Transparent Silicone–Epoxy Coatings with Enhanced Icephobic Properties for Photovoltaic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings
2.2. Synthesis of the Chemical Modifiers
2.3. Analysis of the Chemical Modifiers
2.4. Preparation of the Samples
2.5. Determination of the Optical Properties
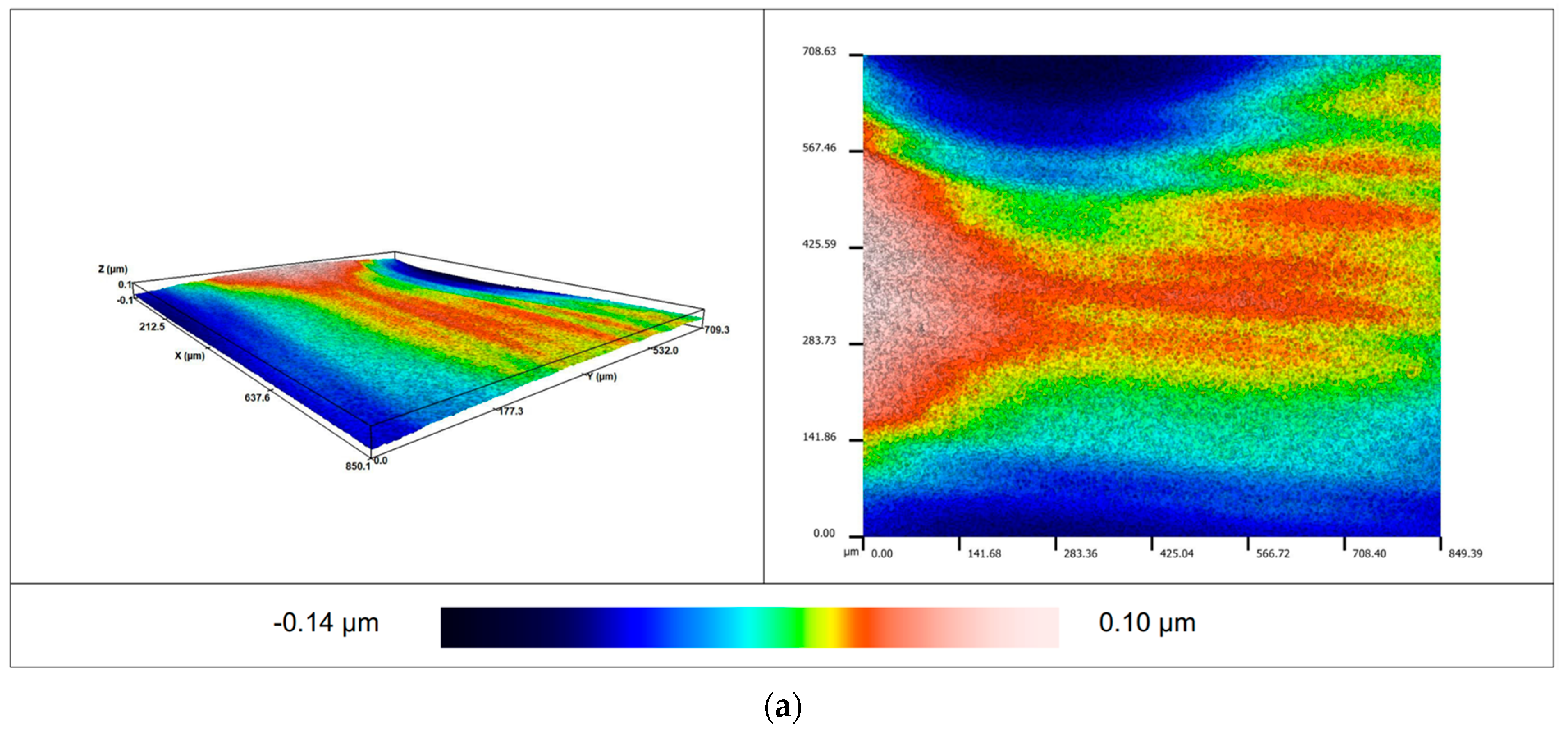
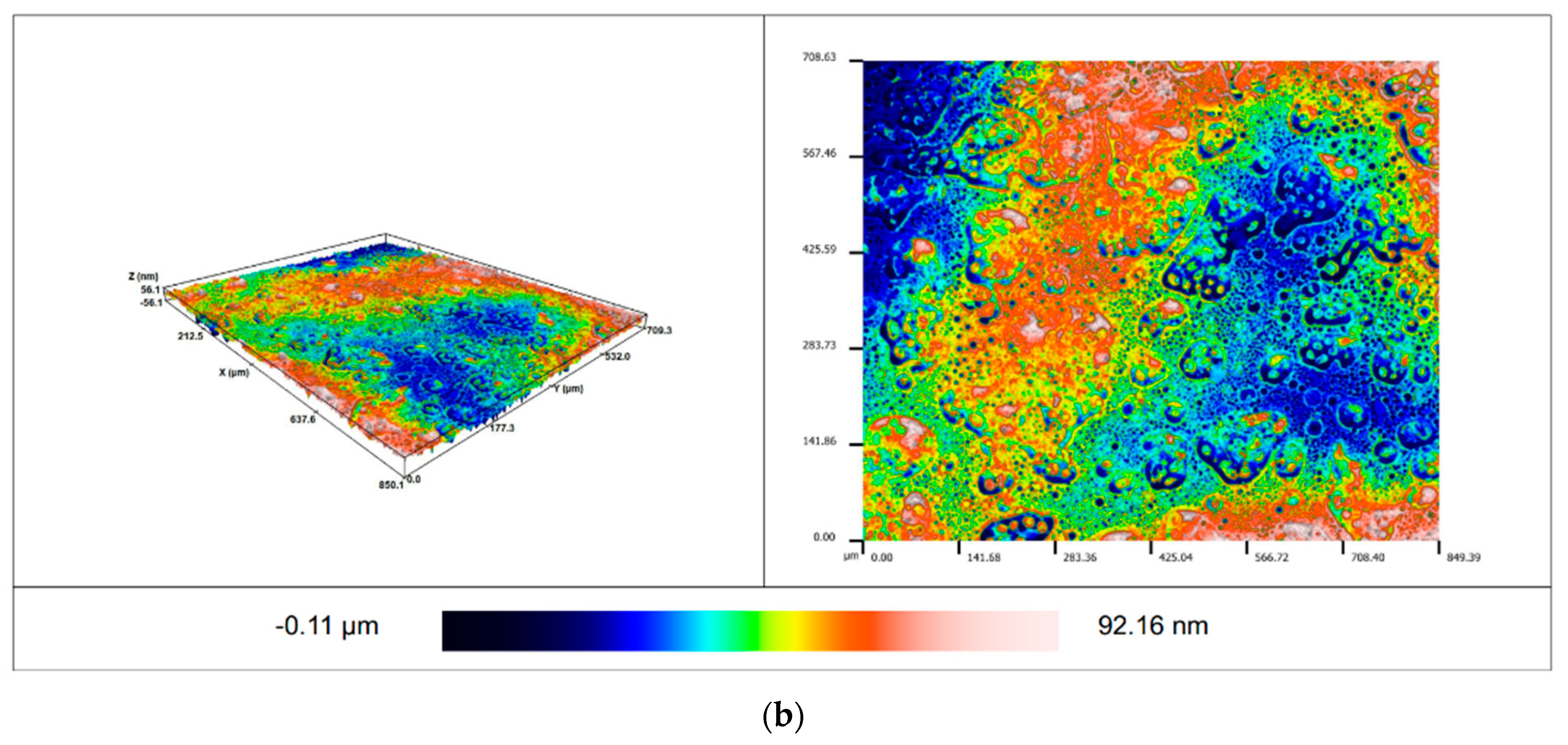
2.6. Determination of Roughness
2.7. Determination of Hydrophobicity
2.8. Determination of Freezing Delay Time (FDT)
2.9. Determination of Ice Adhesion Strength (IA)
3. Results
3.1. Characterization of the Polysiloxanes Derivatives

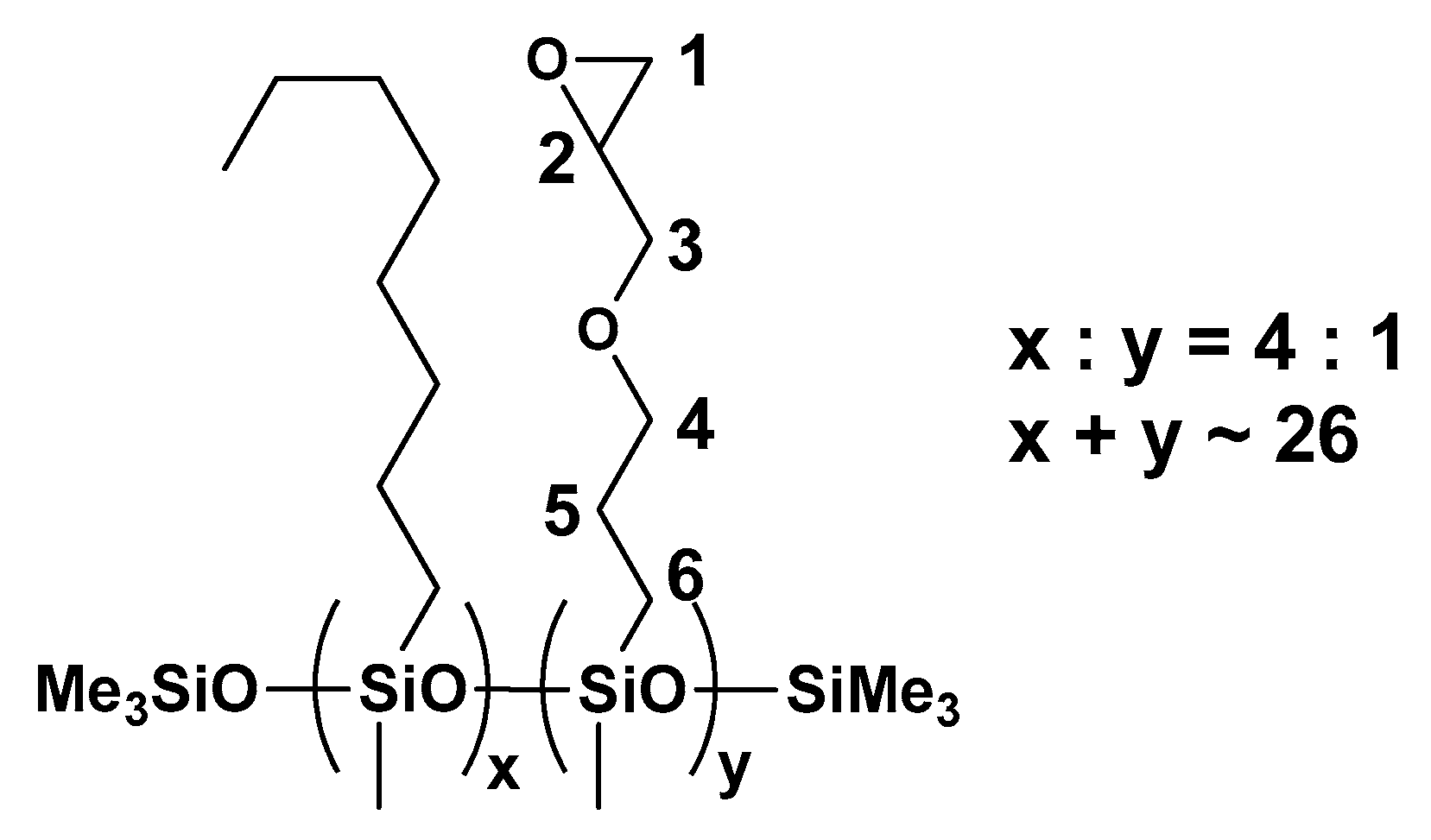
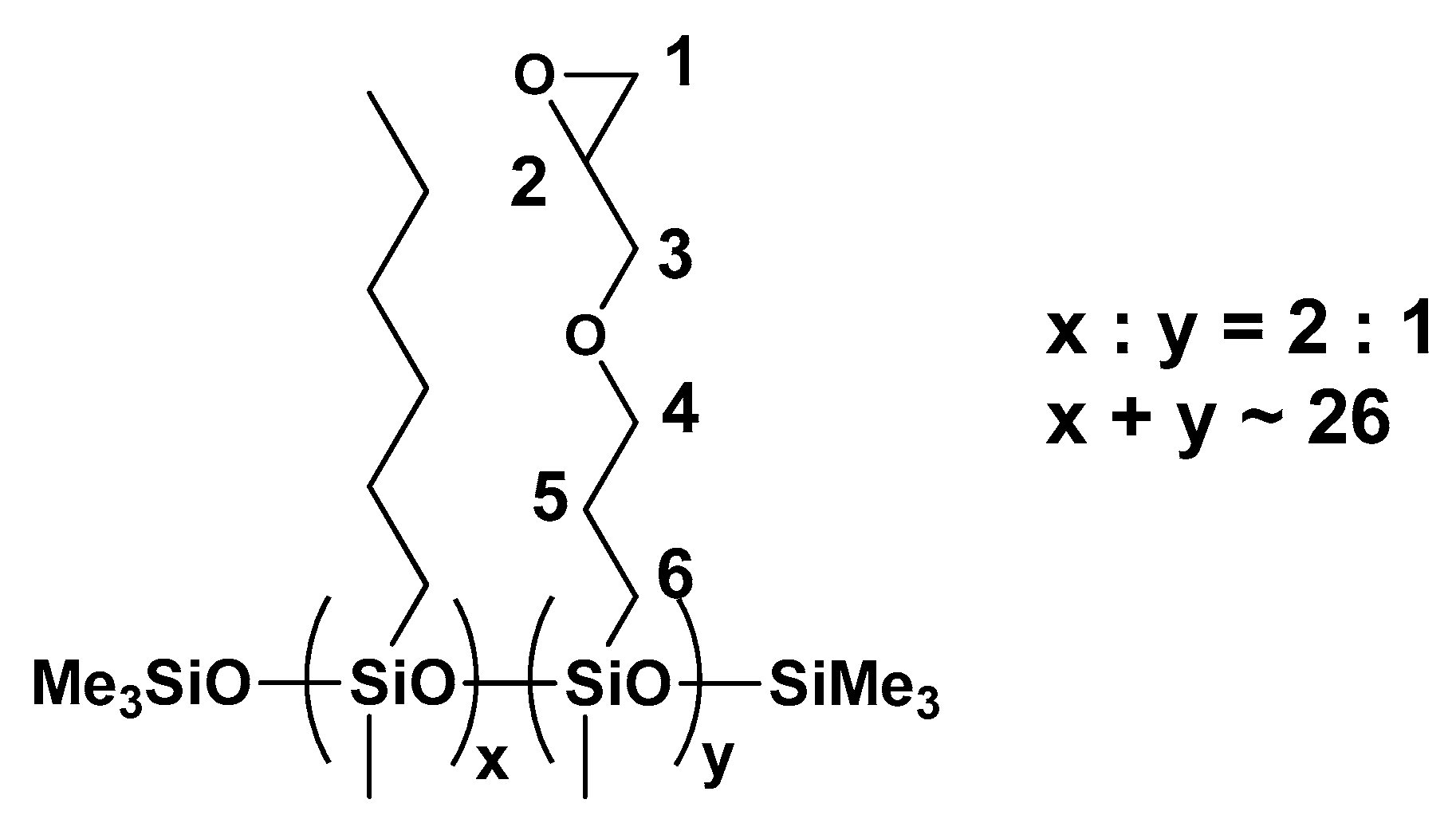
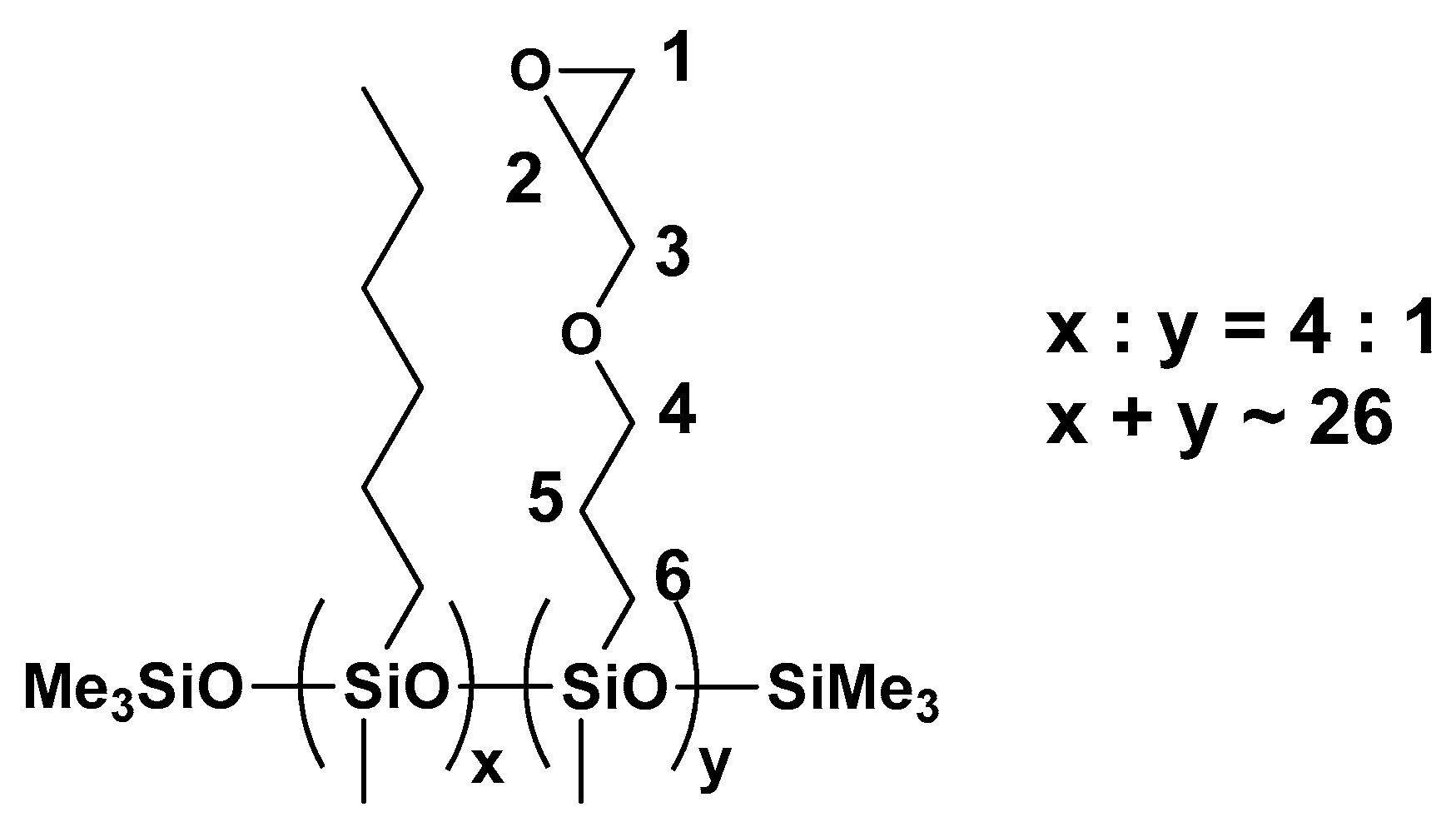
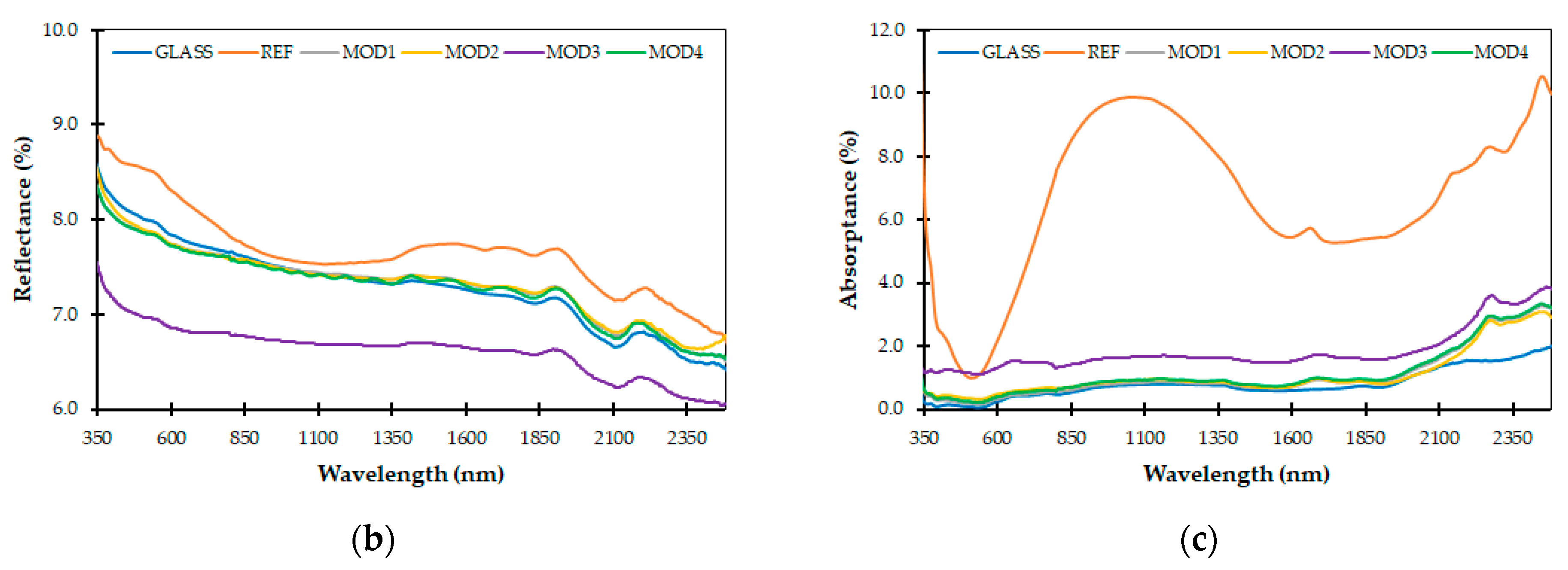
3.2. Optical Properties
3.3. Roughness of the Surface
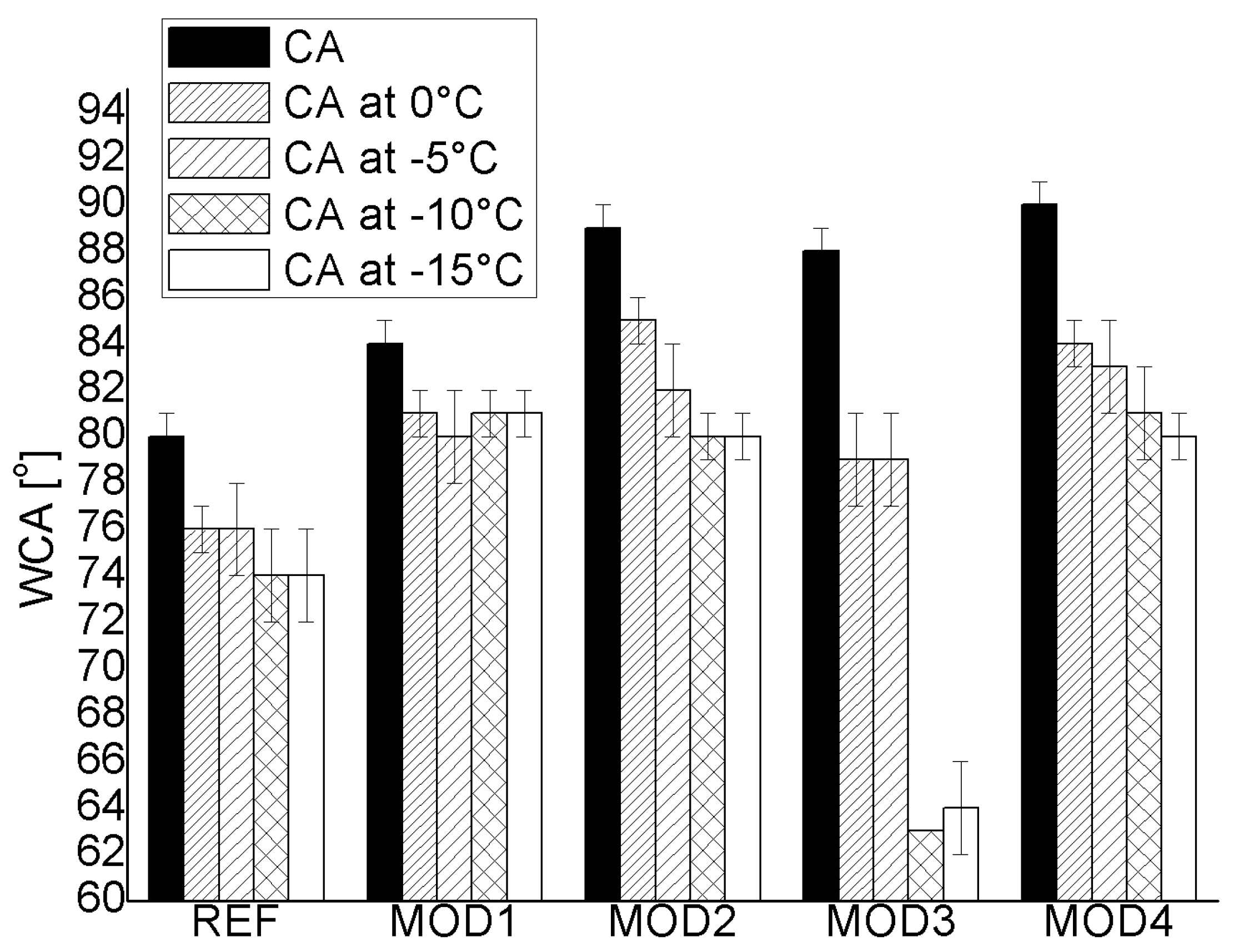
3.4. Hydrophobic Properties
Water Contact Angle at Temperatures Below 0 °C
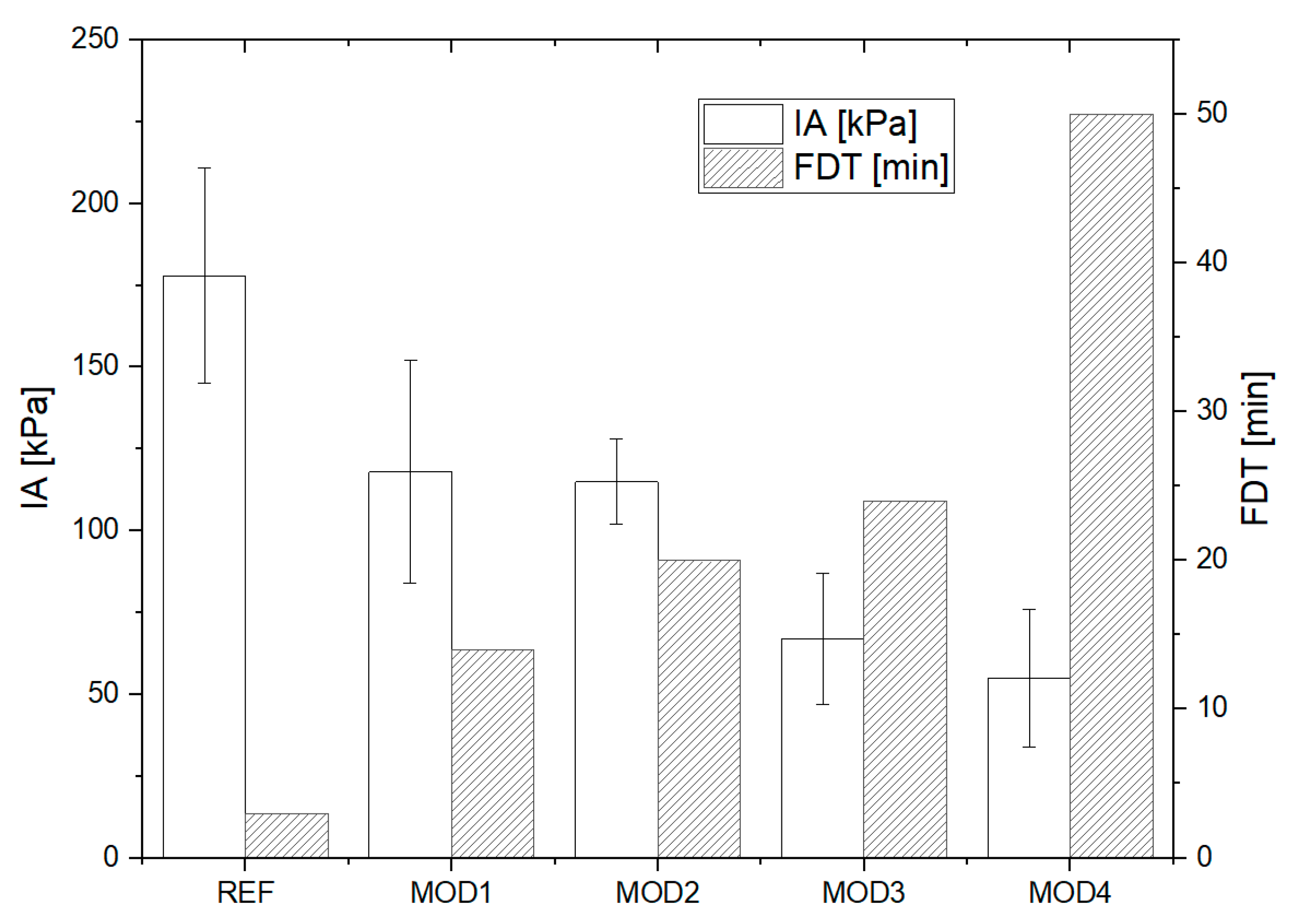
3.5. Icephobic Properties
3.5.1. Ice Adhesion Strength
3.5.2. Freezing Delay Time
3.5.3. Summary of the Icephobic Properties
4. Discussion
4.1. Influence of the Chemical Groups on Wettability and Icephobicity
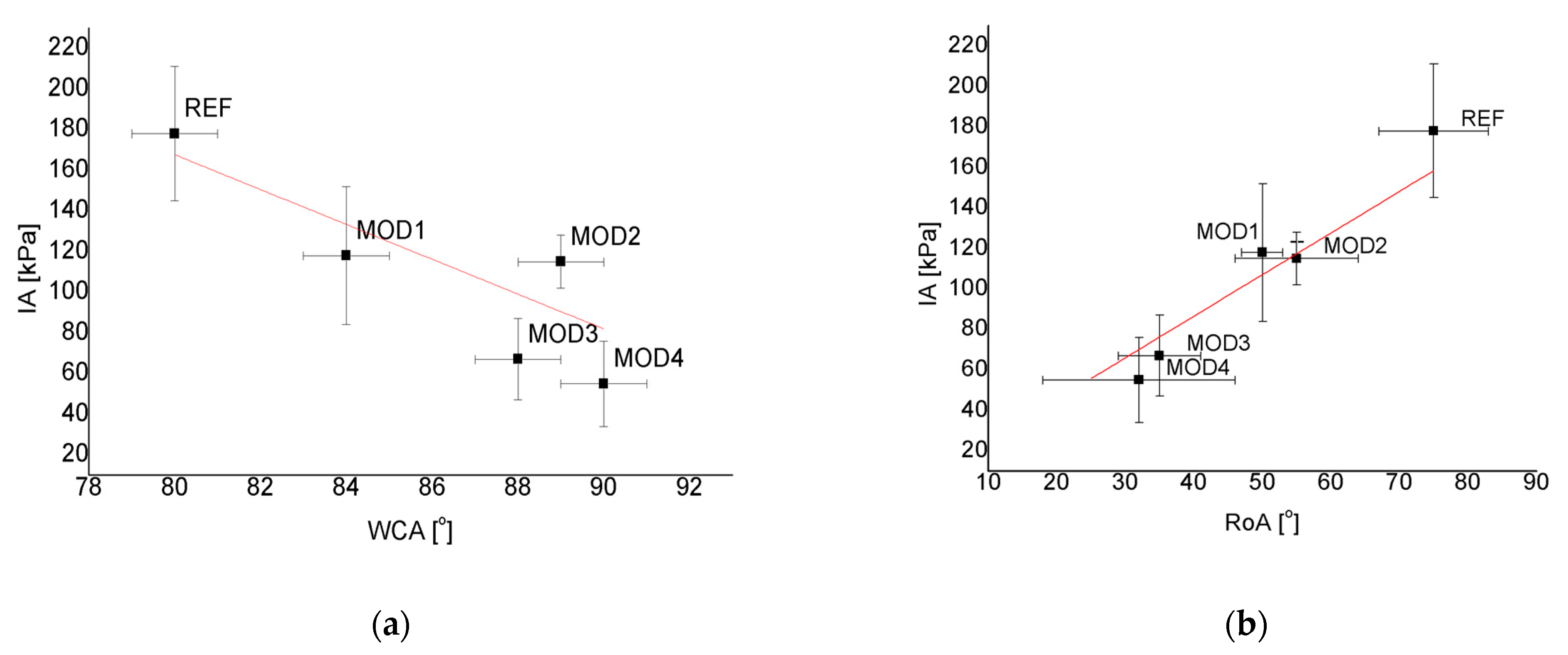
4.2. Relationship between Wettability and Icephobicity
5. Conclusions
- The silicone–epoxy coatings produced in the entire tested spectral range showed similar optical properties (T, R, A) to those of glass. They can be potentially used in photovoltaic panels.
- The chemical modification with polysiloxanes resulted in an increase in surface roughness compared to the unmodified coating.
- The chemical modification with polysiloxanes contributed to an increase in hydrophobicity, with most samples achieving WCA values close to 90°.
- The use of polysiloxanes resulted in a significant reduction in the CAH and RoA values compared to those of the unmodified coating.
- The WCA values at reduced temperatures decreased for all applied modifications compared to the WCA values determined at room temperature. In addition, it was observed that the lower the ambient temperature, the lower the WCA value.
- Double-functionalized polysiloxanes in the epoxy–silicone matrix led to a significant improvement in the anti-icing properties, a reduction in ice adhesion, and an increased freezing delay time of water droplets in comparison to the unmodified silicone–epoxy resin.
- The synergistic effect of the AGE and HEX functional groups at the polysiloxane core yielded better icephobic and hydrophobic results compared to those obtained with the AGE and OCT functional groups when using a silicone–epoxy matrix.
- Two relationships were observed for the modifications used. As the WCA values increased and as the RoA values decreased, the IA values were reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyu, J.; Wu, B.; Wu, N.; Peng, C.; Yang, J.; Meng, Y.; Xing, S. Green preparation of transparent superhydrophobic coatings with persistent dynamic impact resistance for outdoor applications. Chem. Eng. J. 2021, 404, 126456. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, Y.; Du, F. Rational design of superhydrophobic, transparent hybrid coating with superior durability. Prog. Org. Coat. 2021, 157, 106294. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Li, W.; Wang, F.; Ou, J.; Amirfazli, A. One-step fabrication of transparent superhydrophobic surface. Appl. Surf. Sci. 2021, 542, 148534. [Google Scholar] [CrossRef]
- Gorjian, S.; Bousi, E.; Özdemir, E.; Trommsdorff, M.; Kumar, N.M.; Anand, A.; Kant, K.; Chopra, S.S. Progress and challenges of crop production and electricity generation in agrivoltaic systems using semi-transparent photovoltaic technology. Renew. Sustain. Energy Rev. 2022, 158, 112126. [Google Scholar] [CrossRef]
- Bermudez-Garcia, A.; Voarino, P.; Raccurt, O. Environments, needs and opportunities for future space photovoltaic power generation: A review. Appl. Energy 2021, 290, 116757. [Google Scholar] [CrossRef]
- Gorjian, S.; Sharon, H.; Ebadi, H.; Kant, K.; Scavo, F.B.; Tina, G.M. Recent technical advancements, economics and environmental impacts of floating photovoltaic solar energy conversion systems. J. Clean. Prod. 2021, 278, 124285. [Google Scholar] [CrossRef]
- Zahedi, R.; Seraji, M.A.N.; Borzuei, D.; Moosavian, S.F.; Ahmadi, A. Feasibility study for designing and building a zero-energy house in new cities. Sol. Energy 2022, 240, 168–175. [Google Scholar] [CrossRef]
- IEA. Solar PV (IEA); IEA: Paris, France, 2021. [Google Scholar]
- Kant, K.; Pitchumani, R. Fractal textured glass surface for enhanced performance and self-cleaning characteristics of photovoltaic panels. Energy Convers. Manag. 2022, 270, 116240. [Google Scholar] [CrossRef]
- Klugmann-Radziemska, E. Degradation of electrical performance of a crystalline photovoltaic module due to dust deposition in northern Poland. Renew. Energy 2015, 78, 418–426. [Google Scholar] [CrossRef]
- Priyadarshini, B.G.; Sharma, A.K. Design of multi-layer anti-reflection coating for terrestrial solar panel glass. Bull. Mater. Sci. 2016, 39, 683–689. [Google Scholar] [CrossRef]
- Borrebæk, P.O.A.; Jelle, B.P.; Zhang, Z. Avoiding snow and ice accretion on building integrated photovoltaics—Challenges, strategies, and opportunities. Sol. Energy Mater. Sol. Cells 2020, 206, 110306. [Google Scholar] [CrossRef]
- Boutamart, M.; Briche, S.; Nouneh, K.; Rafqah, S.; Agzenai, Y. Transparent and Self-Cleaning Surfaces Based on Nanocomposite Sol-Gel Coatings. ChemistrySelect 2020, 5, 8522–8531. [Google Scholar] [CrossRef]
- Tarquini, S.; Antonini, C.; Amirfazli, A.; Marengo, M.; Palacios, J. Investigation of ice shedding properties of superhydrophobic coatings on helicopter blades. Cold Reg. Sci. Technol. 2014, 100, 50–58. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mourad, A.-H.I.; Pervez, H.; Surkatti, R. Recent developments in multifunctional coatings for solar panel applications: A review. Sol. Energy Mater. Sol. Cells 2019, 189, 75–102. [Google Scholar] [CrossRef]
- Alamri, H.; Al-Shahrani, A.; Bovero, E.; Khaldi, T.; Alabedi, G.; Obaid, W.; Al-Taie, I.; Fihri, A. Self-cleaning superhydrophobic epoxy coating based on fibrous silica-coated iron oxide magnetic nanoparticles. J. Colloid Interface Sci. 2018, 513, 349–356. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Sun, W.; Li, S.; Zhu, T.; Liu, W.; Liu, G. Superhydrophobic epoxy coating modified by fluorographene used for anti-corrosion and self-cleaning. Appl. Surf. Sci. 2017, 401, 146–155. [Google Scholar] [CrossRef]
- Roppolo, I.; Shahzad, N.; Sacco, A.; Tresso, E.; Sangermano, M. Multifunctional NIR-reflective and self-cleaning UV-cured coating for solar cell applications based on cycloaliphatic epoxy resin. Prog. Org. Coat. 2014, 77, 458–462. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as icephobic materials—The past, present and the future. Chem. Eng. J. 2021, 405, 127088. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic Coatings Based on Silicon-Oil-Infused Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Wang, H.; He, G.; Tian, Q. Effects of nano-fluorocarbon coating on icing. Appl. Surf. Sci. 2012, 258, 7219–7224. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, M.; Yi, S.; Liu, X.; Li, H.; Huang, C.; Luo, Y.; Li, Y. Preparation and characterization of silica/fluorinated acrylate copolymers hybrid films and the investigation of their icephobicity. Thin Solid Films 2012, 520, 5644–5651. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Hu, J.; Shu, L.; Shi, X. Anti-icing Performance of a Superhydrophobic PDMS/Modified Nano-silica Hybrid Coating for Insulators. J. Adhes. Sci. Technol. 2012, 26, 665–679. [Google Scholar] [CrossRef]
- Kozera, R.; Przybyszewski, B.; Żołyńska, K.; Boczkowska, A.; Sztorch, B.; Przekop, R.E. Hybrid Modification of Unsaturated Polyester Resins to Obtain Hydro- and Icephobic Properties. Processes 2020, 8, 1635. [Google Scholar] [CrossRef]
- Bessonov, I.V.; Kopitsyna, M.N. New Prepregs Based on Unsaturated Polyester Resins Modified with Polysiloxane Oligomers. Int. Polym. Sci. Technol. 2016, 43, 49–56. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Kumar, D.; Ho, J.W.C.; Kanhere, P.D.; Srikanth, N.; Liu, E.; Wilson, P.; Chen, Z. Development of Sol–Gel Icephobic Coatings: Effect of Surface Roughness and Surface Energy. ACS Appl. Mater. Interfaces 2014, 6, 20685–20692. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Li, H.; Song, Y. Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 2011, 7, 3993–4000. [Google Scholar] [CrossRef]
- Wang, C.-F.; Su, Y.-C.; Kuo, S.-W.; Huang, C.-F.; Sheen, Y.-C.; Chang, F.-C. Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef]
- David, C. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar]
- Ibáñez-Ibáñez, P.F.; Ruiz-Cabello, F.J.M.; Cabrerizo-Vílchez, M.A.; Rodríguez-Valverde, M.A. Mechanical Durability of Low Ice Adhesion Polydimethylsiloxane Surfaces. ACS Omega 2022, 7, 20741–20749. [Google Scholar] [CrossRef]
- Ziętkowska, K.; Kozera, R.; Przybyszewski, B.; Boczkowska, A.; Sztorch, B.; Pakuła, D.; Marciniec, B.; Przekop, R.E. Hydro- and Icephobic Properties and Durability of Epoxy Gelcoat Modified with Double-Functionalized Polysiloxanes. Materials 2023, 16, 875. [Google Scholar] [CrossRef]
- Kozera, R.; Przybyszewski, B.; Krawczyk, Z.D.; Boczkowska, A.; Sztorch, B.; Przekop, R.E.; Barbucha, R.; Tański, M.; Garcia-Casas, X.; Borras, A. Hydrophobic and Anti-Icing Behavior of UV-Laser-Treated Polyester Resin-Based Gelcoats. Processes 2020, 8, 1642. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Water and Ice Adhesion to Solid Surfaces: Common and Specific, the Impact of Temperature and Surface Wettability. Coatings 2020, 10, 648. [Google Scholar] [CrossRef]
- He, Z.; Zhuo, Y.; Zhang, Z.; He, J. Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings 2021, 11, 1343. [Google Scholar] [CrossRef]
- He, Z.; Vågenes, E.T.; Delabahan, C.; He, J.; Zhang, Z. Room Temperature Characteristics of Polymer-Based Low Ice Adhesion Surfaces. Sci. Rep. 2017, 7, 42181. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Li, H.; Yuan, X. Preparation and icephobic properties of polymethyltrifluoropropylsiloxane–polyacrylate block copolymers. Appl. Surf. Sci. 2014, 316, 222–231. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Li, M.; Wang, Q.; Chen, Q. The icephobicity comparison of polysiloxane-modified hydrophobic and su-perhydrophobic surfaces under condensing environments. Appl. Surf. Sci. 2016, 385, 472–480. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]


| Sample No. | MOD Type | PHS | Olefin 1 | Olefin 2 | Molar Ratio |
|---|---|---|---|---|---|
| 1 (REF) | - | - | - | - | - |
| 2 | MOD 1/2 wt.% | PHS992 | AGE | OCT | 1:2 |
| 3 | MOD 2/2 wt.% | PHS992 | AGE | OCT | 1:4 |
| 4 | MOD 3/2 wt.% | PHS992 | AGE | HEX | 1:2 |
| 5 | MOD 4/2 wt.% | PHS992 | AGE | HEX | 1:4 |
| Stage | Spin Speed [rpm] | Spin Accel. [rpm/s] | Spin Time [s] |
|---|---|---|---|
| Dispense | 100 | 1000 | 10 |
| Spread | 2000 | 1000 | 20 |
| EBR | 500 | 1000 | 10 |
| Dry | 4000 | 1000 | 40 |
| Sample No. | MOD Type | Core of MOD | Olefin 1 | Olefin 2 | Molar Ratio | Ra [nm] | WCA [°] | CAH [°] | RoA [°] |
|---|---|---|---|---|---|---|---|---|---|
| 1 (REF) | - | - | - | - | 2 ± 0.1 | 80 ± 1 | 22 ± 2 | 75 ± 8 | |
| 2 | MOD1 | PWS992 | AGE | OCT | 01:02 | 12 ± 1.1 | 84 ± 1 | 9 ± 1 | 50 ± 3 |
| 3 | MOD2 | PWS992 | AGE | OCT | 01:04 | 21 + 1.9 | 89 ± 1 | 7 ± 2 | 55 ± 9 |
| 4 | MOD3 | PWS992 | AGE | HEX | 01:02 | 5 + 0.5 | 88 ± 2 | 9 ± 1 | 35 ± 6 |
| 5 | MOD4 | PWS992 | AGE | HEX | 01:04 | 7 + 0.4 | 90 ± 1 | 11 ± 2 | 32 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętkowska, K.; Przybyszewski, B.; Grzęda, D.; Kozera, R.; Boczkowska, A.; Liszewska, M.; Pakuła, D.; Przekop, R.E.; Sztorch, B. Transparent Silicone–Epoxy Coatings with Enhanced Icephobic Properties for Photovoltaic Applications. Appl. Sci. 2023, 13, 7730. https://doi.org/10.3390/app13137730
Ziętkowska K, Przybyszewski B, Grzęda D, Kozera R, Boczkowska A, Liszewska M, Pakuła D, Przekop RE, Sztorch B. Transparent Silicone–Epoxy Coatings with Enhanced Icephobic Properties for Photovoltaic Applications. Applied Sciences. 2023; 13(13):7730. https://doi.org/10.3390/app13137730
Chicago/Turabian StyleZiętkowska, Katarzyna, Bartłomiej Przybyszewski, Dominik Grzęda, Rafał Kozera, Anna Boczkowska, Malwina Liszewska, Daria Pakuła, Robert Edward Przekop, and Bogna Sztorch. 2023. "Transparent Silicone–Epoxy Coatings with Enhanced Icephobic Properties for Photovoltaic Applications" Applied Sciences 13, no. 13: 7730. https://doi.org/10.3390/app13137730
APA StyleZiętkowska, K., Przybyszewski, B., Grzęda, D., Kozera, R., Boczkowska, A., Liszewska, M., Pakuła, D., Przekop, R. E., & Sztorch, B. (2023). Transparent Silicone–Epoxy Coatings with Enhanced Icephobic Properties for Photovoltaic Applications. Applied Sciences, 13(13), 7730. https://doi.org/10.3390/app13137730









