Ultrasound Elastography for the Differentiation of Benign and Malignant Solid Renal Masses: A Systematic Review and Meta-Analysis
Abstract
:Featured Application
Abstract
1. Introduction
2. Methods
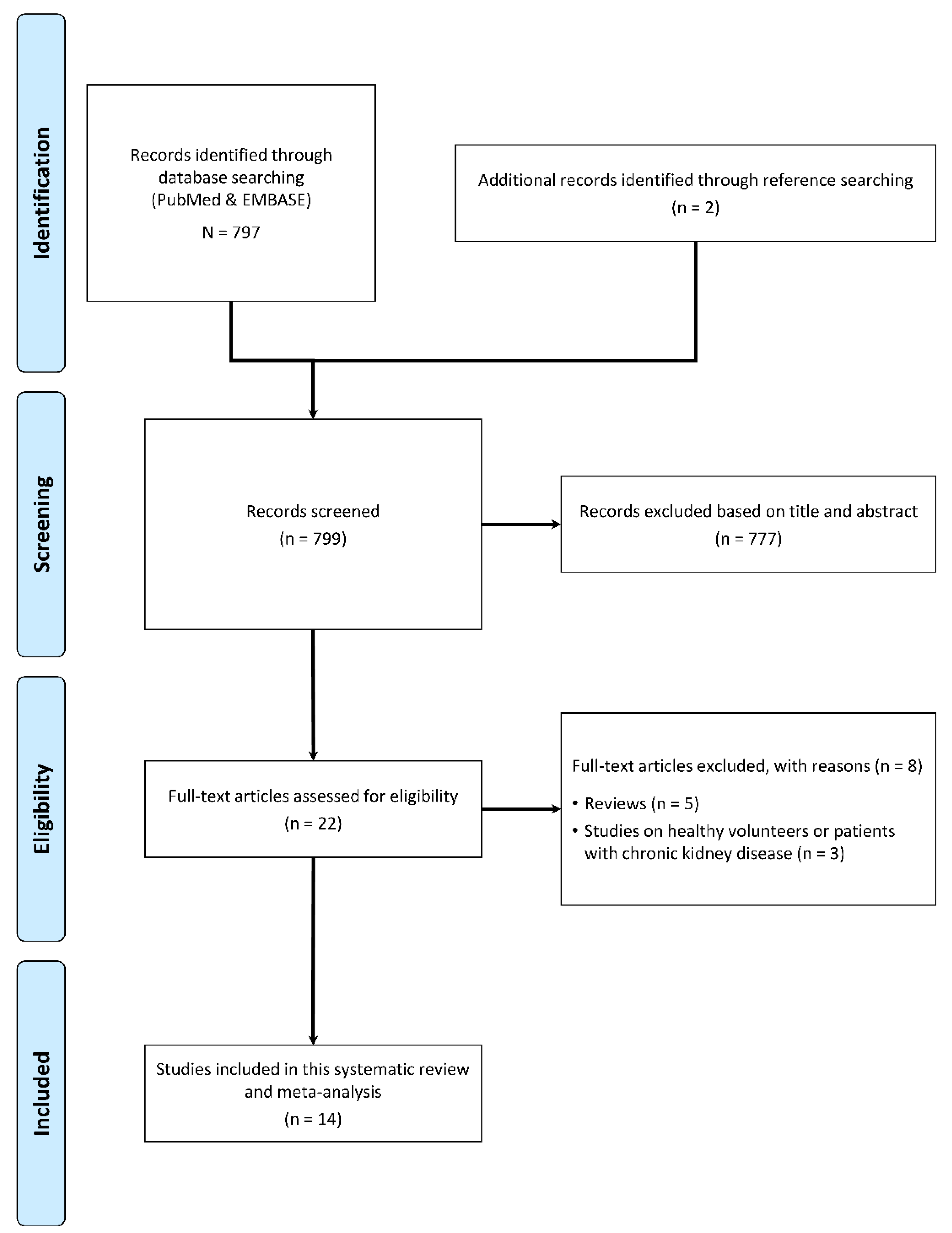
2.1. Article Search and Selection
2.2. Data Extraction and Quality Appraisal
2.3. Statistical Analysis
3. Results
3.1. Qualitative Synthesis
3.2. Quantitative Synthesis
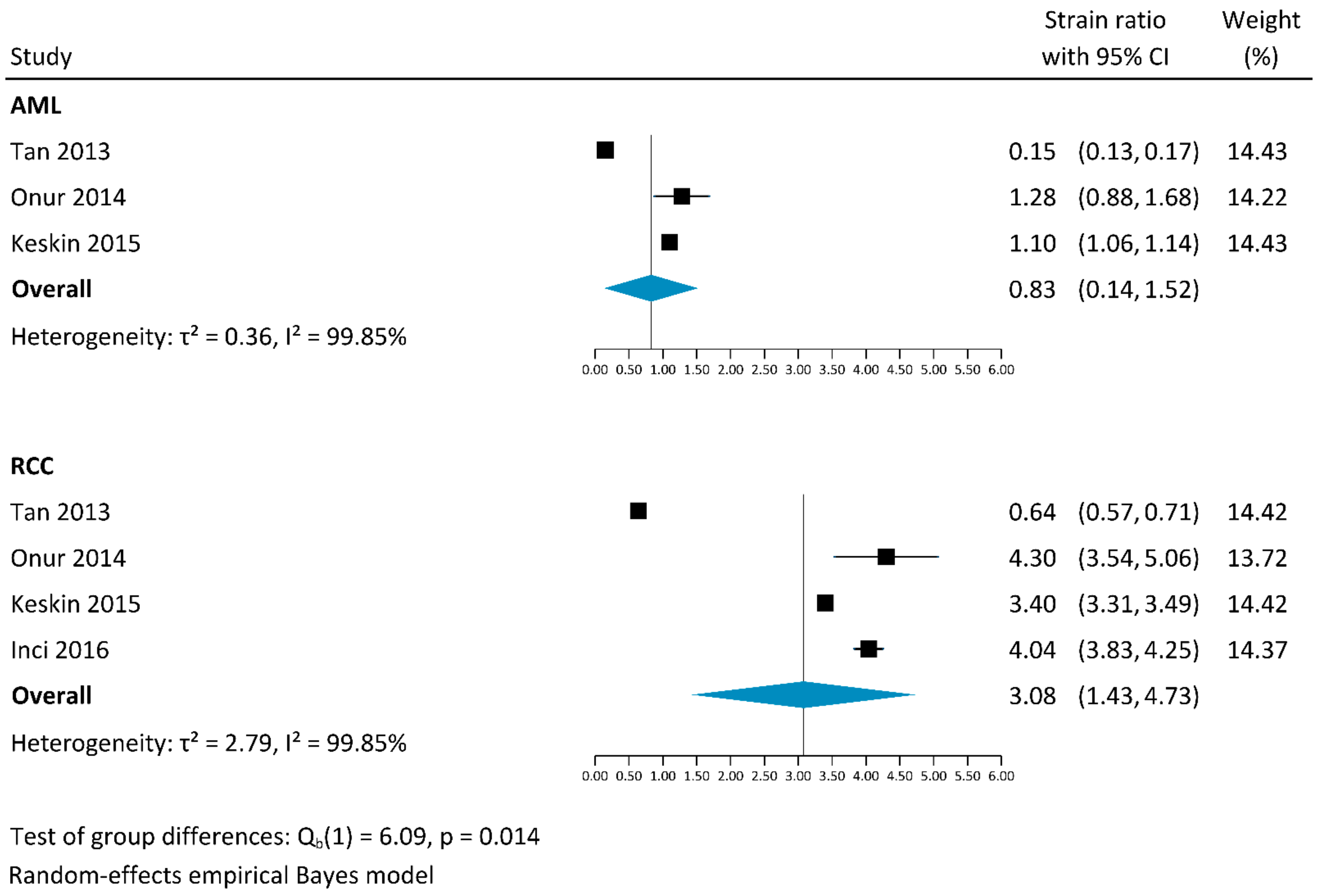
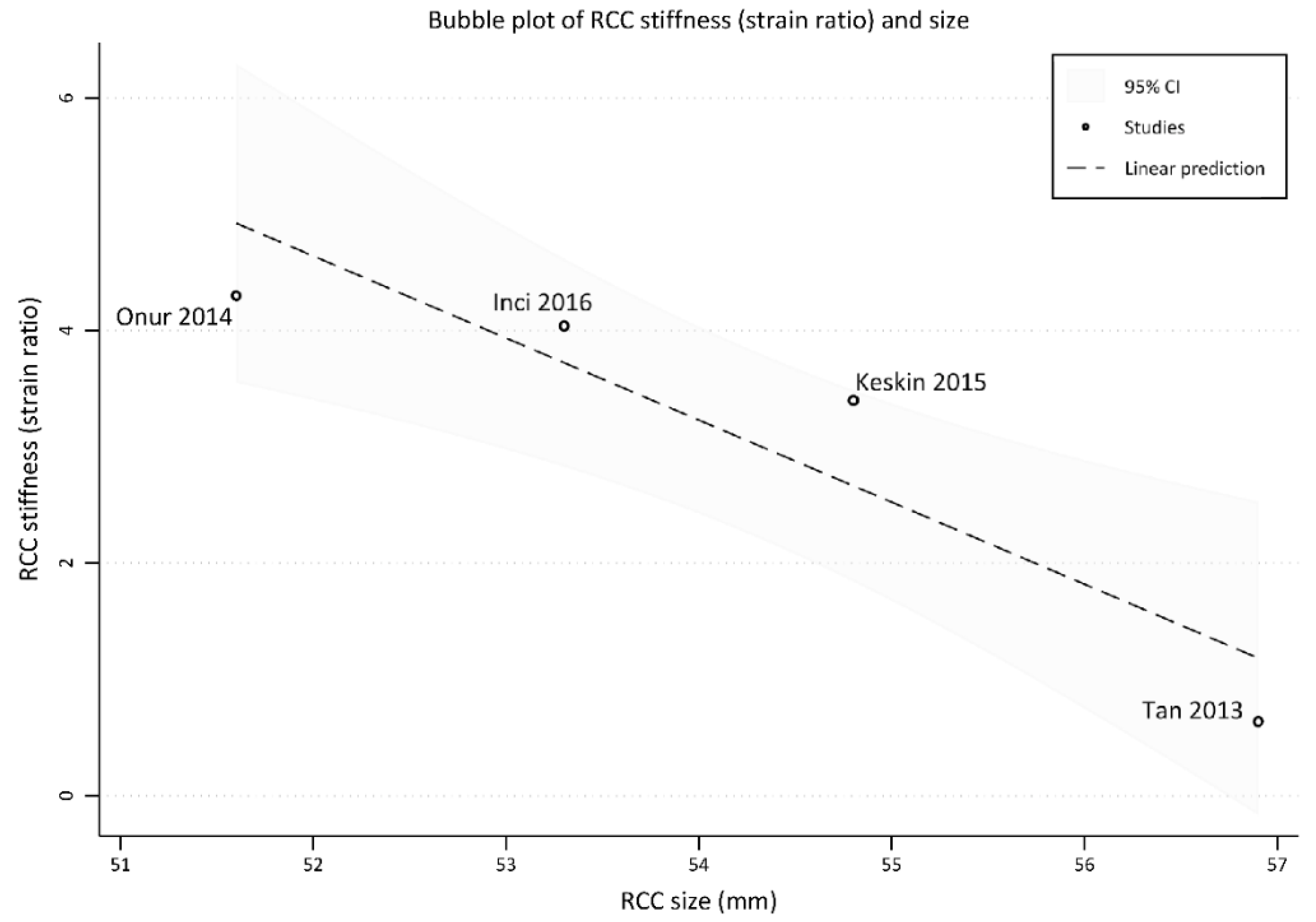
3.2.1. Strain Elastography
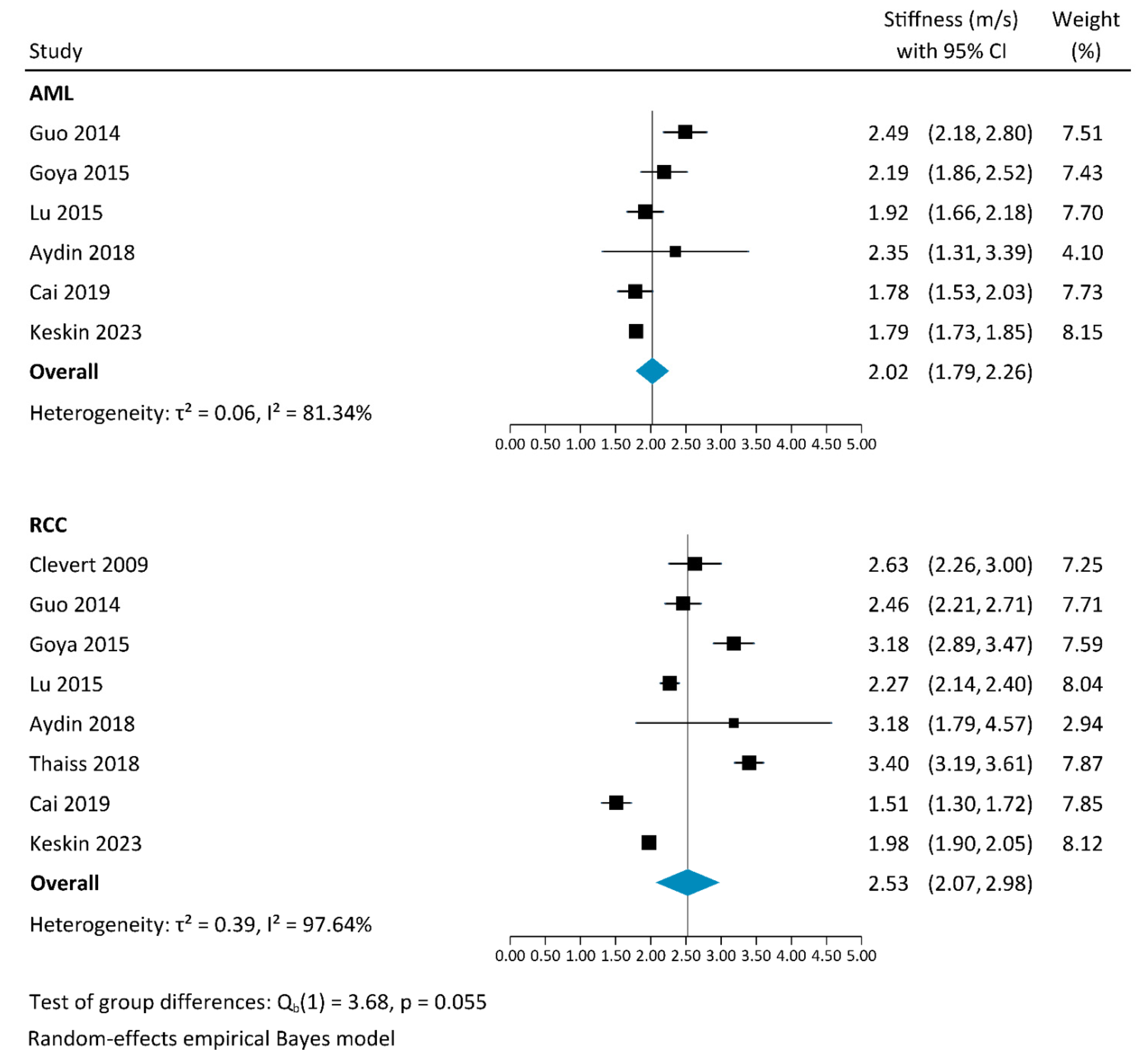
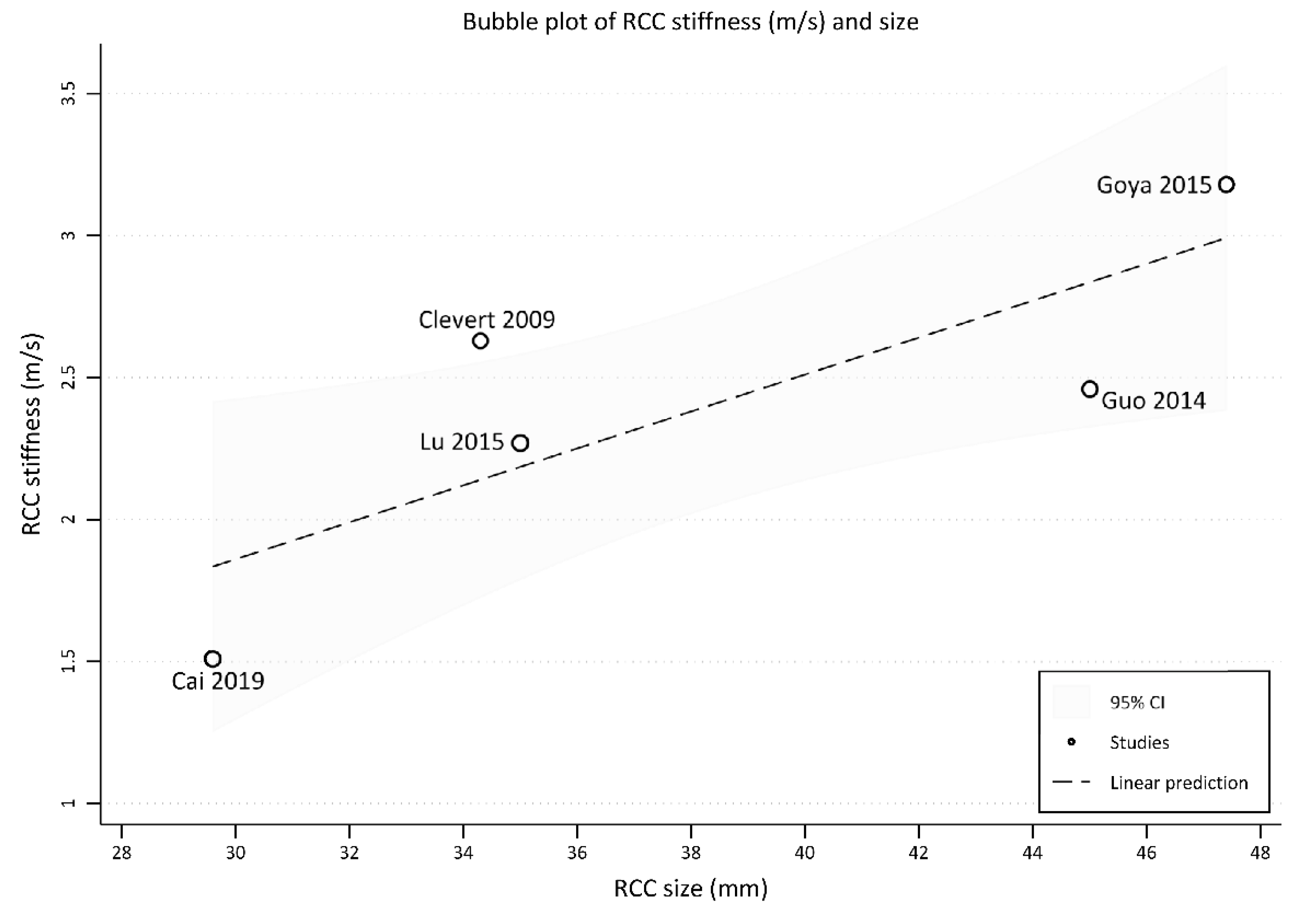
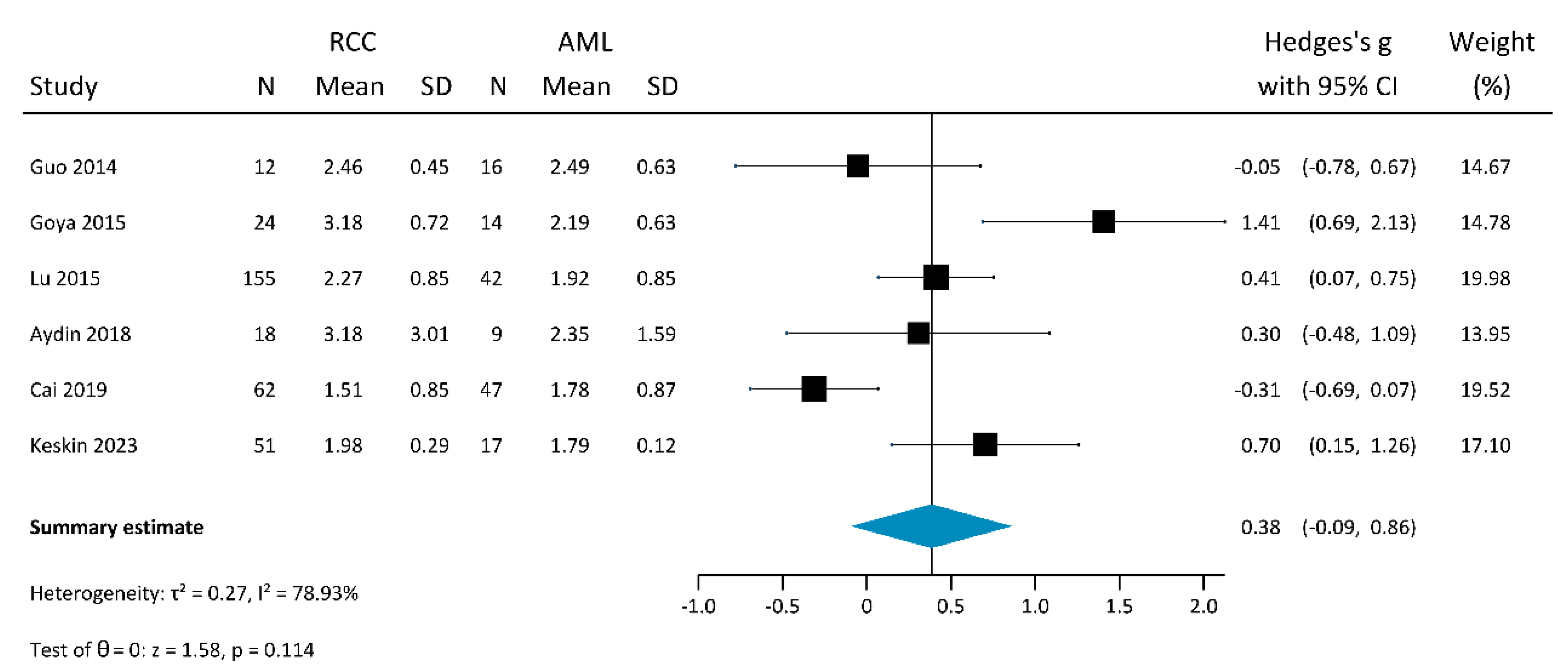
3.2.2. Shear-Wave Elastography
4. Discussion
4.1. Imaging of Renal Masses
4.2. Management of Small Renal Lesions
4.3. US Elastography
4.4. Results of This Systematic Review and Meta-Analysis
4.5. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qayyum, T.; Oades, G.; Horgan, P.; Aitchison, M.; Edwards, J. The Epidemiology and Risk Factors for Renal Cancer. Curr. Urol. 2013, 6, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Tufano, A.; Antonelli, L.; Di Pierro, G.B.; Flammia, R.S.; Minelli, R.; Anceschi, U.; Leonardo, C.; Franco, G.; Drudi, F.M.; Cantisani, V. Diagnostic Performance of Contrast-Enhanced Ultrasound in the Evaluation of Small Renal Masses: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2310. [Google Scholar] [CrossRef]
- Jinzaki, M.; Ohkuma, K.; Tanimoto, A.; Mukai, M.; Hiramatsu, K.; Murai, M.; Hata, J. Small solid renal lesions: Usefulness of power Doppler US. Radiology 1998, 209, 543–550. [Google Scholar] [CrossRef]
- Di Vece, F.; Tombesi, P.; Ermili, F.; Sartori, S. Management of incidental renal masses: Time to consider contrast-enhanced ultrasonography. Ultrasound 2016, 24, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Sahni, V.; Silverman, S. Imaging Management of Incidentally Detected Small Renal Masses. Semin. Intervent. Radiol. 2014, 31, 009–019. [Google Scholar] [CrossRef] [Green Version]
- Park, B.K. Renal Angiomyolipoma: Radiologic Classification and Imaging Features According to the Amount of Fat. Am. J. Roentgenol. 2017, 209, 826–835. [Google Scholar] [CrossRef]
- Doshi, A.M.; Ayoola, A.; Rosenkrantz, A.B. Do Incidental Hyperechoic Renal Lesions Measuring Up to 1 cm Warrant Further Imaging? Outcomes of 161 Lesions. Am. J. Roentgenol. 2017, 209, 346–350. [Google Scholar] [CrossRef]
- Flum, A.S.; Hamoui, N.; Said, M.A.; Yang, X.J.; Casalino, D.D.; McGuire, B.B.; Perry, K.T.; Nadler, R.B. Update on the Diagnosis and Management of Renal Angiomyolipoma. J. Urol. 2016, 195, 834–846. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [Green Version]
- Cè, M.; Felisaz, P.F.; Alì, M.; Re Sartò, G.V.; Cellina, M. Ultrasound elastography in chronic kidney disease: A systematic review and meta-analysis. J. Med. Ultrason. 2023. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 November 2018).
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [Green Version]
- Novianti, P.W.; Roes, K.C.B.; van der Tweel, I. Estimation of between-trial variance in sequential meta-analyses: A simulation study. Contemp. Clin. Trials 2014, 37, 129–138. [Google Scholar] [CrossRef]
- Thaiss, W.M.; Bedke, J.; Kruck, S.; Spira, D.; Stenzl, A.; Nikolaou, K.; Horger, M.; Kaufmann, S. Can contrast-enhanced ultrasound and acoustic radiation force impulse imaging characterize CT-indeterminate renal masses? A prospective evaluation with histological confirmation. World J. Urol. 2019, 37, 1339–1346. [Google Scholar] [CrossRef]
- Aydin, S.; Yildiz, S.; Turkmen, I.; Sharifov, R.; Uysal, O.; Gucin, Z.; Armagan, A.; Kocakoc, E. Value of Shear Wave Elastography for differentiating benign and malignant renal lesions. Med. Ultrason. 2018, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Li, F.; Li, Z.; Du, L.; Wu, R. Diagnostic Performance of Ultrasound Shear Wave Elastography in Solid Small (≤4 cm) Renal Parenchymal Masses. Ultrasound Med. Biol. 2019, 45, 2328–2337. [Google Scholar] [CrossRef]
- Clevert, D.-A.; Stock, K.; Klein, B.; Slotta-Huspenina, J.; Prantl, L.; Heemann, U.; Reiser, M. Evaluation of Acoustic Radiation Force Impulse (ARFI) imaging and contrast-enhanced ultrasound in renal tumors of unknown etiology in comparison to histological findings. Clin. Hemorheol. Microcirc. 2009, 43, 95–107. [Google Scholar] [CrossRef]
- Göya, C.; Daggulli, M.; Hamidi, C.; Yavuz, A.; Hattapoglu, S.; Cetincakmak, M.G.; Teke, M. The role of quantitative measurement by acoustic radiation force impulse imaging in differentiating benign renal lesions from malignant renal tumours. Radiol. Med. 2015, 120, 296–303. [Google Scholar] [CrossRef]
- Guo, L.-H.; Liu, B.-J.; Xu, H.-X.; Liu, C.; Sun, L.-P.; Zhang, Y.-F.; Xu, J.-M.; Wu, J.; Xu, X.-H. Acoustic radiation force impulse elastography in differentiating renal solid masses: A preliminary experience. Int. J. Clin. Exp. Pathol. 2014, 7, 7469–7476. [Google Scholar]
- Inci, M.F.; Kalayci, T.O.; Tan, S.; Karasu, S.; Albayrak, E.; Cakir, V.; Ocal, I.; Ozkan, F. Diagnostic value of strain elastography for differentiation between renal cell carcinoma and transitional cell carcinoma of kidney. Abdom. Radiol. 2016, 41, 1152–1159. [Google Scholar] [CrossRef]
- Keskin, S.; Güven, S.; Keskin, Z.; Özbiner, H.; Kerimoğlu, Ü.; Yeşildağ, A. Strain elastography in the characterization of renal cell carcinoma and angiomyolipoma. Can. Urol. Assoc. J. 2015, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Keskin, Z.; Keskin, S. Shear wave elastography in the characterization of renal cell carcinoma and angiomyolipoma. Acta radiol. 2023, 64, 1272–1279. [Google Scholar] [CrossRef]
- Lu, Q.; Wen, J.-X.; Huang, B.-J.; Xue, L.-Y.; Wang, W.-P. Virtual Touch quantification using acoustic radiation force impulse (ARFI) technology for the evaluation of focal solid renal lesions: Preliminary findings. Clin. Radiol. 2015, 70, 1376–1381. [Google Scholar] [CrossRef]
- Onur, M.R.; Poyraz, A.K.; Bozgeyik, Z.; Onur, A.R.; Orhan, I. Utility of Semiquantitative Strain Elastography for Differentiation Between Benign and Malignant Solid Renal Masses. J. Ultrasound Med. 2015, 34, 639–647. [Google Scholar] [CrossRef]
- Sun, D.; Lu, Q.; Wei, C.; Li, Y.; Zheng, Y.; Hu, B. Differential diagnosis of <3 cm renal tumors by ultrasonography: A rapid, quantitative, elastography self-corrected contrast-enhanced ultrasound imaging mode beyond screening. Br. J. Radiol. 2020, 93, 20190974. [Google Scholar] [CrossRef]
- Tan, S.; Özcan, M.F.; Tezcan, F.; Balcı, S.; Karaoğlanoğlu, M.; Huddam, B.; Arslan, H. Real-Time Elastography for Distinguishing Angiomyolipoma from Renal Cell Carcinoma: Preliminary Observations. Am. J. Roentgenol. 2013, 200, W369–W375. [Google Scholar] [CrossRef]
- Sagreiya, H.; Akhbardeh, A.; Li, D.; Sigrist, R.; Chung, B.I.; Sonn, G.A.; Tian, L.; Rubin, D.L.; Willmann, J.K. Point Shear Wave Elastography Using Machine Learning to Differentiate Renal Cell Carcinoma and Angiomyolipoma. Ultrasound Med. Biol. 2019, 45, 1944–1954. [Google Scholar] [CrossRef]
- Nicolau, C.; Antunes, N.; Paño, B.; Sebastia, C. Imaging Characterization of Renal Masses. Medicina 2021, 57, 51. [Google Scholar] [CrossRef]
- Kang, S.K.; Huang, W.C.; Pandharipande, P.V.; Chandarana, H. Solid Renal Masses: What the Numbers Tell Us. Am. J. Roentgenol. 2014, 202, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Israel, G.M.; Hindman, N.; Bosniak, M.A. Evaluation of Cystic Renal Masses: Comparison of CT and MR Imaging by Using the Bosniak Classification System. Radiology 2004, 231, 365–371. [Google Scholar] [CrossRef]
- Tappouni, R.; Kissane, J.; Sarwani, N.; Lehman, E.B. Pseudoenhancement of Renal Cysts: Influence of Lesion Size, Lesion Location, Slice Thickness, and Number of MDCT Detectors. Am. J. Roentgenol. 2012, 198, 133–137. [Google Scholar] [CrossRef]
- Ishigami, K.; Jones, A.R.; Dahmoush, L.; Leite, L.V.; Pakalniskis, M.G.; Barloon, T.J. Imaging spectrum of renal oncocytomas: A pictorial review with pathologic correlation. Insights Imaging 2015, 6, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Jinzaki, M.; Silverman, S.G.; Akita, H.; Nagashima, Y.; Mikami, S.; Oya, M. Renal angiomyolipoma: A radiological classification and update on recent developments in diagnosis and management. Abdom. Imaging 2014, 39, 588–604. [Google Scholar] [CrossRef] [Green Version]
- Seyam, R.M.; Alkhudair, W.K.; Kattan, S.A.; Alotaibi, M.F.; Alzahrani, H.M.; Altaweel, W.M. The Risks of Renal Angiomyolipoma: Reviewing the Evidence. J. Kidney Cancer VHL 2017, 4, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.P.; Middleton, W.D.; Melson, G.L.; McClennan, B.L. Hyperechoic renal cell carcinomas: Increase in detection at US. Radiology 1993, 188, 431–434. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for Management of the Clinical T1 Renal Mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Ter Riet, G.; Bachmann, L.M.; Kessels, A.G.H.; Khan, K.S. Individual patient data meta-analysis of diagnostic studies: Opportunities and challenges. Evid. Based Med. 2013, 18, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Khenkina, N.; Toto-Brocchi, M.; Martinenghi, C.; Papa, S.; Carrafiello, G. Artificial Intelligence in Lung Cancer Imaging: Unfolding the Future. Diagnostics 2022, 12, 2644. [Google Scholar] [CrossRef]

| Author | Year | Geographic Origin | Study Type | Elastography Technique | Stiffness Measurement | Manufacturer | Model | Probe and Frequency | Measurements per Lesion | Reference Standard | Histopathology Modality | Imaging Modality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clevert et al. | 2009 | Europe | OP | SWE | m/s | Siemens | Acuson S2000 | Convex probe (4C1), 1–4 MHz | - | Histopathology + Imaging | - | US/CT/MR |
| Tan et al. | 2013 | Europe | OP | SE | Strain ratio | GE | LogiQ E9 | Convex probe, 2.8–5 MHz | - | Histopathology + Imaging | Post-operative pathology/biopsy | CT/MR |
| Guo et al. | 2014 | Asia | OP | SWE | m/s | Siemens | Acuson S2000 | Convex probe (4C1), 1–4 MHz | 7 | Histopathology + Imaging | Post-operative pathology/biopsy | CT/MR |
| Onur et al. | 2014 | Europe | OP | SE | Strain ratio | Toshiba | Aplio XG | Convex probe, 3.5 MHz | - | Histopathology + Imaging | Post-operative pathology/biopsy | CT/MR |
| Goya et al. | 2015 | Europe | OP | SWE | m/s | Siemens | Acuson S2000 | Convex probe (4C1), 1–4 MHz | 16 | Histopathology + Imaging | Post-operative pathology | US/CT/MR |
| Keskin et al. | 2015 | Europe | OP | SE | Strain ratio | Toshiba | Aplio XG | Convex probe (PVT-375BT), 2.5–5 MHz | - | Histopathology + Imaging | Post-operative pathology | CT/MR |
| Lu et al. | 2015 | Asia | OP | SWE | m/s | Siemens | Acuson S2000 | Convex probe (4C1), 1–4 MHz | 10 | Histopathology + Imaging | Post-operative pathology | CT/MR |
| Inci et al. | 2016 | Europe | OP | SE | Strain ratio | Toshiba | Aplio 500 | Convex probe, 3.5–5 MHz | - | Histopathology | Post-operative pathology/biopsy | - |
| Aydin et al. | 2018 | Europe | OP | SWE | kPa | Philips | iU22 | Convex probe (C5-1), 1–5 MHz | 3 | Histopathology + Imaging | Post-operative pathology/biopsy | - |
| Thaiss et al. | 2018 | Europe | OP | SWE | m/s | Siemens | Acuson S 3000 HELX | Convex probe (6C1 HD), 1.5–6 MHz | - | Histopathology + Imaging | Post-operative pathology | - |
| Cai et al. | 2019 | Asia | OP | SWE | kPa | Aixplorer | Aixplorer | Convex probe (SC6-1), 1–6 MHz | - | Histopathology + Imaging | Post-operative pathology | CT/MR |
| Sagreiya et al. | 2019 | America | OP | SWE | - | Siemens | Acuson S2000 | Convex probe (6C1 HD), 1.5–6 MHz | 10 | Histopathology + Imaging | Post-operative pathology | CT/MR |
| Sun et al. | 2020 | Asia | OR | SWE | m/s | Siemens | Acuson S2000 | Convex probe (4C1), 1–4 MHz | 5 | - | - | - |
| Keskin et al. | 2023 | Europe | OP | SWE | m/s | Philips | iU22 | Convex probe (C5-1), 2.5 MHz | 2 | Histopathology + Imaging | Post-operative pathology | US/CT/MR |
| Author | Year | Elastography Technique | Total Patients | Mean Age ± Standard Deviation | Patients Excluded for Technical Reasons | Effectively Sampled Lesions | Benign Lesions | Malignant Lesions | Biopsies | Surgery Samples | Imaging | RCC | TCC | MTX | OCY | LYM | AML | SRC | ABS | HE | WIL | PST | HC | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clevert et al. | 2009 | SWE | 15 | 54 | 15 | 4 | 11 | 11 | 2 | 2 | ||||||||||||||
| Tan et al. | 2013 | SE | 52 | 54 ± 12 | 5 | 47 | 28 | 19 | 2 | 19 | 26 | 19 | 28 | |||||||||||
| Guo et al. | 2014 | SWE | 88 | 50 ± 38 | 46 | 42 | 30 | 12 | 19 | 23 | 12 | 1 | 16 | 13 | ||||||||||
| Onur et al. | 2014 | SE | 85 | 58.00 | 14 | 71 | 29 | 42 | 8 | 41 | 22 | 34 | 4 | 3 | 5 | 1 | 24 | |||||||
| Goya et al. | 2015 | SWE | 71 | 50 ± 20 | 11 | 60 | 24 | 36 | 33 | 21 | 24 | 5 | 7 | 14 | 3 | 7 | ||||||||
| Keskin et al. | 2015 | SE | 65 | 56 ± 12 | 65 | 24 | 41 | 41 | 24 | 41 | 24 | |||||||||||||
| Lu et al. | 2015 | SWE | 209 | 12 | 197 | 42 | 155 | 168 | 29 | 155 | 42 | |||||||||||||
| Inci et al. | 2016 | SE | 99 | 61 ± 8 | 28 | 71 | 4 | 67 | 11 | 60 | 44 | 18 | 3 | 3 | 1 | 1 | 1 | |||||||
| Aydin et al. | 2018 | SWE | 40 | 50 ± 16 | 40 | 15 | 25 | 3 | 28 | 18 | 2 | 2 | 1 | 9 | 2 | 1 | 1 | 1 | 3 | |||||
| Thaiss et al. | 2018 | SWE | 123 | 64 | 46 | 77 | 19 | 58 | 77 | 58 | 10 | 1 | 8 | |||||||||||
| Cai et al. | 2019 | SWE | 176 | 57 ± 11 | 59 | 117 | 49 | 68 | 117 | 87 | 30 | 68 | 2 | 47 | ||||||||||
| Sagreiya et al. | 2019 | SWE | 58 | 57 ± 13 | 7 | 52 | 10 | 42 | 44 | 8 | 42 | 10 | ||||||||||||
| Sun et al. | 2020 | SWE | 35 | 47 | 37 | 22 | 15 | 13 | 11 | 13 | ||||||||||||||
| Keskin et al. | 2023 | SWE | 74 | 58 ± 12 | 6 | 68 | 17 | 51 | 51 | 17 | 51 | 17 |
| RCC | TCC | MTX | OCY | LYM | AML | SRC | ABS | HE | WIL | PST | HC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) | N | Size (mm) |
| Clevert et al. | 2009 | 11 | 34 | 2 | 2 | 2 | 38 | ||||||||||||||||||
| Tan et al. | 2013 | 19 | 57 | 28 | 22 | ||||||||||||||||||||
| Guo et al. | 2014 | 12 | 45 | 1 | 29 | 16 | 24 | 13 | 24 | ||||||||||||||||
| Onut et al. | 2014 | 34 | 52 | 4 | 3 | 5 | 1 | 24 | 26 | ||||||||||||||||
| Goya et al. | 2015 | 24 | 47 | 5 | 32 | 7 | 29 | 14 | 25 | 3 | 30 | ||||||||||||||
| Keskin et al. | 2015 | 41 | 55 | 24 | 30 | ||||||||||||||||||||
| Lu et al. | 2015 | 155 | 35 | 42 | 41 | ||||||||||||||||||||
| Inci et al. | 2016 | 44 | 53 | 18 | 40 | 3 | 31 | 3 | 64 | 1 | 49 | 1 | 54 | 1 | 68 | ||||||||||
| Aydin et al. | 2018 | 18 | 2 | 2 | 1 | 9 | 2 | 1 | 1 | 1 | |||||||||||||||
| Thaiss et al. | 2018 | 58 | 10 | 1 | |||||||||||||||||||||
| Cai et al. | 2019 | 68 | 30 | 2 | 23 | 47 | 23 | ||||||||||||||||||
| Sagreiya et al. | 2019 | 42 | 35 | 10 | 22 | ||||||||||||||||||||
| Sun et al. | 2020 | 13 | 11 | ||||||||||||||||||||||
| Keskin et al. | 2023 | 51 | Range 23–180 | 17 | Range 15–98 | ||||||||||||||||||||
| US Elastography | Stiffness Values (Mean ± Standard Deviation) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Elastography Technique | Stiffness Measurement | RCC | TCC | MTX | OCY | LYM | AML | SRC | ABS | HE | WIL | PST | HC |
| Clevert et al. | 2009 | SWE | m/s | 2.63 ± 0.63 | 2.90 ± 0.27 | 3.05 ± 0.35 | |||||||||
| Tan et al. | 2013 | SE | Strain ratio | 0.64 ± 0.15 | 0.15 ± 0.06 | ||||||||||
| Guo et al. | 2014 | SWE | m/s | 2.46 ± 0.45 | 1.60 | 2.49 ± 0.63 | 3.24 ± 0.75 | ||||||||
| Onur et al. | 2014 | SE | Strain ratio | 4.30 ± 2.27 | 2.43 ± 1.03 | 2.54 ± 1.53 | 1.79 ± 0.26 | 4.73 | 1.28 ± 1.01 | ||||||
| Goya et al. | 2015 | SWE | m/s | 3.18 ± 0.72 | 2.33 ± 0.29 | 2.90 ± 1.11 | 2.19 ± 0.63 | 1.20 ± 0.14 | |||||||
| Keskin et al. | 2015 | SE | Strain ratio | 3.40 ± 0.30 | 1.10 ± 0.10 | ||||||||||
| Lu et al. | 2015 | SWE | m/s | 2.27 ± 0.85 | 1.92 ± 0.85 | ||||||||||
| Inci et al. | 2016 | SE | Strain ratio | 4.04 ± 0.72 | 5.18 ± 1.12 | 3.04 ± 1.09 | 1.98 ± 0.43 | 3.32 | 1.42 | 4.13 | |||||
| Aydin et al. | 2018 | SWE | kPa § | 31.80 ± 28.64 | 19.41 ± 10.04 | 8.99 ± 0.72 | 8.05 | 17.46 ± 7.95 | 22.99 ± 7.97 | 32.60 | 3.24 | 5.12 | |||
| Thaiss et al. | 2018 | SWE | m/s | 3.40 ± 0.80 | 2.80 ± 0.40 | ||||||||||
| Cai et al. | 2019 | SWE | kPa § | 7.20 ± 2.50 | 10 ± 2.40 | 10.00 ± 2.40 | |||||||||
| Sagreiya et al. | 2019 | SWE | - | ||||||||||||
| Sun et al. | 2020 | SWE | m/s | ||||||||||||
| Keskin et al. | 2023 | SWE | m/s | 1.98 ± 0.29 | 1.79 ± 0.12 | ||||||||||
| Patient Selection | Comparability | Reference Standard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author/Year | Is the Malignant Case Definition Adequate? | Representativeness of the Malignant Cases | Selection of Benign Cases | Definition of Benign Cases | Comparability of Benign and Malignant Cases on the basis of the Design or Analysis | Reference Standard for Malignancy | Congruence of Reference Standard for Benign and Malignant Cases | Follow-Up Type | Total Score |
| Clevert 2009 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Tan 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Guo 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Onur 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Goya 2015 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 6 |
| Keskin 2015 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 4 |
| Lu 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Inci 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Aydin 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Thaiss 2018 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Cai 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Sagreiya 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Sun 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Keskin 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cè, M.; Cozzi, A.; Cellina, M.; Schifano, E.; Gibelli, D.; Oliva, G.; Papa, S.; Dughetti, L.; Irmici, G.; Carrafiello, G. Ultrasound Elastography for the Differentiation of Benign and Malignant Solid Renal Masses: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 7767. https://doi.org/10.3390/app13137767
Cè M, Cozzi A, Cellina M, Schifano E, Gibelli D, Oliva G, Papa S, Dughetti L, Irmici G, Carrafiello G. Ultrasound Elastography for the Differentiation of Benign and Malignant Solid Renal Masses: A Systematic Review and Meta-Analysis. Applied Sciences. 2023; 13(13):7767. https://doi.org/10.3390/app13137767
Chicago/Turabian StyleCè, Maurizio, Andrea Cozzi, Michaela Cellina, Eliana Schifano, Daniele Gibelli, Giancarlo Oliva, Sergio Papa, Luca Dughetti, Giovanni Irmici, and Gianpaolo Carrafiello. 2023. "Ultrasound Elastography for the Differentiation of Benign and Malignant Solid Renal Masses: A Systematic Review and Meta-Analysis" Applied Sciences 13, no. 13: 7767. https://doi.org/10.3390/app13137767
APA StyleCè, M., Cozzi, A., Cellina, M., Schifano, E., Gibelli, D., Oliva, G., Papa, S., Dughetti, L., Irmici, G., & Carrafiello, G. (2023). Ultrasound Elastography for the Differentiation of Benign and Malignant Solid Renal Masses: A Systematic Review and Meta-Analysis. Applied Sciences, 13(13), 7767. https://doi.org/10.3390/app13137767








