Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application
Abstract
:1. Introduction
2. Experimental
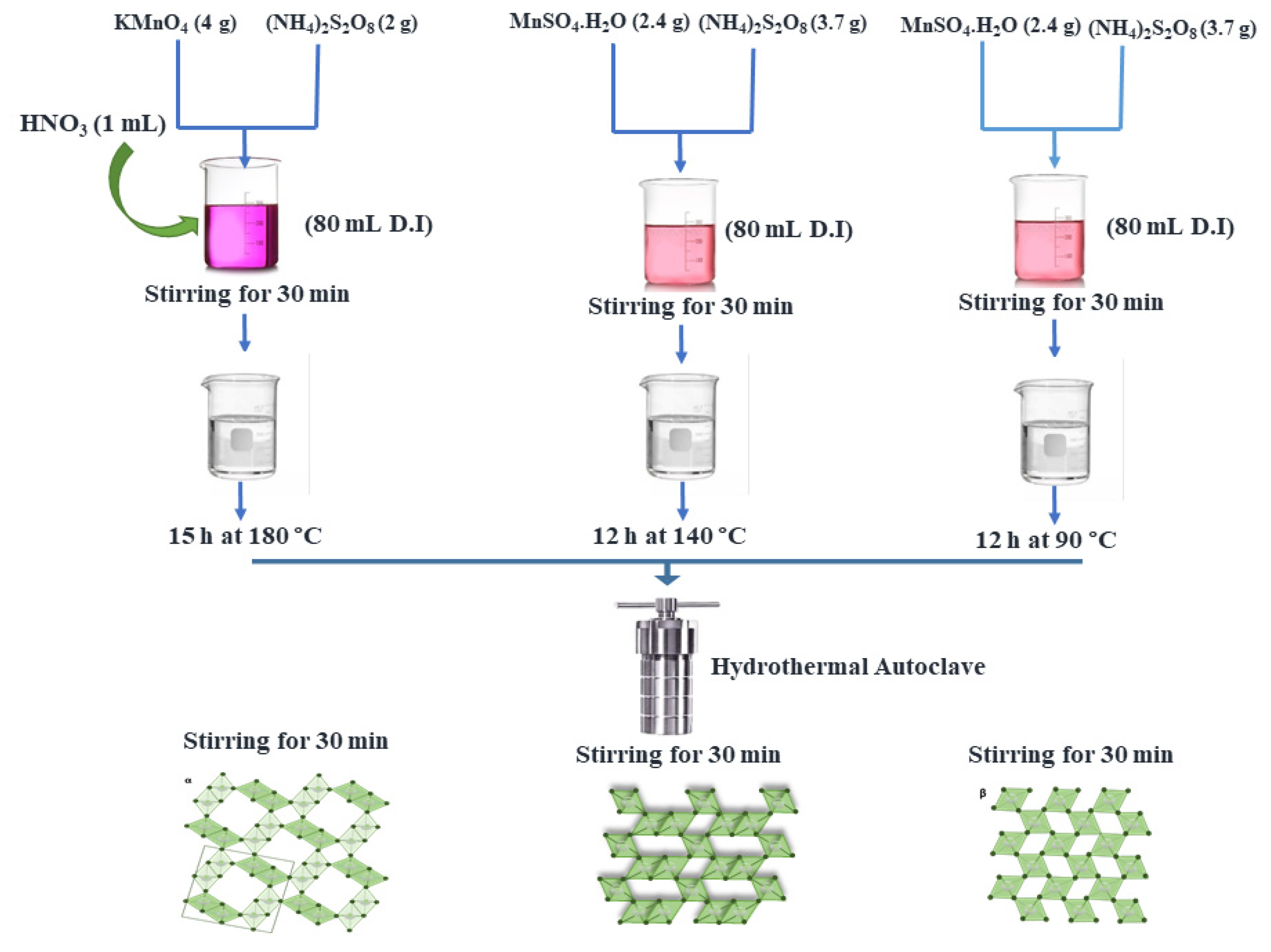
2.1. Material Preparation
2.2. Electrode Preparation and Electrochemical Measurements for Supercapacitor
3. Results and Discussion
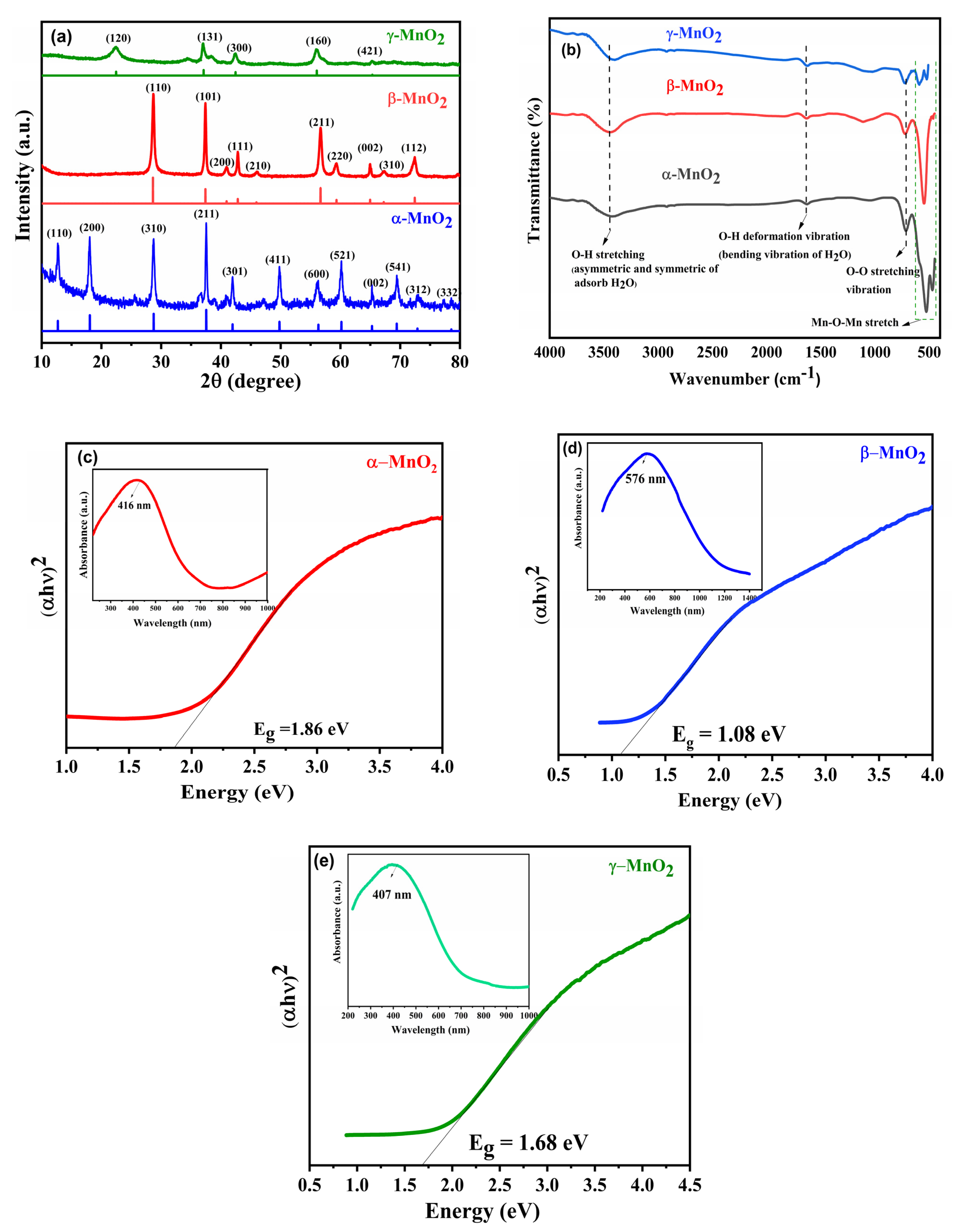
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. UV–Vis Analysis
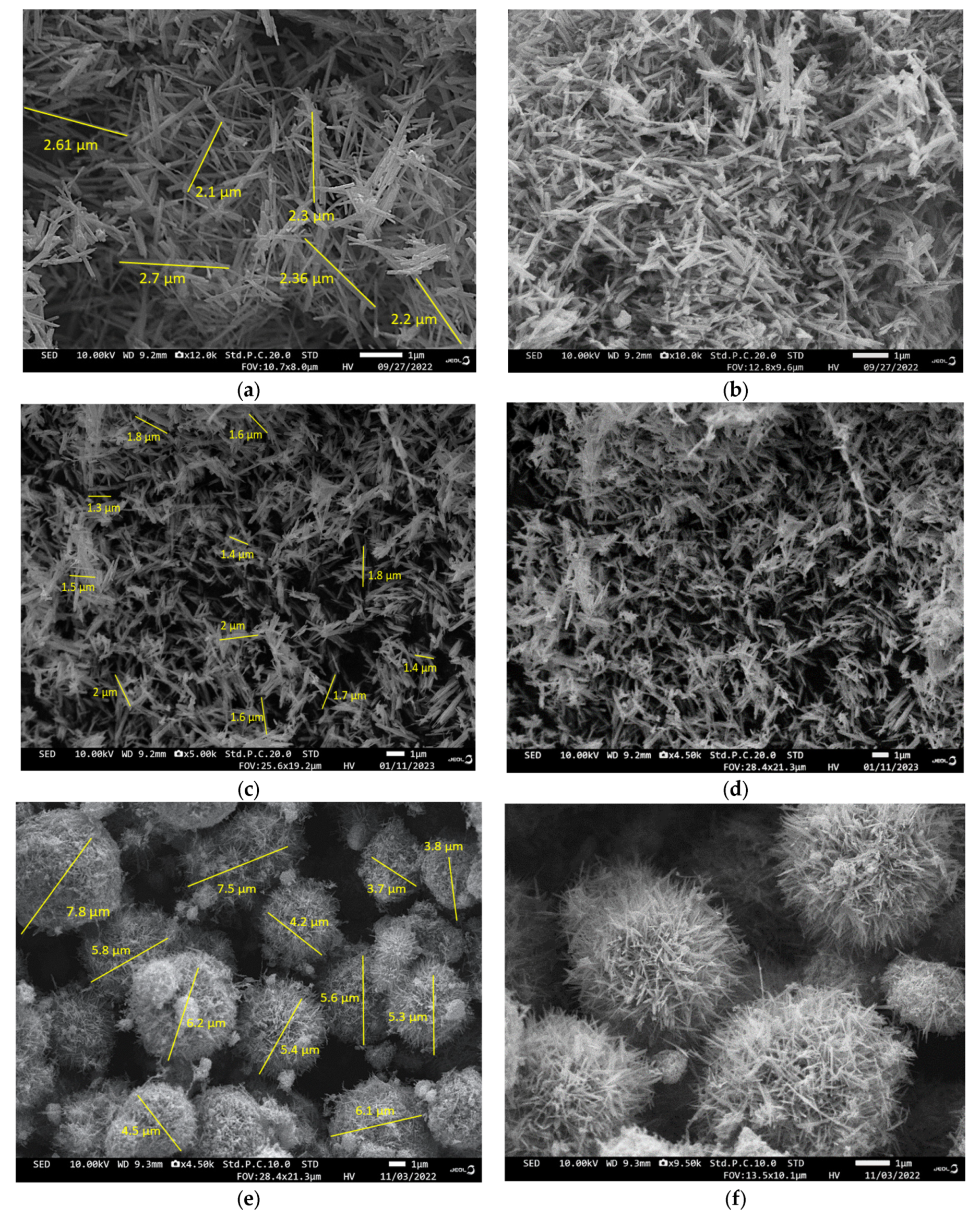
3.4. FE-SEM Analysis
3.5. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musil, M.; Choi, B.; Tsutsumi, A. Morphology and Electrochemical Properties of α-, β-, γ-, and δ-MnO2 Synthesized by Redox Method. J. Electrochem. Soc. 2015, 162, A2058. [Google Scholar] [CrossRef]
- Su, X.; Liang, Z.; He, Q.; Guo, Y.; Luo, G.; Han, S.; Yu, L. Advanced three-dimensional hierarchical porous α-MnO2 nanowires network toward enhanced supercapacitive performance. Nanotechnology 2022. [Google Scholar] [CrossRef] [PubMed]
- Modi, K.H.; Pataniya, P.M.; Siraj, S.; Sahatiya, P.; Patel, V.; Sumesh, C.K. Synergistic effect from Ni2+ ions with SnS for all solid-state type symmetric supercapacitor. J. Energy Storage 2023, 63, 107040. [Google Scholar] [CrossRef]
- Rani, P.; Dahiya, R.; Bulla, M.; Devi, R.; Jeet, K.; Jatrana, A.; Kumar, V. Hydrothermal-assisted green synthesis of reduced graphene oxide nanosheets (rGO) using lemon (Citrus Limon) peel extract. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Jatrana, A.; Agrawal, A.; Singh, P. Development of biochar-based functional materials for electrochemical supercapacitor applications. ECS Trans. 2022, 107, 7979. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Sisodiya, A.K.; Mishra, A.K.; Jatrana, A.; Singh, P. Development of Activated Carbon by Bio Waste Material for Application in Supercapacitor Electrodes. Mater. Lett. 2023, 335, 133830. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Dabhi, S.; Patel, V.; Sumesh, C.K. Liquid phase exfoliated ReS2 nanocrystals on paper based electrodes for hydrogen evolution and supercapacitor applications. Surf. Interfaces 2022, 34, 102318. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Devi, R.; Sisodia, A.K.; Jatrana, A.; Singh, R.B.; Mishra, A.K. Sustainable and Scalable Approach for Enhancing the Electrochemical Performance of Molybdenum Disulfide (MoS2). Adv. Mater. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Chand, P.; Joshi, A.; Lal, S.; Singh, V. Effect of hydrothermal temperature on structural, optical and electrochemical properties of α-MnO2 nanostructures for supercapacitor application. Chem. Phys. Lett. 2021, 777, 138742. [Google Scholar]
- Zhu, S.; Li, L.; Liu, J.; Wang, H.; Wang, T.; Zhang, Y.; Dong, F. Structural directed growth of ultrathin parallel birnessite on β-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 2018, 12, 1033–1042. [Google Scholar] [CrossRef]
- Sivakumar, S.; Prabu, L.N. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 2021, 7, 52–55. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Source 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Toufiq, A.M.; Wang, F.; Javed, Q.U.A.; Li, Q.; Li, Y. Hydrothermal synthesis of MnO2 nanowires: Structural characterizations, optical and magnetic properties. Appl. Phys. A 2014, 116, 1127–1132. [Google Scholar] [CrossRef]
- Chen, W.M.; Qie, L.; Shao, Q.G.; Yuan, L.X.; Zhang, W.X.; Huang, Y.H. Controllable synthesis of hollow bipyramid β-MnO2 and its high electrochemical performance for lithium storage. ACS Appl. Mater. Interfaces 2012, 4, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, G.; Lan, B.; Zheng, X.; Sun, M.; Ye, F.; Cheng, X. Crystallization design of MnO2 via acid towards better oxygen reduction activity. CrystEngComm 2016, 18, 6895–6902. [Google Scholar] [CrossRef]
- Taranu, B.O.; Novaconi, S.D.; Ivanovici, M.; Gonçalves, J.N.; Rus, F.S. α-MnO2 Nanowire Structure Obtained at Low Temperature with Aspects in Environmental Remediation and Sustainable Energy Applications. Appl. Sci. 2022, 12, 6821. [Google Scholar] [CrossRef]
- Gangwar, D.; Rath, C. Structural, optical and magnetic properties of α-and β-MnO2 nanorods. Appl. Surf. Sci. 2021, 557, 149693. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Sato, N. Electrochemistry at Metal and Semiconductor Electrodes; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Khan, Y.; Durrani, S.K.; Mehmood, M.; Khan, M.R. Mild hydrothermal synthesis of γ-MnO2 nanostructures and their phase transformation to α-MnO2 nanowires. J. Mater. Res. 2011, 26, 2268–2275. [Google Scholar] [CrossRef]
- Rajagopal, R.; Ryu, K.S. Synthesis of MnO2 nanostructures with MnS-deposits for high performance supercapacitor electrodes. New J. Chem. 2019, 43, 12987–13000. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, D.; Zhao, J.; Zhang, H. Snowflake-like core-shell α-MnO2@ δ-MnO2 for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 251, 344–354. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Li, S.; Zhu, H.; Ge, Y.; Wang, S.; Liu, Y. Electrochemical synthesis of MnO2 porous nanowires for flexible all-solid-state supercapacitor. New J. Chem. 2017, 41, 3750–3757. [Google Scholar] [CrossRef]
- Singu, B.S.; Hong, S.E.; Yoon, K.R. Ultra-thin and ultra-long α-MnO2 nanowires for pseudocapacitor material. J. Solid State Electrochem. 2017, 21, 3215–3220. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Kumar, Y.; Chandra, R. An efficient α-MnO2 nanorods forests electrode for electrochemical capacitors with neutral aqueous electrolytes. Electrochim. Acta 2016, 220, 712–720. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, C.; Ye, J.; Zhao, D.; Chen, Y.; Zhang, J.M.; Huang, Z. Bi2S3/rGO nanocomposites with covalent heterojunctions as a high-performance aqueous zinc ion battery material. Ceram. Int. 2023, 49, 22160–22169. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Isaikina, O.Y.; Novotortsev, R.Y.; Stolbov, D.N.; Xia, H.; Savilov, S.V. Application of MnO2/MWCNT composite in supercapacitors. Mater. Today Proc. 2022, 60, 1008–1011. [Google Scholar] [CrossRef]
- Aquino, C.L.E.; Gorospe, A.E.; Rezaga, B.F.Y.; Avila, R.A.M.; Macugay, J.K.; Tercero, J.U.; Balela, M.D.L. Effect of ammonium persulfate on the growth of MnO2 nanostructures prepared via hydrothermal synthesis for supercapacitor applications. Mater. Today Proc. 2020, 33, 1945–1948. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Consequences of electrodeposition parameters on the microstructure and electrochemical behavior of electrolytic manganese dioxide (EMD) for supercapacitor. Ceram. Int. 2022, 48, 19913–19924. [Google Scholar] [CrossRef]
- Xie, Y. Electrochemical properties of sodium manganese oxide/nickel foam supercapacitor electrode material. Inorg. Nano-Met. Chem. 2022, 52, 548–555. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Erdemir, F.; Tuzcu, E.; Bilgin, S.; Alver, Ü.; Çanakçı, A. Influence of fluorine doping of zinc oxide on its electrochemical performance in supercapacitors. Mater. Chem. Phys. 2021, 259, 124033. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Sharma, S.; Sharma, A. Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Appl. Sci. 2023, 13, 7907. https://doi.org/10.3390/app13137907
Devi R, Kumar V, Kumar S, Bulla M, Sharma S, Sharma A. Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Applied Sciences. 2023; 13(13):7907. https://doi.org/10.3390/app13137907
Chicago/Turabian StyleDevi, Raman, Vinay Kumar, Sunil Kumar, Mamta Bulla, Shruti Sharma, and Ashutosh Sharma. 2023. "Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application" Applied Sciences 13, no. 13: 7907. https://doi.org/10.3390/app13137907
APA StyleDevi, R., Kumar, V., Kumar, S., Bulla, M., Sharma, S., & Sharma, A. (2023). Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Applied Sciences, 13(13), 7907. https://doi.org/10.3390/app13137907





