Dual-Energy CT Applications in Urological Diseases
Featured Application
Abstract
1. Introduction
2. DECT Principles
3. DECT Benefits
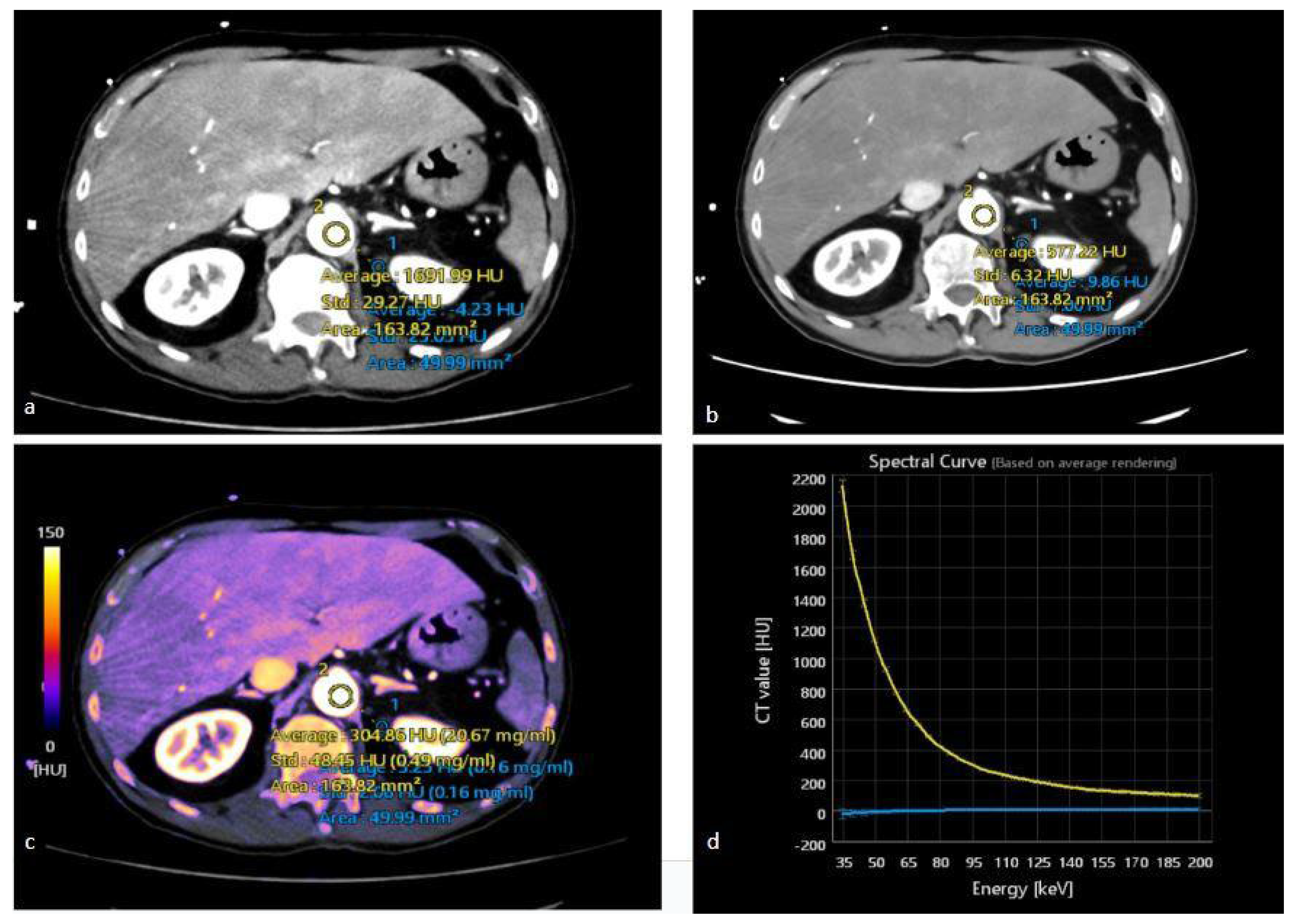
3.1. Virtual Monochromatic Images
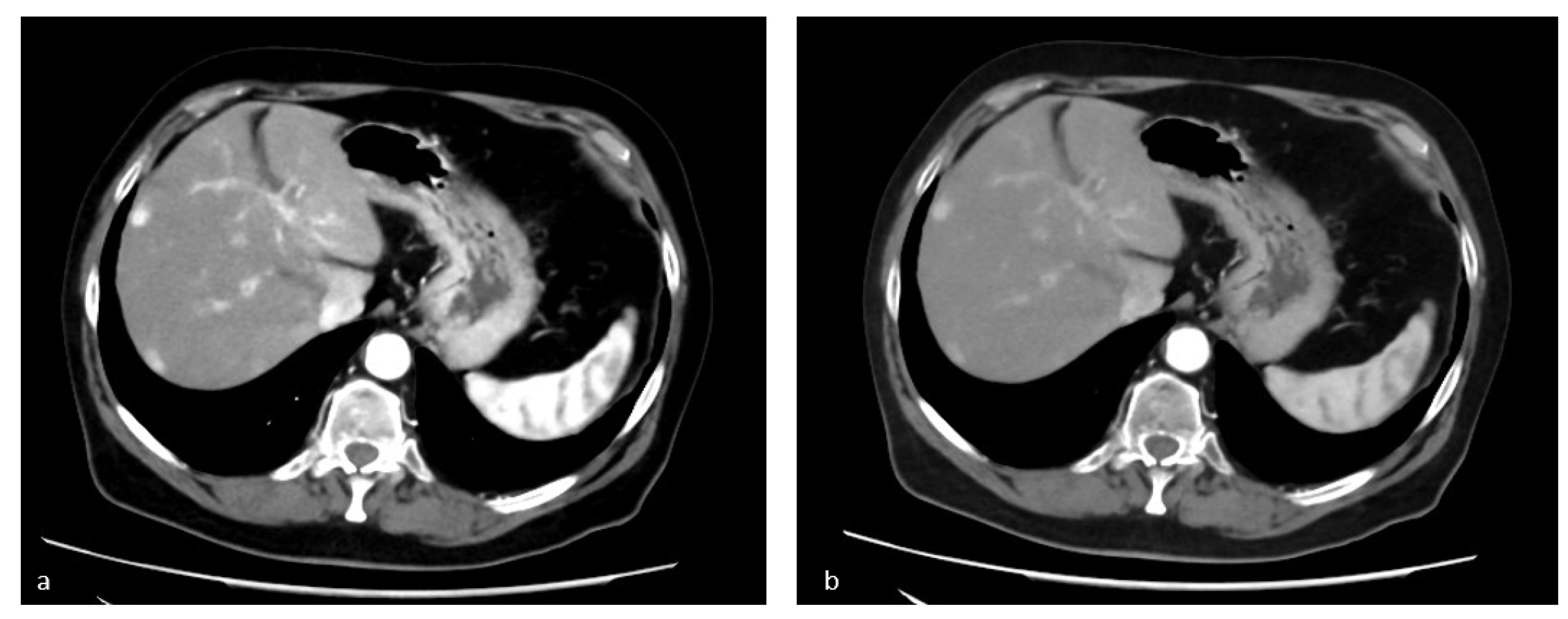
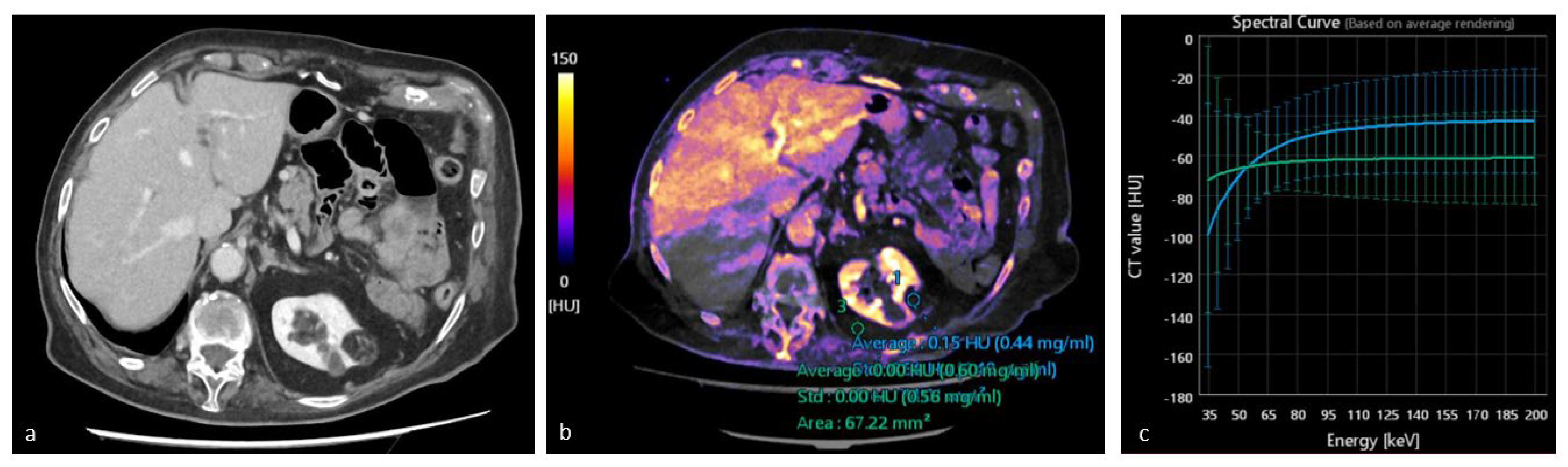
3.2. Virtual Non-Contrast Enhanced Image
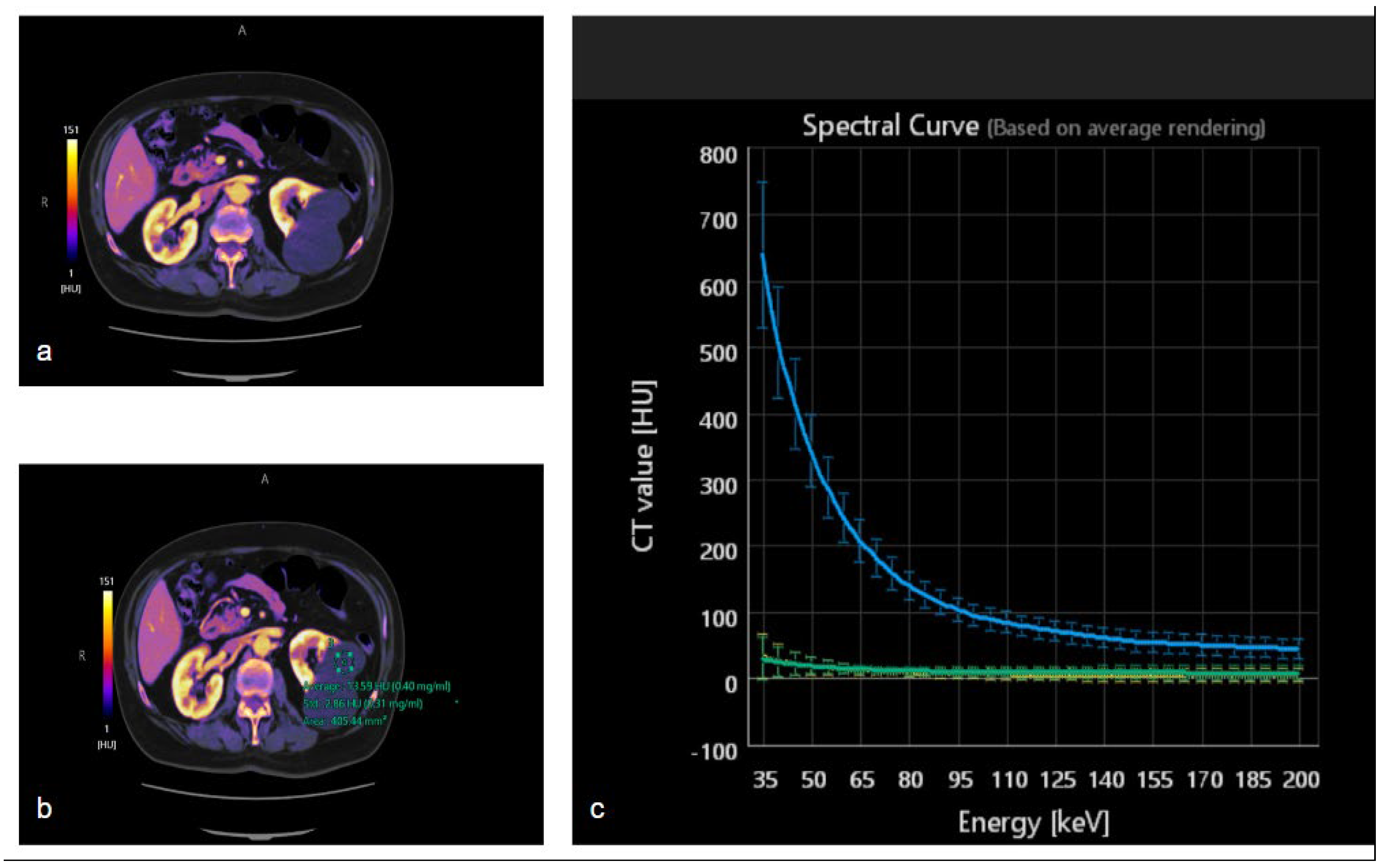
3.3. Iodine Maps
3.4. Spectral HU Curves
4. DECT Clinical Applications
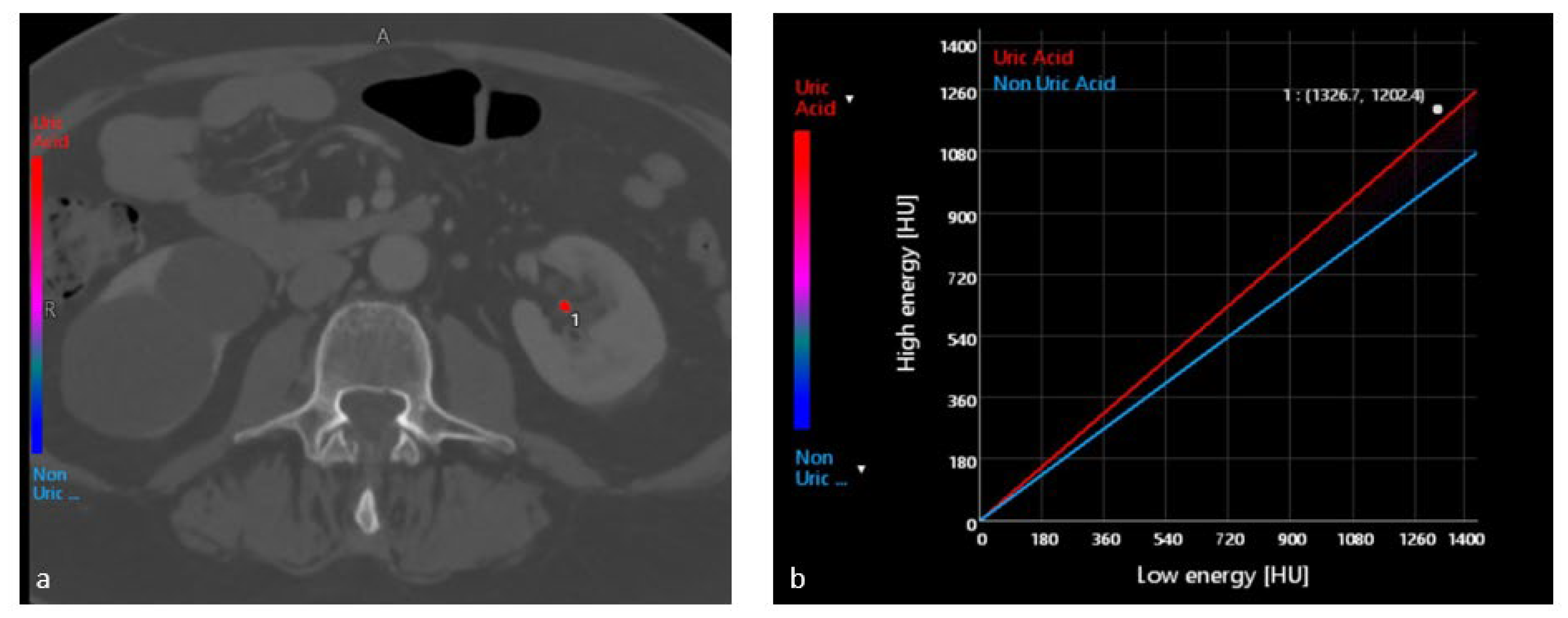
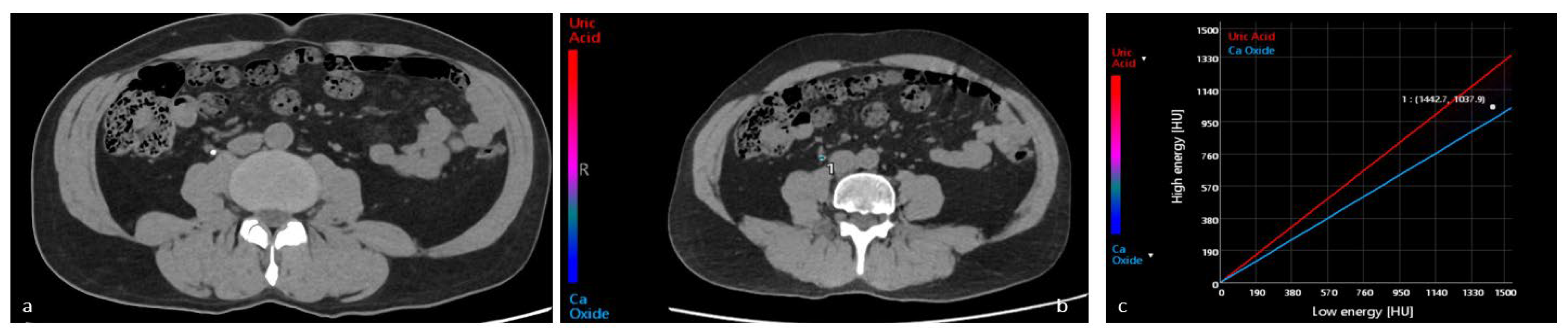
4.1. Characterization of Urinary Stones
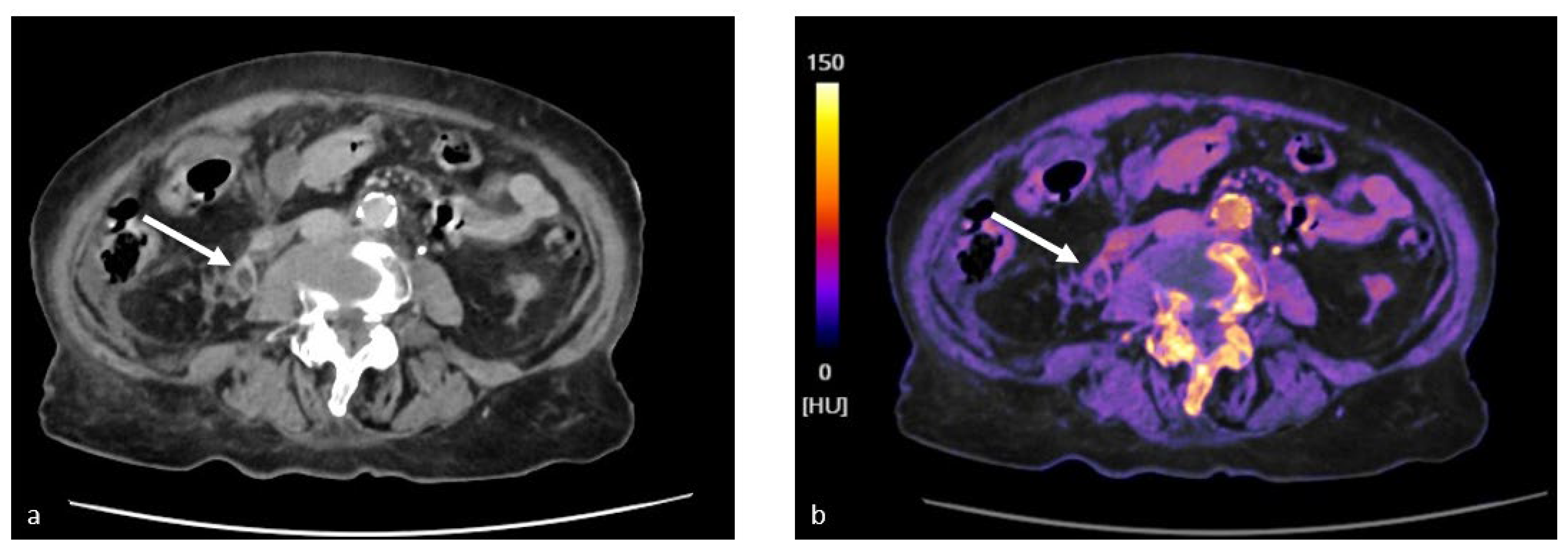
4.2. Study of Renal Lesions
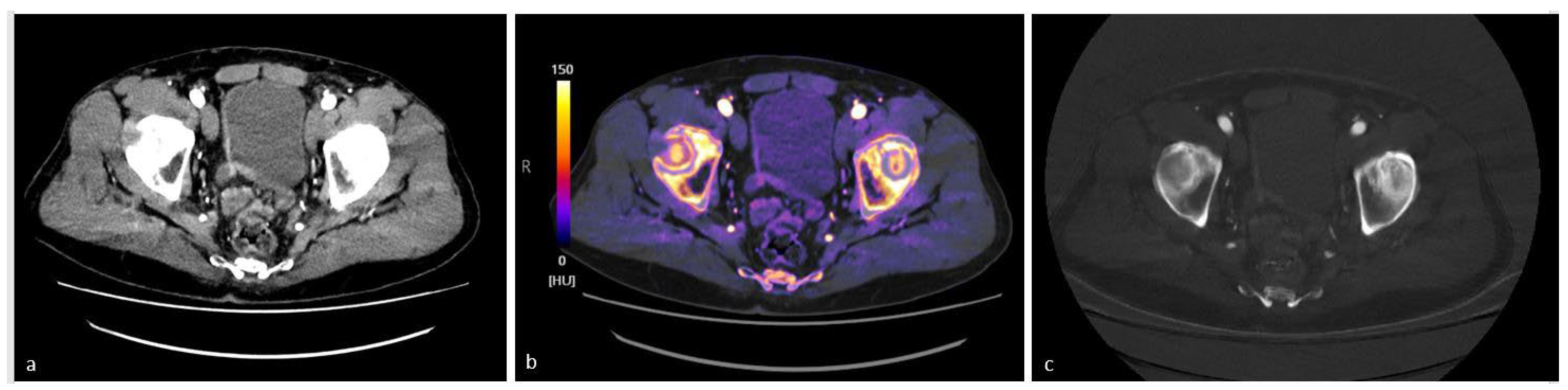
4.3. Study of the Bladder and Ureters
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ascenti, G.; Cicero, G.; Bertelli, E.; Papa, M.; Gentili, F.; Ciccone, V.; Manetta, R.; Gandolfo, N.; Cardone, G.; Miele, V. CT-Urography: A Nationwide Survey by the Italian Board of Urogenital Radiology. Radiol. Med. 2022, 127, 577–588. [Google Scholar] [CrossRef]
- Joffe, S.A.; Servaes, S.; Okon, S.; Horowitz, M. Multi-Detector Row CT Urography in the Evaluation of Hematuria. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2003, 23, 1441–1455, Discussion in 1455–1456. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-Energy CT Quantification of Fractional Extracellular Space in Cirrhotic Patients: Comparison between Early and Delayed Equilibrium Phases and Correlation with Oesophageal Varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Kaza, R.K.; Platt, J.F.; Megibow, A.J. Dual-Energy CT of the Urinary Tract. Abdom. Imaging 2013, 38, 167–179. [Google Scholar] [CrossRef]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-Energy CT: Minimal Essentials for Radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual Monochromatic Spectral Imaging with Fast Kilovoltage Switching: Improved Image Quality as Compared with That Obtained with Conventional 120-KVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, J.; Peng, Y. Dual-Energy Spectral CT Imaging of Pulmonary Embolism with Mycoplasma Pneumoniae Pneumonia in Children. Radiol. Med. 2022, 127, 154–161. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Mileto, A.; Sofue, K.; Marin, D. Imaging the Renal Lesion with Dual-Energy Multidetector CT and Multi-Energy Applications in Clinical Practice: What Can It Truly Do for You? Eur. Radiol. 2016, 26, 3677–3690. [Google Scholar] [CrossRef]
- Ergun, D.L.; Mistretta, C.A.; Brown, D.E.; Bystrianyk, R.T.; Sze, W.K.; Kelcz, F.; Naidich, D.P. Single-Exposure Dual-Energy Computed Radiography: Improved Detection and Processing. Radiology 1990, 174, 243–249. [Google Scholar] [CrossRef]
- Fernández-Pérez, G.C.; Fraga Piñeiro, C.; Oñate Miranda, M.; Díez Blanco, M.; Mato Chaín, J.; Collazos Martínez, M.A. Dual-Energy CT: Technical Considerations and Clinical Applications. Radiol. Engl. Ed. 2022, 64, 445–455. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Rossini, N.; Cacioppa, L.M.; Ascenti, V.; Carrafiello, G.; Floridi, C. Computed Tomography Urography: State of the Art and Beyond. Tomography 2023, 9, 909–930. [Google Scholar] [CrossRef]
- Pomerantz, S.R.; Kamalian, S.; Zhang, D.; Gupta, R.; Rapalino, O.; Sahani, D.V.; Lev, M.H. Virtual Monochromatic Reconstruction of Dual-Energy Unenhanced Head CT at 65–75 KeV Maximizes Image Quality Compared with Conventional Polychromatic CT. Radiology 2013, 266, 318–325. [Google Scholar] [CrossRef]
- Yuan, R.; Shuman, W.P.; Earls, J.P.; Hague, C.J.; Mumtaz, H.A.; Scott-Moncrieff, A.; Ellis, J.D.; Mayo, J.R.; Leipsic, J.A. Reduced Iodine Load at CT Pulmonary Angiography with Dual-Energy Monochromatic Imaging: Comparison with Standard CT Pulmonary Angiography—A Prospective Randomized Trial. Radiology 2012, 262, 290–297. [Google Scholar] [CrossRef]
- Agrawal, M.D.; Pinho, D.F.; Kulkarni, N.M.; Hahn, P.F.; Guimaraes, A.R.; Sahani, D.V. Oncologic Applications of Dual-Energy CT in the Abdomen. RadioGraphics 2014, 34, 589–612. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Darnell, A.; Rengo, M.; Muscogiuri, G.; Bellini, D.; Ayuso, C.; Laghi, A. Dual-Energy CT: Oncologic Applications. AJR Am. J. Roentgenol. 2012, 199 (Suppl. S5), S98–S105. [Google Scholar] [CrossRef]
- Silva, A.C.; Morse, B.G.; Hara, A.K.; Paden, R.G.; Hongo, N.; Pavlicek, W. Dual-Energy (Spectral) CT: Applications in Abdominal Imaging. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2011, 31, 1031–1046, Discussion in 1047–1050. [Google Scholar] [CrossRef]
- Ferda, J.; Novák, M.; Mírka, H.; Baxa, J.; Ferdová, E.; Bednárová, A.; Flohr, T.; Schmidt, B.; Klotz, E.; Kreuzberg, B. The Assessment of Intracranial Bleeding with Virtual Unenhanced Imaging by Means of Dual-Energy CT Angiography. Eur. Radiol. 2009, 19, 2518–2522. [Google Scholar] [CrossRef]
- Hartman, R.; Kawashima, A.; Takahashi, N.; Silva, A.; Vrtiska, T.; Leng, S.; Fletcher, J.; McCollough, C. Applications of Dual-Energy CT in Urologic Imaging: An Update. Radiol. Clin. 2012, 50, 191–205. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT Techniques for Assessing Hepatocellular Carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef]
- Hamid, S.; Nicolaou, S.; Khosa, F.; Andrews, G.; Murray, N.; Abdellatif, W.; Qamar, S.R. Dual-Energy CT: A Paradigm Shift in Acute Traumatic Abdomen. Can. Assoc. Radiol. J. 2020, 71, 371–387. [Google Scholar] [CrossRef]
- Nogel, S.J.; Ren, L.; Yu, L.; Takahashi, N.; Froemming, A.T. Feasibility of Dual-Energy Computed Tomography Imaging of Gadolinium-Based Contrast Agents and Its Application in Computed Tomography Cystography: An Exploratory Study to Assess an Alternative Option When Iodinated Contrast Agents Are Contraindicated. J. Comput. Assist. Tomogr. 2021, 45, 691–695. [Google Scholar] [CrossRef]
- Smith, R.C.; Verga, M.; McCarthy, S.; Rosenfield, A.T. Diagnosis of Acute Flank Pain: Value of Unenhanced Helical CT. Am. J. Roentgenol. 1996, 166, 97–101. [Google Scholar] [CrossRef]
- Ciccarese, F.; Brandi, N.; Corcioni, B.; Golfieri, R.; Gaudiano, C. Complicated Pyelonephritis Associated with Chronic Renal Stone Disease. Radiol. Med. 2021, 126, 505–516. [Google Scholar] [CrossRef]
- Andrabi, Y.; Patino, M.; Das, C.J.; Eisner, B.; Sahani, D.V.; Kambadakone, A. Advances in CT Imaging for Urolithiasis. Indian J. Urol. 2015, 31, 185. [Google Scholar] [CrossRef]
- Nakada, S.Y.; Hoff, D.G.; Attai, S.; Heisey, D.; Blankenbaker, D.; Pozniak, M. Determination of Stone Composition by Noncontrast Spiral Computed Tomography in the Clinical Setting. Urology 2000, 55, 816–819. [Google Scholar] [CrossRef]
- Shahnani, P.S.; Karami, M.; Astane, B.; Janghorbani, M. The Comparative Survey of Hounsfield Units of Stone Composition in Urolithiasis Patients. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 650–653. [Google Scholar]
- Ngo, T.C.; Assimos, D.G. Uric Acid Nephrolithiasis: Recent Progress and Future Directions. Rev. Urol. 2007, 9, 17–27. [Google Scholar]
- Manglaviti, G.; Tresoldi, S.; Guerrer, C.S.; Di Leo, G.; Montanari, E.; Sardanelli, F.; Cornalba, G. In Vivo Evaluation of the Chemical Composition of Urinary Stones Using Dual-Energy CT. AJR Am. J. Roentgenol. 2011, 197, W76–W83. [Google Scholar] [CrossRef]
- Joshi, M.; Langan, D.A.; Sahani, D.S.; Kambadakone, A.; Aluri, S.; Procknow, K.; Wu, X.; Bhotika, R.; Okerlund, D.; Kulkarni, N.; et al. Effective Atomic Number Accuracy for Kidney Stone Characterization Using Spectral CT; SPIE: Bellingham, WA, USA, 2010; Available online: https://spie.org/Publications/Proceedings/Paper/10.1117/12.844372?SSO=1 (accessed on 8 June 2023).
- Flohr, T.G.; McCollough, C.H.; Bruder, H.; Petersilka, M.; Gruber, K.; Süβ, C.; Grasruck, M.; Stierstorfer, K.; Krauss, B.; Raupach, R.; et al. First Performance Evaluation of a Dual-Source CT (DSCT) System. Eur. Radiol. 2006, 16, 256–268. [Google Scholar] [CrossRef]
- Mansouri, M.; Aran, S.; Singh, A.; Kambadakone, A.R.; Sahani, D.V.; Lev, M.H.; Abujudeh, H.H. Dual-Energy Computed Tomography Characterization of Urinary Calculi: Basic Principles, Applications and Concerns. Curr. Probl. Diagn. Radiol. 2015, 44, 496–500. [Google Scholar] [CrossRef]
- Thomas, C.; Patschan, O.; Ketelsen, D.; Tsiflikas, I.; Reimann, A.; Brodoefel, H.; Buchgeister, M.; Nagele, U.; Stenzl, A.; Claussen, C.; et al. Dual-Energy CT for the Characterization of Urinary Calculi: In Vitro and in Vivo Evaluation of a Low-Dose Scanning Protocol. Eur. Radiol. 2009, 19, 1553–1559. [Google Scholar] [CrossRef]
- Jepperson, M.A.; Cernigliaro, J.G.; Ibrahim, E.-S.H.; Morin, R.L.; Haley, W.E.; Thiel, D.D. In Vivo Comparison of Radiation Exposure of Dual-Energy CT Versus Low-Dose CT Versus Standard CT for Imaging Urinary Calculi. J. Endourol. 2015, 29, 141–146. [Google Scholar] [CrossRef]
- Spek, A.; Strittmatter, F.; Graser, A.; Kufer, P.; Stief, C.; Staehler, M. Dual Energy Can Accurately Differentiate Uric Acid-Containing Urinary Calculi from Calcium Stones. World J. Urol. 2016, 34, 1297–1302. [Google Scholar] [CrossRef]
- Lombardo, F.; Bonatti, M.; Zamboni, G.A.; Avesani, G.; Oberhofer, N.; Bonelli, M.; Pycha, A.; Mucelli, R.P.; Bonatti, G. Uric Acid versus Non-Uric Acid Renal Stones: In Vivo Differentiation with Spectral CT. Clin. Radiol. 2017, 72, 490–496. [Google Scholar] [CrossRef]
- Bonatti, M.; Lombardo, F.; Zamboni, G.A.; Pernter, P.; Pycha, A.; Mucelli, R.P.; Bonatti, G. Renal Stones Composition in vivo Determination: Comparison between 100/Sn140 KV Dual-Energy CT and 120 KV Single-Energy CT. Urolithiasis 2017, 45, 255–261. [Google Scholar] [CrossRef]
- Nestler, T.; Nestler, K.; Neisius, A.; Isbarn, H.; Netsch, C.; Waldeck, S.; Schmelz, H.U.; Ruf, C. Diagnostic Accuracy of Third-Generation Dual-Source Dual-Energy CT: A Prospective Trial and Protocol for Clinical Implementation. World, J. Urol. 2019, 37, 735–741. [Google Scholar] [CrossRef]
- Euler, A.; Wullschleger, S.; Sartoretti, T.; Müller, D.; Keller, E.X.; Lavrek, D.; Donati, O. Dual-Energy CT Kidney Stone Characterization—Can Diagnostic Accuracy Be Achieved at Low Radiation Dose? Eur. Radiol. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Kordbacheh, H.; Baliyan, V.; Uppot, R.N.; Eisner, B.H.; Sahani, D.V.; Kambadakone, A.R. Dual-Source Dual-Energy CT in Detection and Characterization of Urinary Stones in Patients with Large Body Habitus: Observations in a Large Cohort. Am. J. Roentgenol. 2019, 212, 796–801. [Google Scholar] [CrossRef]
- Kordbacheh, H.; Baliyan, V.; Singh, P.; Eisner, B.H.; Sahani, D.V.; Kambadakone, A.R. Rapid KVp Switching Dual-Energy CT in the Assessment of Urolithiasis in Patients with Large Body Habitus: Preliminary Observations on Image Quality and Stone Characterization. Abdom. Radiol. 2019, 44, 1019–1026. [Google Scholar] [CrossRef]
- Takahashi, N.; Hartman, R.P.; Vrtiska, T.J.; Kawashima, A.; Primak, A.N.; Dzyubak, O.P.; Mandrekar, J.N.; Fletcher, J.G.; McCollough, C.H. Dual-Energy CT Iodine-Subtraction Virtual Unenhanced Technique to Detect Urinary Stones in an Iodine-Filled Collecting System: A Phantom Study. AJR Am. J. Roentgenol. 2008, 190, 1169–1173. [Google Scholar] [CrossRef]
- Moon, J.W.; Park, B.K.; Kim, C.K.; Park, S.Y. Evaluation of Virtual Unenhanced CT Obtained from Dual-Energy CT Urography for Detecting Urinary Stones. Br. J. Radiol. 2012, 85, e176–e181. [Google Scholar] [CrossRef]
- Wang, J.; Qu, M.; Leng, S.; McCollough, C.H. Differentiation of Uric Acid versus Non-Uric Acid Kidney Stones in the Presence of Iodine Using Dual-Energy CT. In Proceedings of the SPIE Medical Imaging, San Diego, CA, USA, 13–18 February 2010; Volume 7622. [Google Scholar] [CrossRef]
- Yeo, Y.J.; Kim, S.H.; Kim, M.J.; Kim, Y.H.; Cho, S.H.; Lee, E.J. Diagnostic Efficiency of Split-Bolus Dual-Energy Computed Tomography for Patients with Suspected Urinary Stones. J. Comput. Assist. Tomogr. 2015, 39, 25–31. [Google Scholar] [CrossRef]
- Magistro, G.; Bregenhorn, P.; Krauß, B.; Nörenberg, D.; D’Anastasi, M.; Graser, A.; Weinhold, P.; Strittmatter, F.; Stief, C.G.; Staehler, M. Optimized Management of Urolithiasis by Coloured Stent-Stone Contrast Using Dual-Energy Computed Tomography (DECT). BMC Urol. 2019, 19, 29. [Google Scholar] [CrossRef]
- Jepperson, M.A.; Cernigliaro, J.G.; Sella, D.; Ibrahim, E.; Thiel, D.D.; Leng, S.; Haley, W.E. Dual-Energy CT for the Evaluation of Urinary Calculi: Image Interpretation, Pitfalls and Stone Mimics. Clin. Radiol. 2013, 68, e707–e714. [Google Scholar] [CrossRef]
- Wisenbaugh, E.S.; Paden, R.G.; Silva, A.C.; Humphreys, M.R. Dual-Energy vs Conventional Computed Tomography in Determining Stone Composition. Urology 2014, 83, 1243–1247. [Google Scholar] [CrossRef]
- Zhang, G.-M.-Y.; Sun, H.; Xue, H.-D.; Xiao, H.; Zhang, X.-B.; Jin, Z.-Y. Prospective Prediction of the Major Component of Urinary Stone Composition with Dual-Source Dual-Energy CT in Vivo. Clin. Radiol. 2016, 71, 1178–1183. [Google Scholar] [CrossRef]
- Shalini, S.; Kasi Arunachalam, V.; Kumar Varatharajaperumal, R.; Mehta, P.; Thambidurai, S.; Cherian, M. The Role of Third-Generation Dual-Source Dual-Energy Computed Tomography in Characterizing the Composition of Renal Stones with Infrared Spectroscopy as the Reference Standard. Pol. J. Radiol. 2022, 87, 172–176. [Google Scholar] [CrossRef]
- Taha, M.; Shawky, M.; Abd Ella, M.D.; Tarek, F. Role of Dual Energy Computed Tomography in Evaluation of Renal Stones. Med. J. Cairo Univ. 2021, 89, 1349–1357. [Google Scholar] [CrossRef]
- Galluzzo, A.; Danti, G.; Bicci, E.; Mastrorosato, M.; Bertelli, E.; Miele, V. The Role of Dual-Energy CT in the Study of Urinary Tract Tumors: Review of Recent Literature. Semin. Ultrasound CT MRI 2023, 44, 136–144. [Google Scholar] [CrossRef]
- Graser, A.; Becker, C.R.; Staehler, M.; Clevert, D.A.; Macari, M.; Arndt, N.; Nikolaou, K.; Sommer, W.; Stief, C.; Reiser, M.F.; et al. Single-Phase Dual-Energy CT Allows for Characterization of Renal Masses as Benign or Malignant. Investig. Radiol. 2010, 45, 399–405. [Google Scholar] [CrossRef]
- Pourvaziri, A.; Mojtahed, A.; Hahn, P.F.; Gee, M.S.; Kambadakone, A.; Sahani, D.V. Renal Lesion Characterization: Clinical Utility of Single-Phase Dual-Energy CT Compared to MRI and Dual-Phase Single-Energy CT. Eur. Radiol. 2023, 33, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.A.; Ahmad, F.; Sathiadoss, P.; Haroon, M.; McInnes, M.D.; Bossuyt, P.M.; Schieda, N. Direct Comparison of Diagnostic Accuracy of Fast Kilovoltage Switching Dual-Energy Computed Tomography and Magnetic Resonance Imaging for Detection of Enhancement in Renal Masses. J. Comput. Assist. Tomogr. 2022, 46, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Marcon, J.; Graser, A.; Horst, D.; Casuscelli, J.; Spek, A.; Stief, C.G.; Reiser, M.F.; Rübenthaler, J.; Buchner, A.; Staehler, M. Papillary vs Clear Cell Renal Cell Carcinoma. Differentiation and Grading by Iodine Concentration Using DECT-Correlation with Microvascular Density. Eur. Radiol. 2020, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jonisch, A.I.; Rubinowitz, A.N.; Mutalik, P.G.; Israel, G.M. Can High-Attenuation Renal Cysts Be Differentiated from Renal Cell Carcinoma at Unenhanced CT? Radiology 2007, 243, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Graser, A.; Johnson, T.R.C.; Hecht, E.M.; Becker, C.R.; Leidecker, C.; Staehler, M.; Stief, C.G.; Hildebrandt, H.; Godoy, M.C.B.; Finn, M.E.; et al. Dual-Energy CT in Patients Suspected of Having Renal Masses: Can Virtual Nonenhanced Images Replace True Nonenhanced Images? Radiology 2009, 252, 433–440. [Google Scholar] [CrossRef]
- Thiravit, S.; Brunnquell, C.; Cai, L.M.; Flemon, M.; Mileto, A. Use of Dual-Energy CT for Renal Mass Assessment. Eur. Radiol. 2021, 31, 3721–3733. [Google Scholar] [CrossRef]
- Arndt, N.; Staehler, M.; Siegert, S.; Reiser, M.F.; Graser, A. Dual Energy CT in Patients with Polycystic Kidney Disease. Eur. Radiol. 2012, 22, 2125–2129. [Google Scholar] [CrossRef]
- Cha, D.; Kim, C.K.; Park, J.J.; Park, B.K. Evaluation of Hyperdense Renal Lesions Incidentally Detected on Single-Phase Post-Contrast CT Using Dual-Energy CT. Br. J. Radiol. 2016, 89, 20150860. [Google Scholar] [CrossRef]
- Ascenti, G.; Mazziotti, S.; Mileto, A.; Racchiusa, S.; Donato, R.; Settineri, N.; Gaeta, M. Dual-Source Dual-Energy CT Evaluation of Complex Cystic Renal Masses. AJR Am. J. Roentgenol. 2012, 199, 1026–1034. [Google Scholar] [CrossRef]
- Moleesaide, A.; Maneegarn, A.; Kaewlai, R.; Thiravit, S. Virtual Monochromatic Spectral Attenuation Curve Analysis for Evaluation of Incidentally Detected Small Renal Lesions Using Rapid Kilovoltage-Switching Dual-Energy Computed Tomography. Abdom. Radiol. 2022, 47, 3817–3827. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Zhang, X.; Wang, D.; Zhang, W.; Wang, Z.; Zhou, J. Analysis of Dual Energy Spectral CT and Pathological Grading of Clear Cell Renal Cell Carcinoma (CcRCC). PLoS ONE 2018, 13, e0195699. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Relationship between Visceral Adipose Tissue and Genetic Mutations (VHL and KDM5C) in Clear Cell Renal Cell Carcinoma. Radiol. Med. 2021, 126, 645–651. [Google Scholar] [CrossRef]
- Walker, D.; Udare, A.; Chatelain, R.; McInnes, M.; Flood, T.; Schieda, N. Utility of Material-Specific Fat Images Derived from Rapid-KVp-Switch Dual-Energy Renal Mass CT for Diagnosis of Renal Angiomyolipoma. Acta Radiol. 2021, 62, 1263–1272. [Google Scholar] [CrossRef]
- Çamlıdağ, İ.; Nural, M.S.; Danacı, M.; Özden, E. Usefulness of Rapid KV-Switching Dual Energy CT in Renal Tumor Characterization. Abdom. Radiol. 2019, 44, 1841–1849. [Google Scholar] [CrossRef]
- Alanee, S.; Dynda, D.I.; Hemmer, P.; Schwartz, B. Low Enhancing Papillary Renal Cell Carcinoma Diagnosed by Using Dual Energy Computerized Tomography: A Case Report and Review of Literature. BMC Urol. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Uroweb—European Association of Urology. Non-Muscle-Invasive Bladder Cancer—Introduction—Uroweb. Available online: https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer (accessed on 14 May 2023).
- Takeuchi, M.; McDonald, J.S.; Takahashi, N.; Frank, I.; Thompson, R.H.; King, B.F.; Kawashima, A. Cancer Prevalence and Risk Stratification in Adults Presenting with Hematuria: A Population-Based Cohort Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 308–319. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Buffa, V.; Fedeli, S.; Vallone, A.; Ruopoli, R.; Luzietti, M.; Miele, V.; Rengo, M.; Maurizi Enrici, M.; Fina, P.; et al. Preliminary Experience with Abdominal Dual-Energy CT (DECT): True versus Virtual Nonenhanced Images of the Liver. Radiol. Med. 2010, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Buffa, V.; Solazzo, A.; D’Auria, V.; Del Prete, A.; Vallone, A.; Luzietti, M.; Madau, M.; Grassi, R.; Miele, V. Dual-Source Dual-Energy CT: Dose Reduction after Endovascular Abdominal Aortic Aneurysm Repair. Radiol. Med. 2014, 119, 934–941. [Google Scholar] [CrossRef]
- Helenius, M.; Dahlman, P.; Magnusson, M.; Lönnemark, M.; Magnusson, A. Contrast Enhancement in Bladder Tumors Examined with CT Urography Using Traditional Scan Phases. Acta Radiol. 2014, 55, 1129–1136. [Google Scholar] [CrossRef]
- Raman, S.P.; Fishman, E.K. Bladder Malignancies on CT: The Underrated Role of CT in Diagnosis. Am. J. Roentgenol. 2014, 203, 347–354. [Google Scholar] [CrossRef]
- Sahni, V.A.; Shinagare, A.B.; Silverman, S.G. Virtual Unenhanced CT Images Acquired from Dual-Energy CT Urography: Accuracy of Attenuation Values and Variation with Contrast Material Phase. Clin. Radiol. 2013, 68, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C.; Kwan, S.W.; Olcott, E.W.; Sommer, G. Split-Bolus MDCT Urography with Synchronous Nephrographic and Excretory Phase Enhancement. AJR Am. J. Roentgenol. 2007, 189, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tsai, T.-H.; Jaw, T.-S.; Lai, M.-L.; Chao, M.-F.; Liu, G.-C.; Hsu, J.-S. Diagnostic Performance of Split-Bolus Portal Venous Phase Dual-Energy CT Urography in Patients with Hematuria. AJR Am. J. Roentgenol. 2016, 206, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.C. Dual-Energy CT: General Principles. AJR Am. J. Roentgenol. 2012, 199 (Suppl. S5), S3–S8. [Google Scholar] [CrossRef]
- Zopfs, D.; Laukamp, K.R.; dos Santos, D.P.; Sokolowski, M.; Hokamp, N.G.; Maintz, D.; Borggrefe, J.; Persigehl, T.; Lennartz, S. Low-KeV Virtual Monoenergetic Imaging Reconstructions of Excretory Phase Spectral Dual-Energy CT in Patients with Urothelial Carcinoma: A Feasibility Study. Eur. J. Radiol. 2019, 116, 135–143. [Google Scholar] [CrossRef]
- Uroweb—European Association of Urology. EAU Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma—Introduction—Uroweb. Available online: https://uroweb.org/guidelines/upper-urinary-tract-urothelial-cell-carcinoma (accessed on 14 May 2023).
- Lee, K.Y.G.; Cheng, H.M.J.; Chu, C.Y.; Tam, C.W.A.; Kan, W.K. Metal Artifact Reduction by Monoenergetic Extrapolation of Dual-Energy CT in Patients with Metallic Implants. J. Orthop. Surg. 2019, 27, 2309499019851176. [Google Scholar] [CrossRef]
- Bamberg, F.; Dierks, A.; Nikolaou, K.; Reiser, M.F.; Becker, C.R.; Johnson, T.R.C. Metal Artifact Reduction by Dual Energy Computed Tomography Using Monoenergetic Extrapolation. Eur. Radiol. 2011, 21, 1424–1429. [Google Scholar] [CrossRef]
- Lewis, M.; Reid, K.; Toms, A.P. Reducing the Effects of Metal Artefact Using High KeV Monoenergetic Reconstruction of Dual Energy CT (DECT) in Hip Replacements. Skelet. Radiol. 2013, 42, 275–282. [Google Scholar] [CrossRef]
- Higashigaito, K.; Angst, F.; Runge, V.M.; Alkadhi, H.; Donati, O.F. Metal Artifact Reduction in Pelvic Computed Tomography with Hip Prostheses: Comparison of Virtual Monoenergetic Extrapolations from Dual-Energy Computed Tomography and an Iterative Metal Artifact Reduction Algorithm in a Phantom Study. Investig. Radiol. 2015, 50, 828–834. [Google Scholar] [CrossRef]
- Bicci, E.; Mastrorosato, M.; Danti, G.; Lattavo, L.; Bertelli, E.; Cozzi, D.; Pradella, S.; Agostini, S.; Miele, V. Dual-Energy CT Applications in Urinary Tract Cancers: An Update. Tumori 2023, 109, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Naiki, T.; Naiki-Ito, A.; Ozawa, Y.; Shimohira, M.; Ohnishi, M.; Shibamoto, Y. Usefulness of Advanced Monoenergetic Reconstruction Technique in Dual-Energy Computed Tomography for Detecting Bladder Cancer. Jpn. J. Radiol. 2022, 40, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Becker, C.D.; Montet, X.; Botsikas, D. Diagnosis of Urothelial Tumors with a Dedicated Dual-Source Dual-Energy MDCT Protocol: Preliminary Results. Am. J. Roentgenol. 2014, 202, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Wrixon, A.D. New ICRP Recommendations. J. Radiol. Prot. 2008, 28, 161–168. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-Generation Iterative Reconstruction on a Dual-Source, High-Pitch, Low-Dose Chest CT Protocol with Tin Filter for Spectral Shaping at 100 KV: A Study on a Small Series of COVID-19 Patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef]
- Apfaltrer, G.; Dutschke, A.; Baltzer, P.A.T.; Schestak, C.; Özsoy, M.; Seitz, C.; Veser, J.; Petter, E.; Helbich, T.H.; Ringl, H.; et al. Substantial Radiation Dose Reduction with Consistent Image Quality Using a Novel Low-Dose Stone Composition Protocol. World J. Urol. 2020, 38, 2971–2979. [Google Scholar] [CrossRef]
- Dewes, P.; Frellesen, C.; Scholtz, J.-E.; Fischer, S.; Vogl, T.J.; Bauer, R.W.; Schulz, B. Low-Dose Abdominal Computed Tomography for Detection of Urinary Stone Disease—Impact of Additional Spectral Shaping of the X-ray Beam on Image Quality and Dose Parameters. Eur. J. Radiol. 2016, 85, 1058–1062. [Google Scholar] [CrossRef]
- Marin, D.; Boll, D.T.; Mileto, A.; Nelson, R.C. State of the Art: Dual-Energy CT of the Abdomen. Radiology 2014, 271, 327–342. [Google Scholar] [CrossRef]
- Mileto, A.; Marin, D.; Nelson, R.C.; Ascenti, G.; Boll, D.T. Dual Energy MDCT Assessment of Renal Lesions: An Overview. Eur. Radiol. 2014, 24, 353–362. [Google Scholar] [CrossRef]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2021, 41, 509–523. [Google Scholar] [CrossRef]
- Lambert, J.W.; FitzGerald, P.F.; Edic, P.M.; Sun, Y.; Bonitatibus, P.J.; Colborn, R.E.; Yeh, B.M. The Effect of Patient Diameter on the Dual-Energy Ratio of Selected Contrast-Producing Elements. J. Comput. Assist. Tomogr. 2017, 41, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Dual-Energy CT: Is It What the Doctor Ordered for the Cost-Conscious Community Hospital? Available online: https://radiologybusiness.com/sponsored/1081/hitachi-healthcare-americas/topics/healthcare-management/healthcare-economics/dual (accessed on 14 May 2023).


| Authors | Number of Patients | Number of Stones | Stone Composition | Gold Standard | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Spek et al. [35] | 64 | 213 | 9 patients with uric acid, 26 patients with calcium oxalate monohydrate, 4 patients with calcium oxalate dihydrate, 7 patients with calcium hydroxyapatite, 1 patient with brushite, 1 patient with cystine, 2 patients with mixed uric acid 14 patients with mixed non-uric acid | Spectroscopy | 98.4% | 98% |
| Lombardo et al. [36] | 33 | 62 | 15 uric acid, 39 calcium oxalate or phosphate, 8 cysteine | Chemical Analysis Urinary Calculi Analysis Kit, DiaSys, Diagnostic System GmbH | 100% | 100% |
| Bonatti et al. [37] | 30 | 50 | 17 uric acid, 29 calcium oxalate or phosphate, 4 cysteine | Infrared spectroscopy | 100% | 93.9% |
| Wisenbaugh et al. [48] | Vitro | 27 | 12 uric acid, 6 struvite, 5 cysteine, 4 calcium oxalate | Infrared spectroscopy | 92.3% | 93% |
| Nestler et al. [38] | 84 | 144 | 10 patients uric acid 74 patients non-UA or mixed | Infrared spectroscopy | 84.6% | 100% |
| Zhang et al. [49] | 67 | 81 | 5 uric acid 38 non-UA (31 calcium oxalate 5 uric acid, 2 cysteine) 38 mixed | Fourier-transform infrared spectroscopy | 100% (calcium oxalate, cysteine) 77.8% (uric acid) | 88.24% (CaOx) 100% (HA-Cys-UA) |
| Shalini et al. [50] | 50 | 7 uric acid, 43 calcium containing | Infrared spectroscopy | |||
| Shwaky et al. [51] | 30 | 37 | 8 uric acid, 21 calcium oxalate, 2 calcium phosphates, 4 cysteine, 2 mixed | Crystallography | ||
| Euler et al. [39] | 203 | 227 | 15 uric acid 175 calcium oxalate, 26 calcium oxalate—apatite, 4 cystine, 3 apatite, 2 calcium oxalate—calcium hydrogen phosphate, 1 triple phosphate, 1 apatite—triple phosphate | X-ray diffraction | 100% | 93% |
| Author | Investigated Pathology | N° of Patients | Gold Standard | Acquisition Phase in DECT | Main Results |
|---|---|---|---|---|---|
| Graser et al. [53] | Ultrasound-based suspicion of a renal mass | 202 | Histopathology | Nephrographic phase DECT | -96.0% patients with malignancy and 93.2% without malignancy were correctly identified -overall accuracy: 94.6% -The omission of the true unenhanced phase led to a 48.9 ± 7.0% dose reduction. |
| Pourvaziri et al. [54] | Renal lesions | 69 | Simple cysts: all distinct features of a simple cyst on both CT and MR. Proteinaceous/hemorrhagic cyst: the characteristic appearance of it on both CT and MRI. Enhancing lesions: histopathology or unequivocal signs of enhancement on both CT and MRI. | Nephrographic phase DECT | -The pooled diagnostic confidence scores for the three readers were comparable for the DECT and MRI scans (pooled DECT mean = 4.78, SD: 0.48 vs. pooled MRI mean = 4.78, SD: 0.51, p-value: 0.96). -The inter-reader agreement was almost perfect for DECT and MRI (kappa: 0.8–1) -Comparable diagnostic accuracy between single energy CT, DECT, and MRI (p-value > 0.05). |
| McGrath et al. [55] | Renal masses | 24 | Histopathology | Corticomedullary phase (CMP) and late nephrographic phase (LNP) | -No significant difference in diagnostic accuracy comparing subjective enhancement by MRI and DECT iodine map. |
| Alanee et al. [68] | Papillary renal-cell carcinoma (pRCC) | 1 | Histopathology | Not specified | -Iodine maps allow for their accurate characterization as solid masses. |
| Graser et al. [58] | Renal masses | 110 | Histopathology, contrast-enhanced US, follow-up CT | Nephrographic phase DECT | -In all but three patients, radiologists accepted virtual non-contrast images as a replacement for true non-contrast images. -Mean dose reduction by omitting the true non-contrast scan was 35.05%. |
| Cha et al. [61] | Hyperdense renal lesions incidentally detected on single-phase postcontrast CT | 79 | In patients who underwent surgical resection or biopsy, histopathological reports were used as the reference standard. If histopathology was not confirmed, follow-up imaging was used to make a final diagnosis. | Corticomedullary phase (CMP) and late nephrographic phase (LNP) | -For differentiating between solid and benign cystic lesions, the specificity and accuracy of all lesions and lesions < 1.5 cm were statistically lower in iodine images than in linearly blended images; for lesions ≥ 1.5 cm, they were not statistically different. -For all types of lesions ≥ 1.5 cm, the CT numbers between linearly blended and iodine images and between true non-contrast and virtual non-contrast images were not statistically different (p > 0.05). |
| Ascenti et al. [62] | Complex cystic renal masses | 79 | Surgical resections, biopsy, percutaneous drainage, and imaging follow-up for a minimum of 12 months were used to determine the outcome. | Corticomedullary phase DECT | -Virtual non-contrast and true unenhanced evaluation to identify complex cystic renal masses was judged acceptable in 97.2% of cases. -Color-coded iodine-overlay images allowed the exclusion of enhancement with a significantly (p < 0.03) higher level of confidence (score 1, n = 22; score 2, n = 3) than on true unenhanced and blended images. |
| Moleesaide et al. [63] | Renal lesions | 46 | Histopathology, interval size-change, US, follow-up CT, MRI T2 sequences | Nephrographic phase DECT | -All datasets yielded a high diagnostic accuracy of 96% for reader 1 and 95% for reader 2. -A ∆HU threshold of 20 HU yielded an accuracy of 100%. -Visual analysis of the curve pattern also yielded high accuracy of 94%. |
| Wei et al. [64] | Clear cell RCC (ccRCC) | 62 | Histopathology | Cortex phase (CP) and parenchymal phase (PP) | -In the qualitative analysis of the imaging features observed during the combined CP and PP, sensitivity and specificity of 80% and 77.8%, respectively, were achieved for differentiating between low- and high-grade ccRCC. -The quantitative parameters analysis with CT spectral imaging compared with qualitative CT image analysis improved the sensitivity from 80% to 90.3% and the specificity from 77.8% to 87.5%. |
| Marcon et al. [56] | Clear-cell RCC and papillary RCC | 53 patients with clear-cell RCC (ccRCC) and 15 with papillary RCC (pRCC) | Histopathology | Nephrographic phase DECT | -Analysis of iodine concentration (IC) showed a significant difference between pRCC and ccRCC (p < 0.001). -Mean IC for ccRCC was 4.83 ± 1.75 mg/mL (range 2.2–11.5 mg/mL); for pRCC, it was 2.53 ± 1.59 (range 0.4–6.6 mg/mL). -ROC analysis revealed an ideal cutoff value of ≤3.1 mg/mL (AUC 0.866, p < 0.001) regarding the distinction between pRCCs and ccRCCs. -The calculated specificity was 90.6%, with a sensitivity of 73.3% and an accuracy of 86.8%. |
| Walker et al. [66] | Angiomyolipoma (AML) | 25 patients with diagnosed AML and 44 patients with renal masses (control group) | Presence of internal macroscopic fat at true 120 kVp non-contrast-enhanced CT (NECT) with an area of macroscopic fat measuring <−15 HU in density (24/25) or histopathology from 18 G core needle biopsy when macroscopic fat was not present (1/25). | Corticomedullary phase DECT | -At DECT, fat concentration was higher in AML (p < 0.001). -AUC to diagnose AML using −206.0 mg/mL threshold was 0.98 (95% CI 0.95–1.0) with sensitivity/specificity of 92.0%/96.7%. -Of AML, 8.0% (2/25)were incorrectly classified; one of these was fat-poor. |
| Authors | Pathologies | N° pz | Acquisition Phase in DECT | Main Results |
|---|---|---|---|---|
| Chen et al. [77] | Hematuria | 171 | Late-venous phase | -Sensitivity, specificity, and accuracy were 98.7%, 98.9%, and 98.8%, respectively, for the one-step approach to malignant mass detection and 98.7%, 97.9%, and 98.3% for the dual-phase approach (p > 0.05 for all comparisons). -Omitting the unenhanced scan reduced the mean radiation dose from 15.4 to 6.7 mSv. |
| Zopfs et al. [79] | Urothelial carcinoma | 26 | Late-venous and excretory phase | -In comparison to venous-phase CT, attenuation, and signal-to-noise-ratio in excretory-phase VMI 40 keV were higher (p < 0.001). -Regarding image noise, no significant difference was found between venous-phase CT and excretory-phase VMI 40 keV (p-range: 0.08–1.00). -Contrast-to-noise ratio of urothelial carcinoma to circumjacent bladder wall was significantly higher in excretory-phase VMI40 keV compared to venous-phase CT. -Subjective vessel contrast and delineation of primary tumor and distant metastases received equivalent or higher Likert scores in excretory-phase VMI40 keV than in venous phase. |
| Nakagawa et al. [86] | Bladder cancer | 52 | Nephrogenic phases | -The mean difference in CT number between the cancer and bladder wall value at 40 keV was significantly higher than that of virtual 120 kVp (80.5 ± 54 vs. 11.4 ± 12.5 HU, p < 0.01). -Average scores of subjective evaluations in the virtual-120 kVp and 40 keV images were 1.7 ± 1.2 and 2.1 ± 1.2, respectively (p < 0.001). |
| Hansen et al. [87] | Macroscopic hematuria and known or suspected neoplastic disease | 56 | Arterial and excretory phase | -Urothelial tumors were identified on 35 s series, 8 min series, and both series combined, with sensitivity of 91.9%, 83.4%, and 97.3%, respectively. -Urothelial tumors showed stronger virtual enhancement (p = 0.02) and higher iodine concentration (p = 0.03) than lesions of other origin. -Distinction between urothelial tumors and nontumoral lesions was possible with sensitivity of 91.9%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cellina, M.; Bausano, M.V.; Pais, D.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Cè, M.; Martinenghi, C.; Oliva, G.; Carrafiello, G. Dual-Energy CT Applications in Urological Diseases. Appl. Sci. 2023, 13, 7653. https://doi.org/10.3390/app13137653
Cellina M, Bausano MV, Pais D, Chiarpenello V, Costa M, Vincenzo Z, Cè M, Martinenghi C, Oliva G, Carrafiello G. Dual-Energy CT Applications in Urological Diseases. Applied Sciences. 2023; 13(13):7653. https://doi.org/10.3390/app13137653
Chicago/Turabian StyleCellina, Michaela, Maria Vittoria Bausano, Daniele Pais, Vittoria Chiarpenello, Marco Costa, Zakaria Vincenzo, Maurizio Cè, Carlo Martinenghi, Giancarlo Oliva, and Gianpaolo Carrafiello. 2023. "Dual-Energy CT Applications in Urological Diseases" Applied Sciences 13, no. 13: 7653. https://doi.org/10.3390/app13137653
APA StyleCellina, M., Bausano, M. V., Pais, D., Chiarpenello, V., Costa, M., Vincenzo, Z., Cè, M., Martinenghi, C., Oliva, G., & Carrafiello, G. (2023). Dual-Energy CT Applications in Urological Diseases. Applied Sciences, 13(13), 7653. https://doi.org/10.3390/app13137653







