An Evaluation of the Usability of Argon Plasma-Treated Bacterial Cellulose as a Carrier for Controlled Releases of Glycoside Hydrolases PelAh and PslGh, Which Are Able to Eradicate Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning, Expression, and Purification of PelAh and PslGh
2.2. Preparation of BC Membranes
2.3. Modification of BC with Low-Pressure Argon Plasma (LPArP)
2.4. Characterization of BC Surfaces after LPArP Treatment
2.4.1. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR–FTIR)
2.4.2. X-ray Photoelectron Spectroscopy (XPS)
2.4.3. Scanning Electron Microscopy (SEM)
2.5. PelAh and PslGh Stability—Differential Scanning Fluorimetry (DSF)
2.6. Analysis of the Cytotoxicity of PslGh Hydrolase
2.7. The Loading of PelAh and PslGh on BC and Ar_BC
2.8. Analysis of In Vitro Release of PelAh and PslGh from Ar_BC and BC
2.9. Antibiofilm Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Influence of LPArP Treatment on BC Membrane Surface Properties
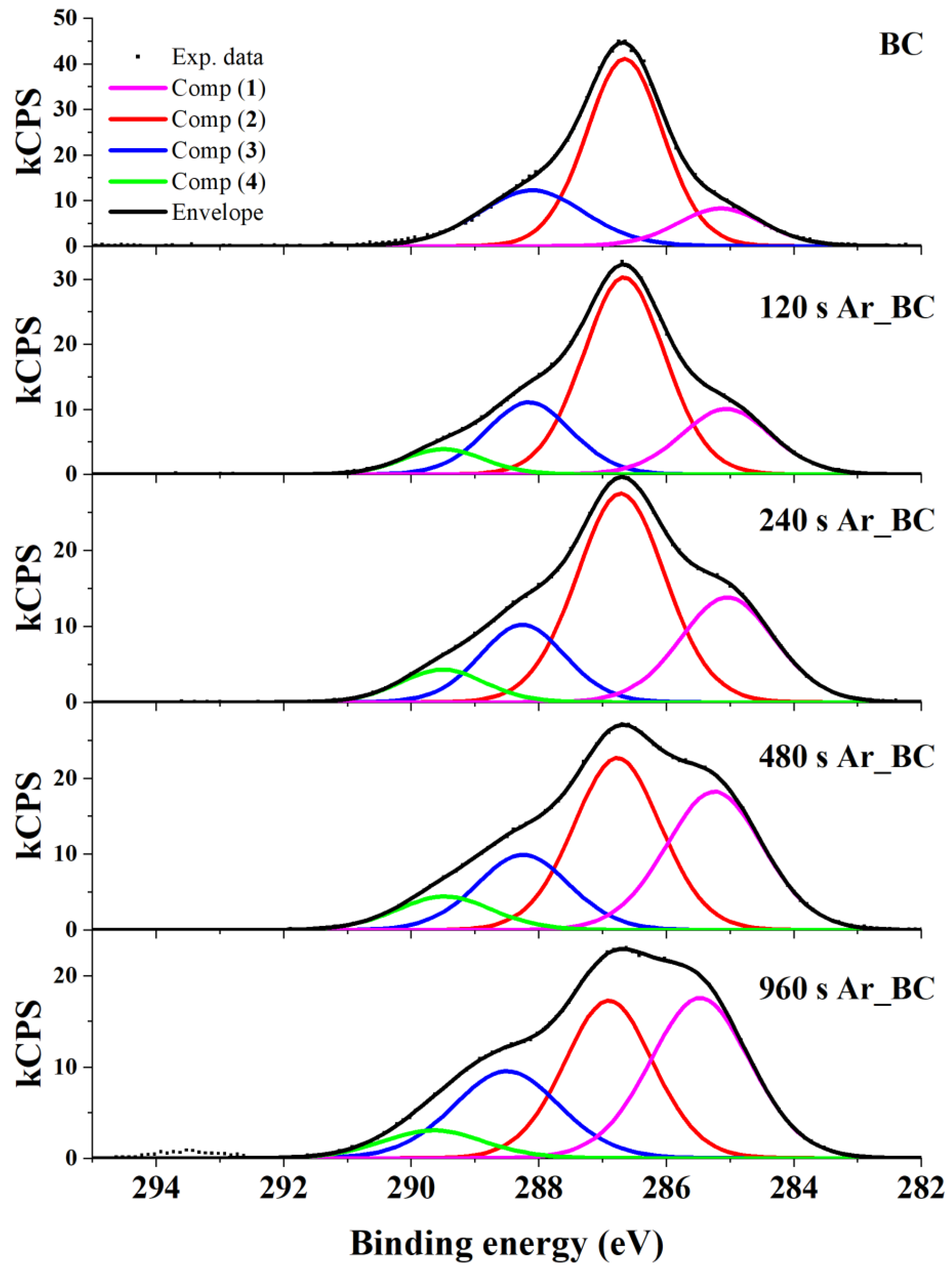
3.2. X-ray Photoelectron Spectroscopy (XPS)
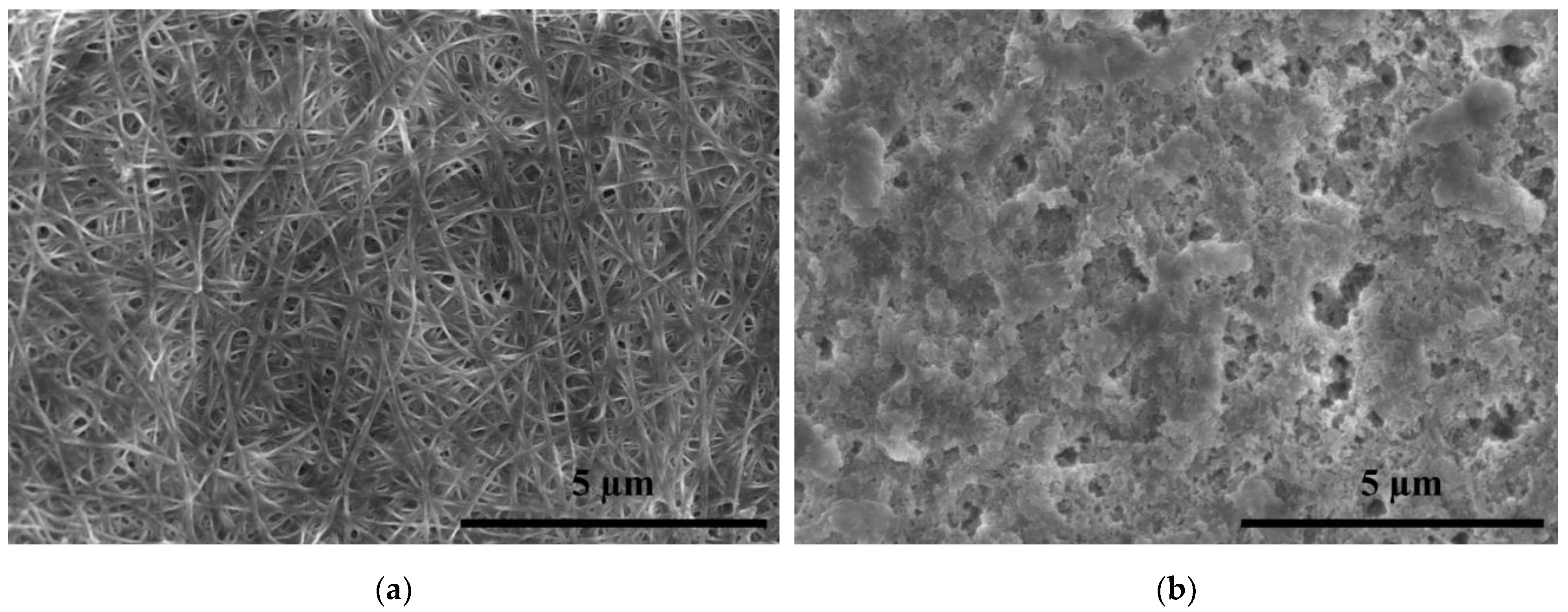
3.3. Influence of LPArP on the BC Surface Morphology
3.4. Differential Scanning Fluorimetry
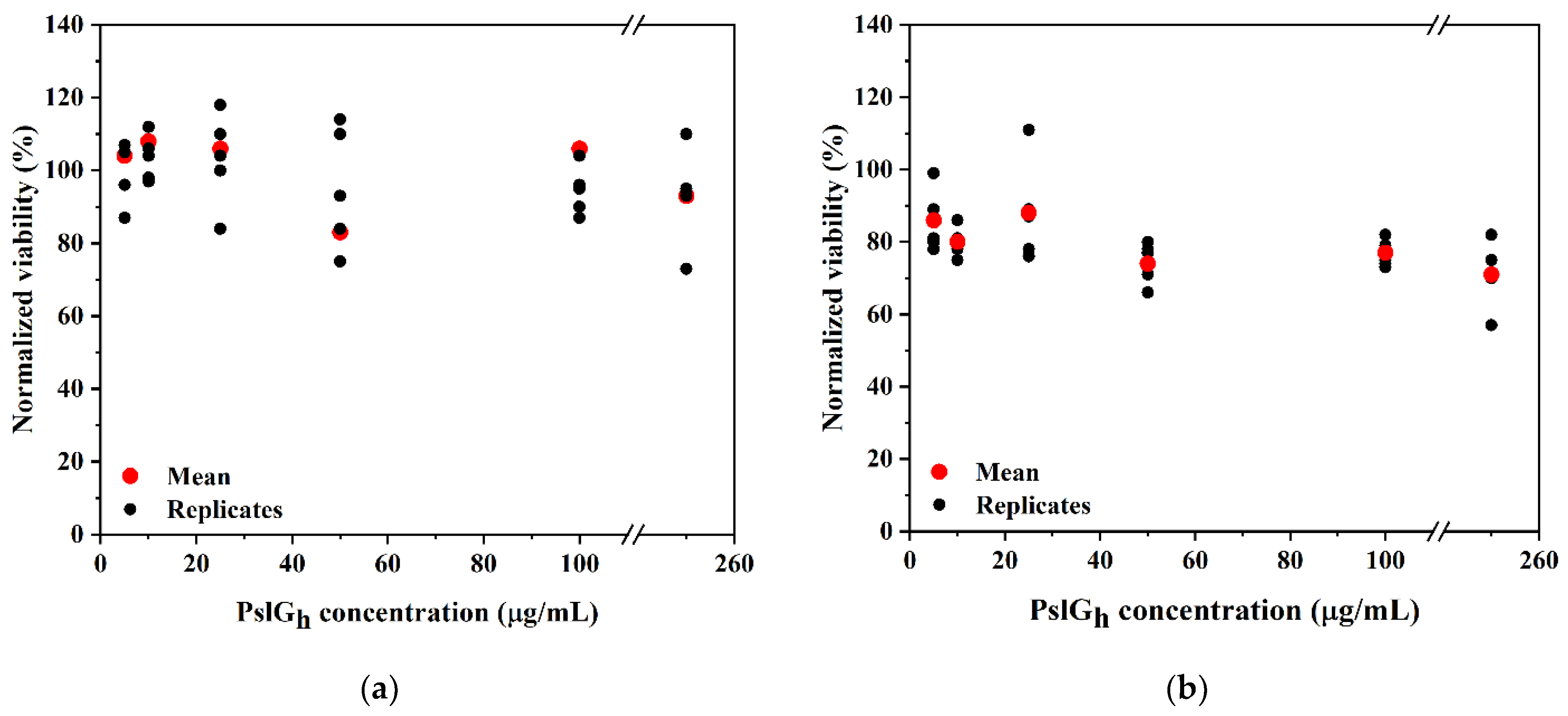
3.5. Cytotoxicity of the PslGh
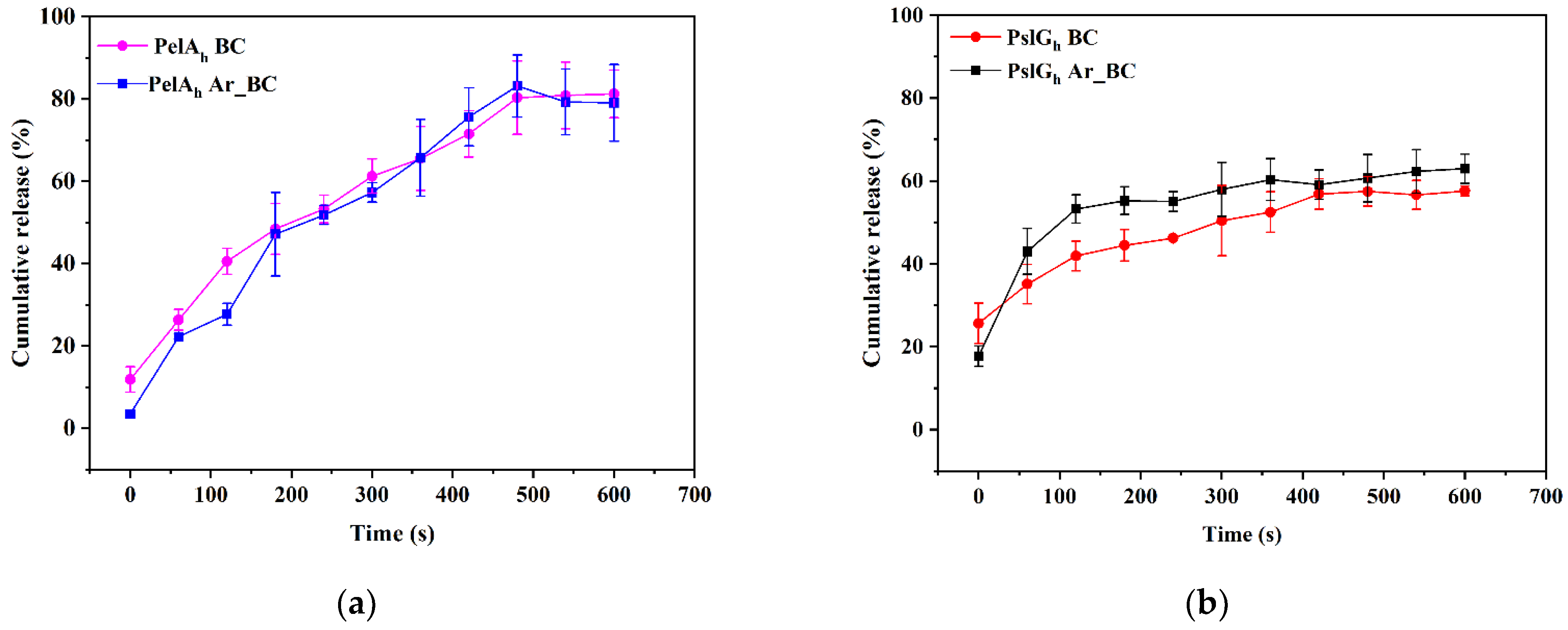
3.6. In Vitro Release Study of PelAh and PslGh from Pristine and LPArP-Modified BC
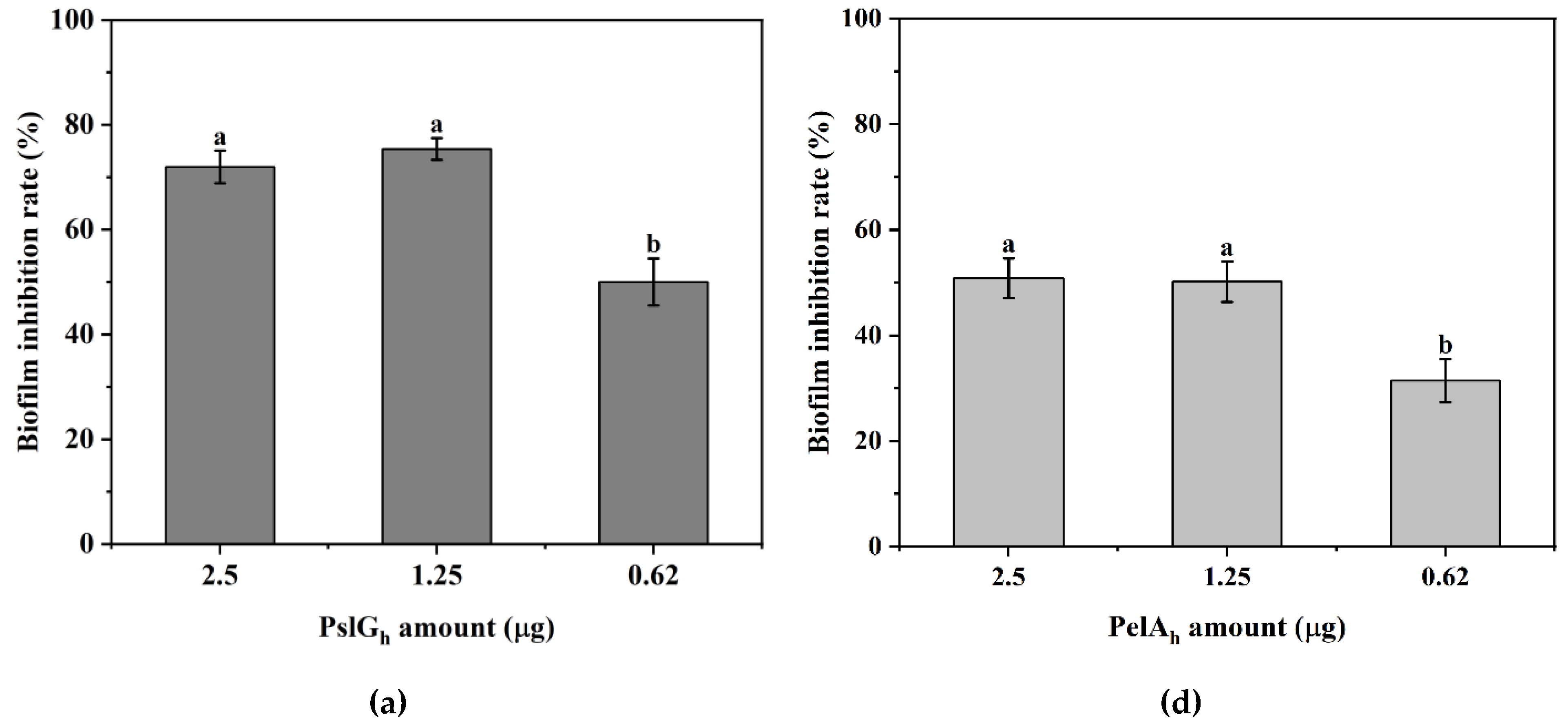
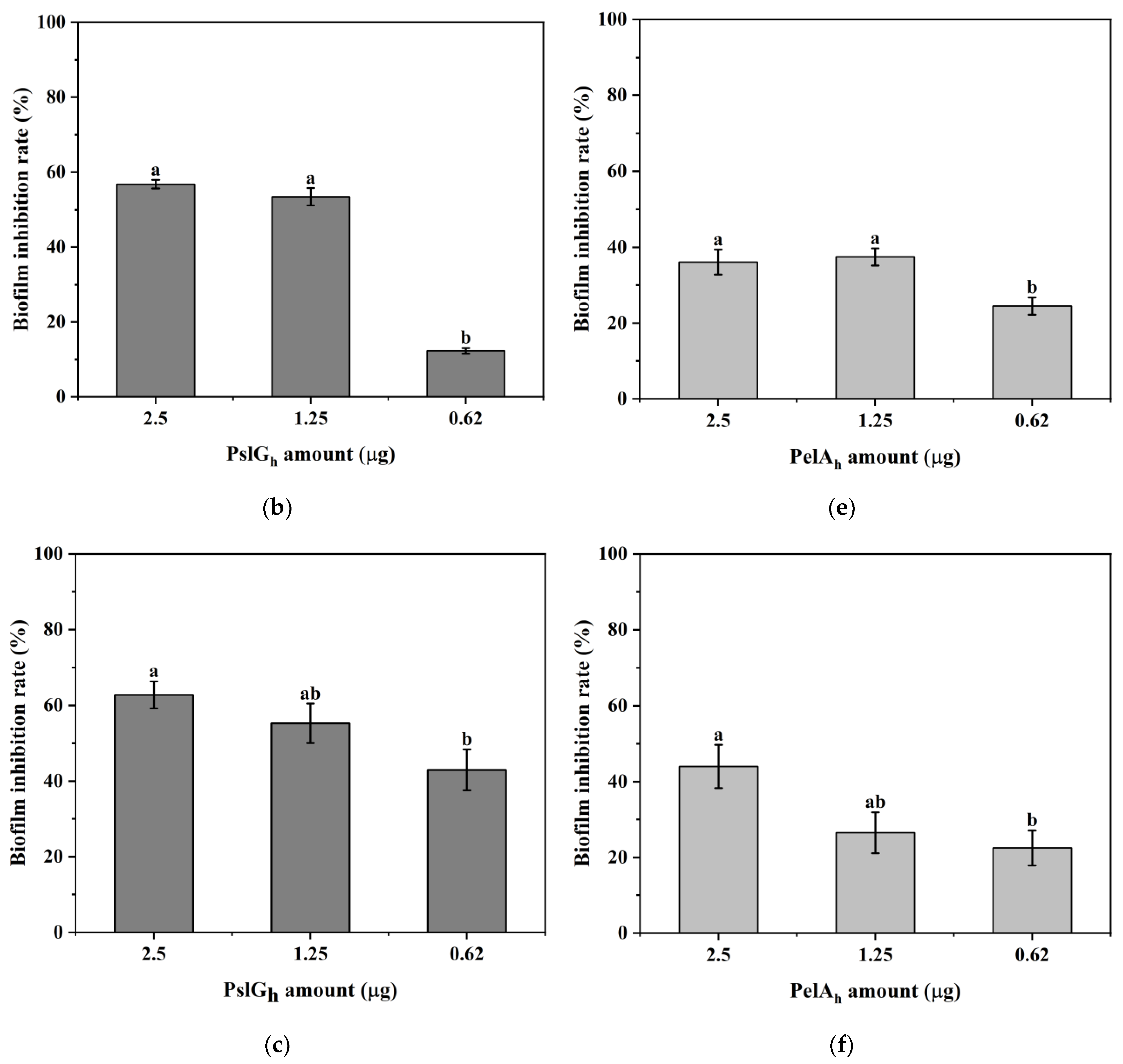
3.7. Influence of the Immobilization of PelAh and PslGh on P. aeruginosa PAO-1 Biofilm Eradication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, S.; Cai, X.; Qu, X.; Yu, B.; Yan, C.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Preparation of biocompatible wound dressings with long-term antimicrobial activity through covalent bonding of antibiotic agents to natural polymers. Int. J. Biol. Macromol. 2019, 123, 1320–1330. [Google Scholar] [CrossRef]
- Talikowska, M.; Fu, X.; Lisak, G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens. Bioelectron. 2019, 135, 50–63. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Improving bacterial cellulose films by ex-situ and in-situ modifications: A review. Food Hydrocoll. 2021, 113, 106514. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, A.; Yeung, K.W.K.; Kao, R.Y.T.; Wu, G.; Hu, T.; Xu, Z.; Chu, P.K. Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl. Mater. Interfaces 2011, 3, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, M.; Hoppe, J.; Dutkiewicz, M.; Sobolewski, P.; Palacz, M.; Janus, E.; Zielińska, B.; Drozd, R. Silicone polyether surfactant enhances bacterial cellulose synthesis and water holding capacity. Int. J. Biol. Macromol. 2022, 208, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Farber, R.; Dabush-Busheri, I.; Chaniel, G.; Rozenfeld, S.; Bormashenko, E.; Multanen, V.; Cahan, R. Biofilm grown on wood waste pretreated with cold low-pressure nitrogen plasma: Utilization for toluene remediation. Int. Biodeterior. Biodegrad. 2019, 139, 62–69. [Google Scholar] [CrossRef]
- Pertile, R.A.; Andrade, F.K.; Alves, C.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Żywicka, A.; Ciecholewska-Juśko, D.; Szymańska, M.; Drozd, R.; Sobolewski, P.; Junka, A.; Gorgieva, S.; El Fray, M.; Fijałkowski, K. Argon plasma-modified bacterial cellulose filters for protection against respiratory pathogens. Carbohydr. Polym. 2023, 302, 120322. [Google Scholar] [CrossRef] [PubMed]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface modification of bacterial cellulose membrane by oxygen plasma treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K.K. Atmospheric pressure plasmas: Infection control and bacterial responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Hasebe, T.; Hotta, A. Effects of plasma treatments on the controlled drug release from poly(ethylene-co-vinyl acetate). Surf. Coat. Technol. 2013, 216, 318–323. [Google Scholar] [CrossRef]
- Canal, C.; Modic, M.; Cvelbar, U.; Ginebra, M.-P. Regulating the antibiotic drug release from β-tricalcium phosphate ceramics by atmospheric plasma surface engineering. Biomater. Sci. 2016, 4, 1454–1461. [Google Scholar] [CrossRef]
- Kasza, K.; Gurnani, P.; Hardie, K.R.; Cámara, M.; Alexander, C. Challenges and solutions in polymer drug delivery for bacterial biofilm treatment: A tissue-by-tissue account. Adv. Drug Deliv. Rev. 2021, 178, 113973. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Vanderwoude, J.; Fleming, D.; Azimi, S.; Trivedi, U.; Rumbaugh, K.P.; Diggle, S.P. The evolution of virulence in Pseudomonas aeruginosa during chronic wound infection. Proc. Biol. Sci. 2020, 287, 20202272. [Google Scholar]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- Al-Wrafy, F.; Brzozowska, E.; Górska, S.; Gamian, A. Pathogenic factors of Pseudomonas aeruginosa—The role of biofilm in pathogenicity and as a target for phage therapy. Postepy Hig. Med. Dosw. 2017, 71, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Da Passos Silva, D.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas aeruginosa lectin LecB binds to the exopolysaccharide Psl and stabilizes the biofilm matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Whitfield, G.B.; Hill, P.J.; Little, D.J.; Pestrak, M.J.; Robinson, H.; Wozniak, D.J.; Howell, P.L. Characterization of the Pseudomonas aeruginosa Glycoside Hydrolase PslG Reveals That Its Levels Are Critical for Psl Polysaccharide Biosynthesis and Biofilm Formation. J. Biol. Chem. 2015, 290, 28374–28387. [Google Scholar] [CrossRef] [PubMed]
- Vandana; Das, S. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydr. Polym. 2022, 291, 119536. [Google Scholar] [CrossRef]
- Le Mauff, F.; Razvi, E.; Reichhardt, C.; Sivarajah, P.; Parsek, M.R.; Howell, P.L.; Sheppard, D.C. The Pel polysaccharide is predominantly composed of a dimeric repeat of α-1,4 linked galactosamine and N-acetylgalactosamine. Commun. Biol. 2022, 5, 502. [Google Scholar] [CrossRef]
- Szymańska, M.; Karakulska, J.; Sobolewski, P.; Kowalska, U.; Grygorcewicz, B.; Böttcher, D.; Bornscheuer, U.T.; Drozd, R. Glycoside hydrolase (PelAh) immobilization prevents Pseudomonas aeruginosa biofilm formation on cellulose-based wound dressing. Carbohydr. Polym. 2020, 246, 116625. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Baker, P.; Dellos-Nolan, S.; Hill, P.J.; Da Passos Silva, D.; Silver, H.; Lacdao, I.; Raju, D.; Parsek, M.R.; Wozniak, D.J.; et al. Treatment with the Pseudomonas aeruginosa Glycoside Hydrolase PslG Combats Wound Infection by Improving Antibiotic Efficacy and Host Innate Immune Activity. Antimicrob. Agents Chemother. 2019, 63, e00234-19. [Google Scholar] [CrossRef]
- Snarr, B.D.; Baker, P.; Bamford, N.C.; Sato, Y.; Liu, H.; Lehoux, M.; Gravelat, F.N.; Ostapska, H.; Baistrocchi, S.R.; Cerone, R.P.; et al. Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc. Natl. Acad. Sci. USA 2017, 114, 7124–7129. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Raju, D.; Sanchez, H.; Lacdao, I.; Gilbert, S.; Sivarajah, P.; Andes, D.R.; Sheppard, D.C.; Howell, P.L.; et al. Preventing Pseudomonas aeruginosa Biofilms on Indwelling Catheters by Surface-Bound Enzymes. ACS Appl. Bio Mater. 2021, 4, 8248–8258. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Baker, P.; Howell, P.L.; Hatton, B.D. Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Charęza, M.; Przygrodzka, K.; Żywicka, A.; Grygorcewicz, B.; Sobolewski, P.; Mozia, S.; Śmiglak, M.; Drozd, R. Enhancement of Inhibition of the Pseudomonas sp. Biofilm Formation on Bacterial Cellulose-Based Wound Dressing by the Combined Action of Alginate Lyase and Gentamicin. Int. J. Mol. Sci. 2023, 24, 4740. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Chichester, UK, 1992; ISBN 0471935921. [Google Scholar]
- Przygrodzka, K.; Charęza, M.; Banaszek, A.; Zielińska, B.; Ekiert, E.; Drozd, R. Bacterial Cellulose Production by Komagateibacter xylinus with the Use of Enzyme-Degraded Oligo- and Polysaccharides as the Substrates. Appl. Sci. 2022, 12, 12673. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M.; Kordas, M.; Konopacki, M.; Rakoczy, R. Modulation of Cellular Response to Different Parameters of the Rotating Magnetic Field (RMF)-An In Vitro Wound Healing Study. Int. J. Mol. Sci. 2021, 22, 5785. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Pietrella, D.; Donnadio, A.; Latterini, L.; Di Michele, A.; Luffarelli, I.; Ricci, M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110863. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Żywicka, A.; Kowalska, U.; Rakoczy, R.; Kordas, M.; Konopacki, M.; Junka, A.F.; Fijałkowski, K. Exposure to non-continuous rotating magnetic field induces metabolic strain-specific response of Komagataeibacter xylinus. Biochem. Eng. J. 2021, 166, 107855. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr. Polym. 2020, 229, 115513. [Google Scholar] [CrossRef]
- Abral, H.; Chairani, M.K.; Rizki, M.D.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021, 11, 896–904. [Google Scholar] [CrossRef]
- Dayal, M.S.; Goswami, N.; Sahai, A.; Jain, V.; Mathur, G.; Mathur, A. Effect of media components on cell growth and bacterial cellulose production from Acetobacter aceti MTCC 2623. Carbohydr. Polym. 2013, 94, 12–16. [Google Scholar] [CrossRef]
- Srikandace, Y.; Apriyana, A.Y.; Zahrad, S.A.; Ramdhani, W.; Asri, P.P.P.; Andriani, D.; Abdullah, A.H.D.; Syampurwadi, A.; Satoto, R.; Karina, M. Bacterial Cellulose Production by Komagataeibacter xylinus Using Rice-washed Water and Tofu Processing Wastewater with the Addition of Sodium Glutamate. Fibers Polym. 2022, 23, 1190–1196. [Google Scholar] [CrossRef]
- Chashmejahanbin, M.R.; Salimi, A.; Ershad Langroudi, A. The study of the coating adhesion on PP surface modified in different plasma/acrylic acid solution. Int. J. Adhes. Adhes. 2014, 49, 44–50. [Google Scholar] [CrossRef]
- Hua, Z.Q.; Sitaru, R.; Denes, F.; Young, R.A. Mechanisms of oxygen- and argon-RF-plasma-induced surface chemistry of cellulose. Plasmas Polym. 1997, 2, 199–224. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. JMPC 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Brassolatti, P.; Kido, H.W.; Bossini, P.S.; Gabbai-Armelin, P.R.; Otterço, A.N.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; de Da Avó, L.R.S.; Forato, L.A.; et al. Bacterial cellulose membrane used as biological dressings on third-degree burns in rats. Biomed. Mater. Eng. 2018, 29, 29–42. [Google Scholar] [CrossRef]
- Arias, S.L.; Cheng, M.K.; Civantos, A.; Devorkin, J.; Jaramillo, C.; Allain, J.P. Ion-Induced Nanopatterning of Bacterial Cellulose Hydrogels for Biosensing and Anti-Biofouling Interfaces. ACS Appl. Nano Mater. 2020, 3, 6719–6728. [Google Scholar] [CrossRef]
- Tang, S.; Lu, N.; Wang, J.K.; Ryu, S.-K.; Choi, H.-S. Novel Effects of Surface Modification on Activated Carbon Fibers Using a Low Pressure Plasma Treatment. J. Phys. Chem. C 2007, 111, 1820–1829. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Budnikov, A.; Gromovykh, T.; Pigaleva, M.; Sadykova, V.; Arkharova, N.; Naumkin, A. Effect of Bacterial Cellulose Plasma Treatment on the Biological Activity of Ag Nanoparticles Deposited Using Magnetron Deposition. Polymers 2022, 14, 3907. [Google Scholar] [CrossRef]
- Nosworthy, N.J.; McKenzie, D.R.; Bilek, M.M. A new surface for immobilizing and maintaining the function of enzymes in a freeze-dried state. Biomacromolecules 2009, 10, 2577–2583. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Gao, K.; Oerlemans, R.; Groves, M.R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys. Rev. 2020, 12, 85–104. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Khandelwal, M. Ex-situ modification of bacterial cellulose for immediate and sustained drug release with insights into release mechanism. Carbohydr. Polym. 2020, 249, 116816. [Google Scholar] [CrossRef] [PubMed]
- Jantarat, C.; Muenraya, P.; Srivaro, S.; Nawakitrangsan, A.; Promsornpason, K. Comparison of drug release behavior of bacterial cellulose loaded with ibuprofen and propranolol hydrochloride. RSC Adv. 2021, 11, 37354–37365. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
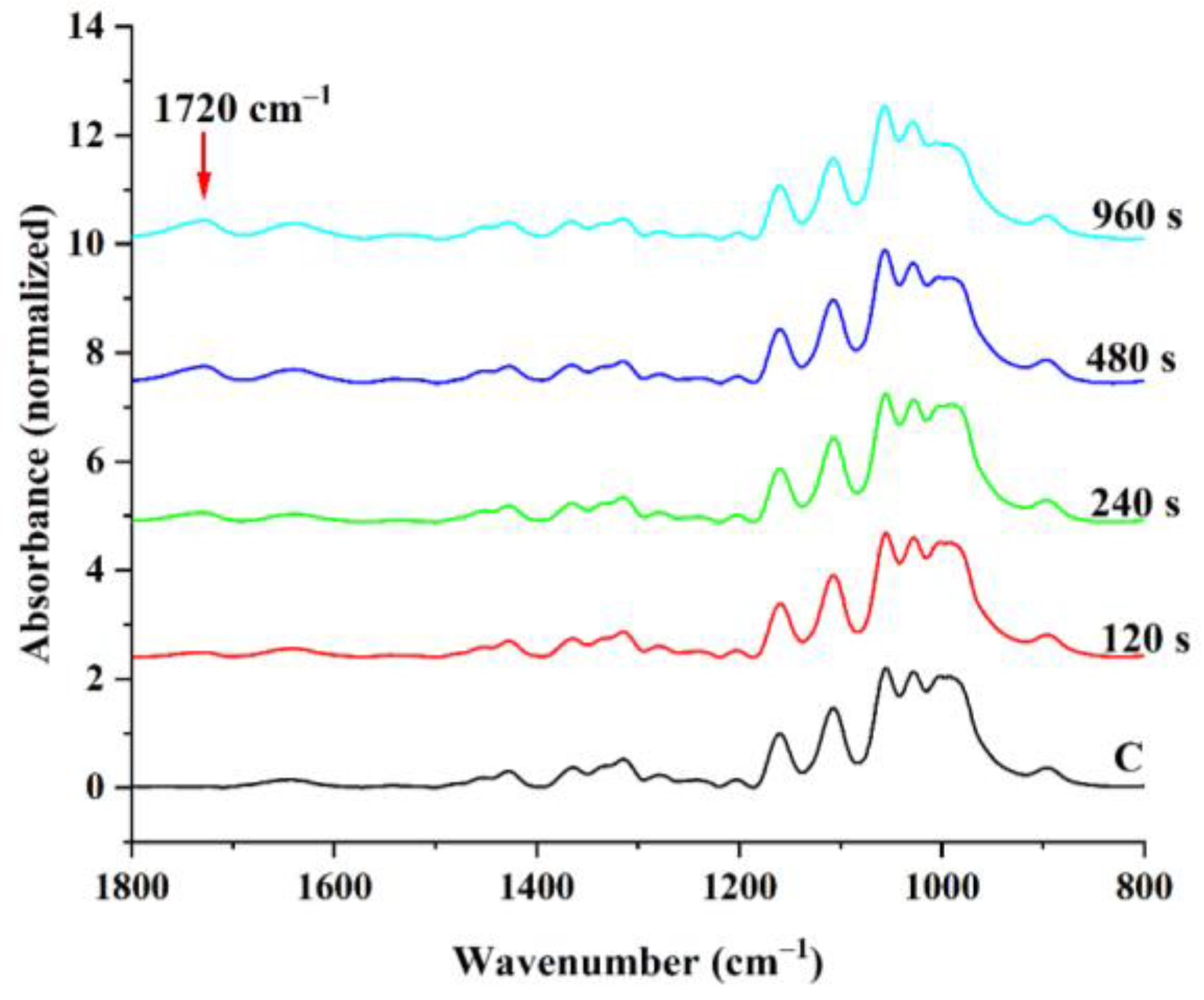


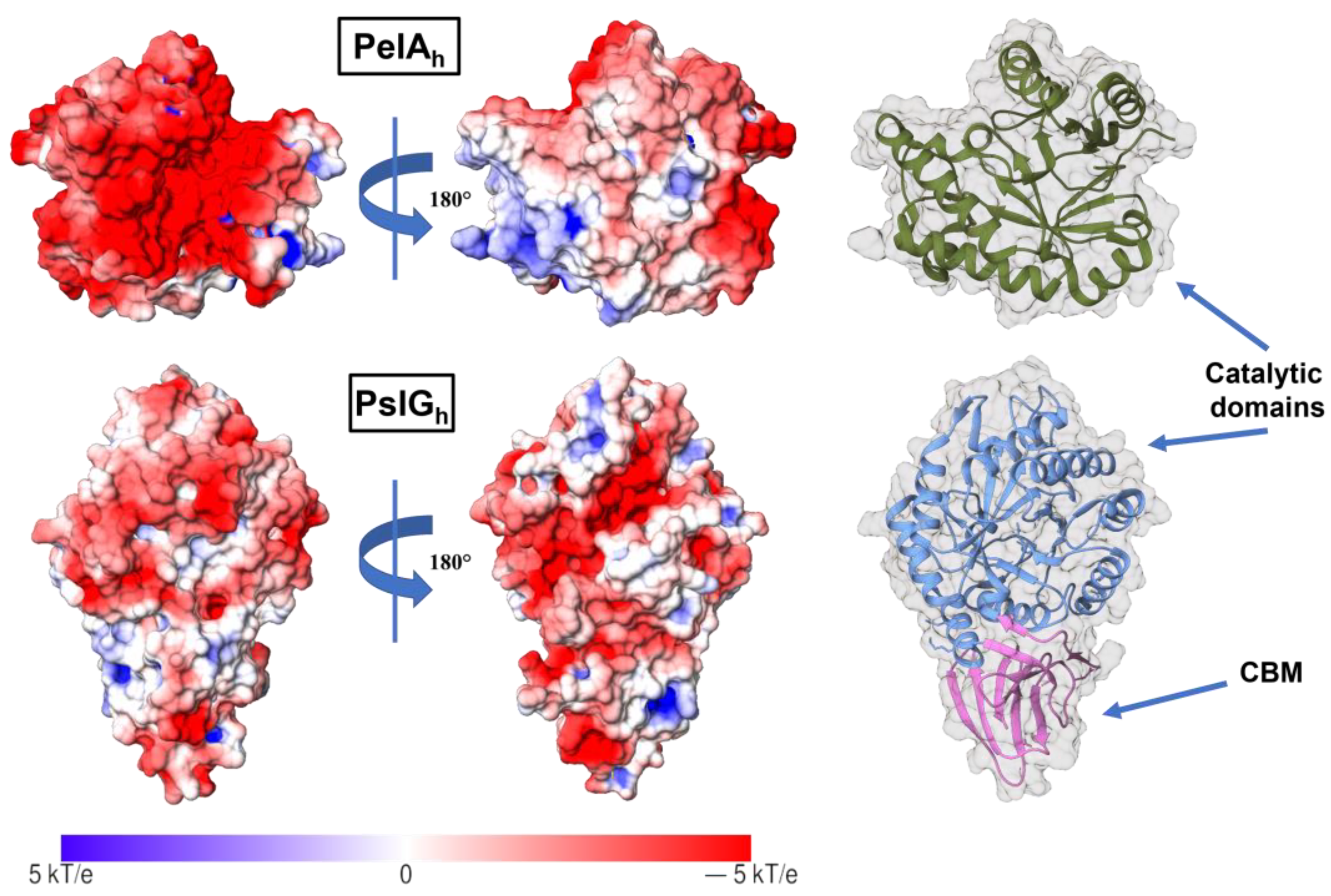
| Sample | IR Crystallinity | HBI | Cellulose Iα Index | |
|---|---|---|---|---|
| A1372/A2900 (TCI) | A1429/A897 (LOI) | A3400/A1320 | ||
| BC | 1.683 ± 0.20 a | 0.993 ± 0.17 a | 0.801 ± 0.08 a | 0.683 ± 0.02 a |
| 120 s Ar_BC | 1.685 ± 0.04 a | 0.877 ± 0.10 ab | 0.751 ± 0.03 a | 0.667 ± 0.01 ab |
| 240 s Ar_BC | 1.673 ± 0.08 a | 0.849 ± 0.04 ab | 0.899 ± 0.10 a | 0.650 ± 0.02 bc |
| 480 s Ar_BC | 1.492 ± 0.10 b | 0.818 ± 0.09 b | 1.266 ± 0.15 b | 0.637 ± 0.02 c |
| 960 s Ar_BC | 1.351 ± 0.07 b | 0.766 ± 0.04 b | 1.468 ± 0.16 c | 0.611 ± 0.006 d |
| Sample | Components of XPS C 1s Signal (Total C 1s Intensity = 100) | ||||
|---|---|---|---|---|---|
| C-C/C-H (Comp (1)) | C-OH (Comp (2)) | COC (Comp (3)) | O=C-O (Comp (4)) | O/C Ratio | |
| BC | 14 | 61 | 25 | 0 | 0.69 |
| 120 s Ar_BC | 19 | 53 | 20 | 7 | 0.73 |
| 240 s Ar_BC | 26 | 49 | 17 | 7 | 0.62 |
| 480 s Ar_BC | 34 | 39 | 18 | 8 | 0.56 |
| 960 s Ar_BC | 38 | 33 | 21 | 6 | 0.64 |
| Sample | Mathematical Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | ||||||
| K0 | R02 | K1 | R12 | KH | RH2 | KK-P | RK-P2 | n | |
| PelAh—Ar_BC | 1.24 × 10−3 | 0.92 | −2.7 × 10−3 | 0.98 | 0.034 | 0.97 | 0.016 | 0.93 | 0.63 |
| PelAh—BC | 1.13 × 10−3 | 0.94 | −2.7 × 10−3 | 0.98 | 0.031 | 0.98 | 0.033 | 0.98 | 0.51 |
| PslGh—Ar_BC | 5.1 × 10−4 | 0.60 | −9.8 × 10−4 | 0.71 | 0.016 | 0.85 | 0.15 | 0.90 | 0.25 |
| PslGh—BC | 4.9 × 10−4 | 0.88 | −9.1 × 10−4 | 0.92 | 0.014 | 0.98 | 0.14 | 0.98 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charęza, M.; Ekiert, E.; Moszyński, D.; Madej, M.; Jędrzejczak-Silicka, M.; Drozd, R. An Evaluation of the Usability of Argon Plasma-Treated Bacterial Cellulose as a Carrier for Controlled Releases of Glycoside Hydrolases PelAh and PslGh, Which Are Able to Eradicate Biofilm. Appl. Sci. 2023, 13, 7797. https://doi.org/10.3390/app13137797
Charęza M, Ekiert E, Moszyński D, Madej M, Jędrzejczak-Silicka M, Drozd R. An Evaluation of the Usability of Argon Plasma-Treated Bacterial Cellulose as a Carrier for Controlled Releases of Glycoside Hydrolases PelAh and PslGh, Which Are Able to Eradicate Biofilm. Applied Sciences. 2023; 13(13):7797. https://doi.org/10.3390/app13137797
Chicago/Turabian StyleCharęza, Magdalena, Ewa Ekiert, Dariusz Moszyński, Mariusz Madej, Magdalena Jędrzejczak-Silicka, and Radosław Drozd. 2023. "An Evaluation of the Usability of Argon Plasma-Treated Bacterial Cellulose as a Carrier for Controlled Releases of Glycoside Hydrolases PelAh and PslGh, Which Are Able to Eradicate Biofilm" Applied Sciences 13, no. 13: 7797. https://doi.org/10.3390/app13137797
APA StyleCharęza, M., Ekiert, E., Moszyński, D., Madej, M., Jędrzejczak-Silicka, M., & Drozd, R. (2023). An Evaluation of the Usability of Argon Plasma-Treated Bacterial Cellulose as a Carrier for Controlled Releases of Glycoside Hydrolases PelAh and PslGh, Which Are Able to Eradicate Biofilm. Applied Sciences, 13(13), 7797. https://doi.org/10.3390/app13137797







