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed. Mater. 2012, 7, 045017. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Yu, H.; Green, C.R.; Chang, J. Bioglass promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 2016, 84, 64–75. [Google Scholar] [CrossRef]
- Hu, H.; Tang, Y.; Pang, L.; Lin, C.; Huang, W.; Wang, D.; Jia, W. Angiogenesis and Full-Thickness Wound Healing Efficiency of a Copper-Doped Borate Bioactive Glass/Poly(lactic-co-glycolic acid) Dressing Loaded with Vitamin E in Vivo and in Vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef]
- Matter, M.T.; Starsich, F.; Galli, M.; Hilber, M.; Schlegel, A.A.; Bertazzo, S.; Pratsinis, S.E.; Herrmann, I.K. Developing a tissue glue by engineering the adhesive and hemostatic properties of metal oxide nanoparticles. Nanoscale 2017, 9, 8418–8426. [Google Scholar] [CrossRef]
- Hu, G.; Xiao, L.; Tong, P.; Bi, D.; Wang, H.; Ma, H.; Zhu, G.; Liu, H. Antibacterial hemostatic dressings with nanoporous bioglass containing silver. Int. J. Nanomed. 2012, 7, 2613–2620. [Google Scholar] [CrossRef]
- Mendonca, A.; Rahman, M.S.; Alhalawani, A.; Rodriguez, O.; Gallant, R.C.; Ni, H.; Clarkin, O.M.; Towler, M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon–calcium–phosphorous mesoporous bioactive glasses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Vasudevaraj Naveen, S.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71–86. [Google Scholar] [CrossRef]
- Li, D.; Jiao, G.; Zhang, W.; Chen, X.; Ning, R.; Du, C. Hybrid scaffolding strategy for dermal tissue reconstruction: A bioactive glass/chitosan/silk fibroin composite. RSC Adv. 2016, 6, 19887–19896. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, F.; Cui, Y.; Ren, H.; Xie, Y.; Li, A.; Ji, L.; Qu, X.; Qiu, D.; Yang, Z. An easy-to-use wound dressing gelatin-bioactive nanoparticle gel and its preliminary in vivo study. J. Mater. Sci. Mater. Med. 2016, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H. Incorporation of Bioglass Improved the Mechanical Stability and Bioactivity of Alginate/Carboxymethyl Chitosan Hydrogel Wound Dressing. ACS Appl. Bio Mater. 2021, 4, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Wang, C.; Yuan, Y.; Qu, X.; Wei, J.; Lin, Z.; Zhou, H.; Liu, C. Novel porous silica granules for instant hemostasis. RSC Adv. 2016, 6, 78930–78935. [Google Scholar] [CrossRef]
- Sergi, R.; Cannillo, V.; Boccaccini, A.R.; Liverani, L. Incorporation of Bioactive Glasses Containing Mg, Sr, and Zn in Electrospun PCL Fibers by Using Benign Solvents. Appl. Sci. 2020, 10, 5530. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Rao, Z.-F.; Liu, Y.-J.; Liu, X.-S.; Liu, Y.-F.; Xu, L.-J.; Wang, Z.-Q.; Guo, J.-Y.; Zhang, L.; Dong, Y.-S.; et al. Multifunctional Injectable Hydrogel Loaded with Cerium-Containing Bioactive Glass Nanoparticles for Diabetic Wound Healing. Biomolecules 2021, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Schuhladen, K.; Lukasiewicz, B.; Basnett, P.; Roy, I.; Boccaccini, A.R. Comparison of the Influence of 45S5 and Cu-Containing 45S5 Bioactive Glass (BG) on the Biological Properties of Novel Polyhydroxyalkanoate (PHA)/BG Composites. Materials 2020, 13, 2607. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F.; Liu, C.; Guan, J.; Hu, X.; Du, G.; Yao, X.; Wu, J.; Tian, F. Blood clot initiation by mesoporous silica nanoparticles: Dependence on pore size or particle size? J. Mater. Chem. B 2016, 4, 7146–7154. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the Blood Coagulation System. In Handbook of Immunological Properties of Engineered Nanomaterials; in Frontiers in Nanobiomedical Research; World Scientific: Singapore, 2016; Volume 6, pp. 261–302. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef]
- Shi, Q.; Luo, X.; Huang, Z.; Midgley, A.C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.K.; Lali, F.V.; Autefage, H.; Agarwal, S.; Nommeots-Nomm, A.; Metcalfe, A.D.; Stevens, M.M.; Jones, J.R. Bioactive glasses and electrospun composites that release cobalt to stimulate the HIF pathway for wound healing applications. Biomater. Res. 2021, 25, 1. [Google Scholar] [CrossRef]
- Nagrath, M.; Bince, D.; Rowsell, C.; Polintan, D.; Rezende-Neto, J.; Towler, M. Porcine liver injury model to assess tantalum-containing bioactive glass powders for hemostasis. J. Mater. Sci. Mater. Med. 2022, 33, 53. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sha, S.; Lu, L.; Wu, G.; Jiang, H.; Boccaccini, A.R.; Zheng, K.; Xu, R. Cerium-Containing Bioactive Glasses Promote In Vitro Lymphangiogenesis. Pharmaceutics 2022, 14, 225. [Google Scholar] [CrossRef]
- Bioactive Glass Implants and Wound Care. News-Medical.net. 3 October 2018. Available online: https://www.news-medical.net/health/Bioactive-Glass-Implants-and-Wound-Care.aspx (accessed on 20 February 2023).
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Abbasi, Z.; Bahrololoom, M.E.; Shariat, M.H.; Bagheri, R. Bioactive Glasses in Dentistry: A Review. J. Dent. Biomater. 2015, 2, 1–9. [Google Scholar]
- Hench, L.L. An Introduction to Bioceramics; World Scientific: Singapore, 1993. [Google Scholar]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C.; Tozar, T.; Dinu, M.; Parau, A.C.; Grumezescu, V.; Hapenciuc, C.; Popa, M.; Stan, M.S.; Marutescu, L.; et al. Implant Surfaces Containing Bioglasses and Ciprofloxacin as Platforms for Bone Repair and Improved Resistance to Microbial Colonization. Pharmaceutics 2022, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Floroian, L.; Ristoscu, C.; Candiani, G.; Pastori, N.; Moscatelli, M.; Mihailescu, N.; Negut, I.; Badea, M.; Gilca, M.; Chiesa, R.; et al. Antimicrobial thin films based on ayurvedic plants extracts embedded in a bioactive glass matrix. Appl. Surf. Sci. 2017, 417, 224–233. [Google Scholar] [CrossRef]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci. Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Mirza Rosca, J.C.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials 2020, 10, 385. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.; Urzica, I.; Dyia, H.; Bleotu, C. Functionalized antimicrobial composite thin films printing for stainless steel implant coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef]
- Venkateshbabu, N.; Anand, S.; Abarajithan, M.; Sheriff, S.O.; Jacob, P.S.; Sonia, N. Natural Therapeutic Options in Endodontics—A Review. Open Dent. J. 2016, 10, 214–226. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2015.00194 (accessed on 20 February 2023). [CrossRef]
- Mancuso, E.; Bretcanu, O.A.; Marshall, M.; Birch, M.A.; McCaskie, A.W.; Dalgarno, K.W. Novel bioglasses for bone tissue repair and regeneration: Effect of glass design on sintering ability, ion release and biocompatibility. Mater. Des. 2017, 129, 239–248. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2015.00202 (accessed on 20 February 2023). [CrossRef]
- Entezari, A.; Roohani-Esfahani, S.-I.; Zhang, Z.; Zreiqat, H.; Dunstan, C.R.; Li, Q. Fracture behaviors of ceramic tissue scaffolds for load bearing applications. Sci. Rep. 2016, 6, 28816. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).