1. Introduction
Infant milk is the first product in a baby’s diet that must be of high-quality to ensure healthy growth and development. Of the two sources of infant milk, the main one is breast milk, recognized as the ideal form of infant feeding, and providing multiple benefits for a child’s health. The promotion, protection, and support of breastfeeding is therefore critical. All organizations worldwide strongly recommend breastfeeding because of its numerous benefits, but it might not always be appropriate, feasible, or adequate for all children [
1]. For infants incapable of breastfeeding, who should not receive breast milk, or for whom breast milk is unavailable, high-quality infant formula milk (IFM) is the other preferred option [
2]. When the quality of IFM is high, it can be an effective substitute for breastfeeding. For the first six months of a child’s life, breastfeeding should be the sole source of nutrition. However, only 38% of infants worldwide are exclusively breastfed, and among Americans, only 13% initiate breastfeeding exclusively for a period not less than six months after birth [
1].
With life demands increasing and women’s need to work, many mothers rely on IFMs to feed their infants directly after delivery. Because IFM is such an essential product, no home that needs it should be without it, especially when infants require food to meet their nutritional needs. Even though a product identical to breast milk cannot be produced, every effort has been made to mimic and replicate the nutrition profile of breast milk to fulfill infants’ needs.
IFM is available in three main formula types: (1) cow milk protein-base, the most common type, widely available, digestible, and modified to mimic breast milk; (2) soy-based version, which is suitable for infants who are allergic to cow milk, lactose, and carbohydrate that is naturally found in cow milk, or for parents who would like to eliminate animal proteins from their child’s diet; and (3) a protein hydrolysate formula used for infants with severe allergies to protein-based formulas and formulas based on soy, in which the protein is partially or entirely hydrolyzed and broken down into smaller pieces to facilitate digestion [
3]. There are also different types of preparation forms for IFMs: (1) Powder. The most common and economical form of infant formula that must be mixed with water before feeding; (2) Liquid. A concentrated liquid that must be mixed with an equal amount of water; and (3) Ready-to-feed. The most expensive form of infant formula that does not require mixing [
1].
IFM has faced many issues throughout its history and has been a target for smugglers and those seeking to make illegal high profits due to its critical nature. In October 2008, the world witnessed the worst case of a Chinese scandal involving IFM trading. A substance known as melamine was illegally added to milk to deceptively increase its purported protein concentration, as assessed by nitrogen measurement, and appears to meet the national standard for milk protein. The fraud adversely affected approximately 294,000 children. Around 52,000 infants were hospitalized due to melamine-related urinary stones (MUS), and at least six died [
4]. In February 2022, Abbott Laboratories issued a massive recall because some versions of Similac, Alimentum, and EleCare baby formula contained Salmonella Newport and Cronobacter Sakazakii bacteria. Laboratory reports confirmed that IFMs were contaminated, and the U.S. Food and Drug Administration (FDA) found Abbott failed to maintain sanitary conditions procedures at the Michigan production plant [
5].
Infant formula milk supply chain (IFMSC) actors are crucial to ensure IFM quality and safety, particularly raw material suppliers and manufacturers. They must continue improving their performance to meet the community’s demands per the agreed-upon standards. The specific guidelines outlined in the US FDA’s current Good Manufacturing Practices rules require that formulas must satisfy the quality factors of normal physical growth and adequate biological quality of protein components.
To evaluate the performance measurement of IFM in the supply chain, this study aimed to identify the most critical risks that can affect IFM quality, focus on the essential criteria and mitigate them appropriately. This research has identified a gap in the literature on overcoming the concerns and challenges in the IFMSC to ensure its quality that has never been addressed. The current study aimed to develop a model that maximizes IFM safety and eliminates adulteration and contamination rates. Using the analytic hierarchy process (AHP) model, mitigation strategies were ranked and prioritized based on expert feedback.
The steps that will help in achieving the aim of this study include the following:
Identifying the importance of IFMs for infant nutrition and the risks they pose;
Determining the mitigation criteria for evaluating the IFMSC’s performance to maximize its quality;
Analyzing the priorities and importance of each mitigation criterion to maximize IFM safety.
There are five sections in this study.
Section 1 presents the articles, the background, the problem statement, and the aim and objectives of the current study. In
Section 2, we review the literature. In
Section 3, we describe the methods used to collect the data for the study. This description includes the research approach, design, data collection method, research philosophy, and data analysis method. The findings of the research are presented in
Section 4. A triangulation process is also used to ensure that findings and results are aligned with the literature from relevant studies.
Section 5 is the last part of the study, which presents the research’s conclusion, implications, and recommendations.
2. Literature Review
This section presents the facts about IFMs, the well-known issues that IFMs suffer, including adulteration and microbial contamination, and the importance of regular quality control of infant milk. The second sub-section discusses risk factors affecting the safety and quality of IFMs and their important classification from the literature. Finally, the section presents a framework with key points to facilitate assessing and managing IFM risks listing the mitigation criteria.
2.1. Literature Identification and Collection
The literature identification and collection phase comprised three steps, as illustrated in
Figure 1.
Step 1: Define the preliminary outline.
An outline for the paper was created by exploring the issues associated with IFM, identifying the associated risk factors, and identifying areas to be discussed to ensure that IFM is safe for consumption. The dimensions of IFMSC risk factors were determined by reviewing various sources and articles and adequately using the recurrent concepts and phases of the IFM in different research papers. The risk factors were categorized into three categories: environmental, operational, and quality.
Step 2: Define the academic sources.
Library search engines such as Google Scholar, Researchgate, Science Direct, Scopus, and Springer Link were used to conduct the literature search. Book chapters were also used to enhance the search results and add more value to the review. The use of all these search engines provided a diversified knowledge base with a range of relevant concepts, as well as enhanced recognition of risk factors and mitigation strategies.
Step 3: Identify the keywords.
We focused our search on secondary sources published in English only, since primary sources were insufficient. Different keywords covering the objectives of the review were identified.
The keywords were categorized into two areas. The first area looked into the issues with infant formula milk and the risk factors, while the other focused on risk mitigation criteria for ensuring safe infant formula milk. Different search strings were used with different combinations of keywords, such as “infant formula milk AND milk quality AND infant formula milk supply chain”, “supply chain risk (OR risks) AND risk mitigation”.
2.2. Facts and Issues of IFM
Food safety and high-quality is one pillar of public health that ensures a healthy community. In 1939, Cicely Williams raised the issue of low-income countries using IFMs as a health risk when she spoke on Milk and Murder, and concerns over IFMs increased in 1974 after the publication of The Baby Killer report [
6]. Adulteration, contamination, and other IFM issues are certainly not limited to low-income countries; they are common, and cases were discovered in high-income countries too. A recent example is Abbott Laboratories’ massive 2022 recall of IFMs due to Salmonella Newport and
Cronobacter sakazakii bacteria, also known as
Enterobacter sakazakii (E.S.).
It can be argued that IFMs are more associated with biological food safety issues, such as parasites, pathogenic bacteria such as Salmonella or pathogenic
Escherichia coli, also known as
E. coli, and foodborne viruses such as Norovirus that cause vomiting and diarrhea [
7]. In addition, Bacillus cereus contamination in IFMs has been well-documented in past literature [
8,
9]. IFM, whether in liquid or powder form, is an ideal medium for bacterial growth that poses a potential risk of foodborne illness [
10]. Consequently, such pathogens are more likely to infect babies and infants than adults because their immune systems are less developed, and their intestinal flora is less competitive [
11]. Several factors determine the microflora of dried milk powders, including raw milk or milk by-products, preheating temperature, operating conditions, evaporator and/or dryer, and plant hygiene [
12].
Food adulteration poses several food safety concerns. These issues affect IFM, including incidents such as the 2008 China milk scandal, where melamine was added to milk to appear more protein-rich. Melamine is a triazine compound with the formula C
3H
6N
6 used to manufacture household utensils and ornaments [
13]. Melamine is a component of flame retardants, glues, and plastics but is prohibited for human or animal consumption [
14]. Nevertheless, it has been used illegally as an additive to food such as rice [
15] and IFMs [
16]. As a legal ingredient in packaging materials and feeds for poultry and livestock, melamine can be present but at low levels.
It could also be a metabolite of the cyromazine pesticide [
17]. It has harmful effects on infants, melamine can retard their growth, cause urinary stones, and damage their kidneys. Children exposed to this kind of toxicity at an early age may suffer uncertain long-term effects [
18]. Therefore, milk safety regulators should focus their monitoring resources on supervising milk sold in reputedly trustworthy stores and not allow exemptions from inspections [
19].
There is limited potential for regulating the IFM industry globally, but improvements can be made. Global regulation must be based on a negotiated consensus among nations. These regulations are not intended to set forth detailed, binding requirements for these nations but to define fundamental principles that national governments will follow and implement through their national laws. Therefore, a global governance system exists even sans a global government [
6]. In addition, IFM producers and distributors must adhere to a high level of microbiological quality control while producing, distributing, and using IFM for newborn infants. Several extrinsic indicators can be used to determine a product’s safety, including venue, brand, and company names or certifications concerning safety. Producers, processors, retailers, or safety supervisors often attach indicators like these during food production and supply processes rather than integrating them into physical structures. A consumer’s confidence in extrinsic indicators heavily depends on their credibility as safety indicators [
20]. IFM must be prepared with good hygienic procedures, quickly cooled, and consumed as soon as possible to minimize the risk of these adulterations and microbial contamination [
21].
2.3. Risk Factors of IFM
Risk is the possibility of something disrupting normal operations or activities, representing the uncertainties associated with a given activity [
22]. William Kannel, one of the pioneers of the Framingham Heart Study, coined the term “risk factor” despite the concept being widely discussed and applied. It was first used in medical literature in 1961 [
23]. For the risks to be sufficient to generate a crisis, they, if left unattended, can become a catastrophe [
24].
Various scholars have defined supply chain risk (SCR) differently, and no consensus exists on what it means [
25,
26]. According to Kumar et al., an SCR is any deviation from the primary goal that may lead to an overall reduction in the value-added process [
27]. As Zsidisin [
28] defined it, risk in supply chains occurs when an incident in inbound supply occurs due to supplier or supply market failures, which results in the purchasing firm being unable to meet customer demands or poses an extreme threat to customer safety. Disruptive and operational risks are the two main types of risk categorized by Kern et al. [
29]. An example of operational risk is a failure that can cause supply demand inconsistency [
30]. Operational risks include malfunctioning equipment, failures of supplies, and strategy failures. Disruptive risks, however, are caused by human-made or natural catastrophes, such as terrorist attacks and natural disasters [
31]. Unlike operational risks, disruptive risks are harder to control. According to Jüttner et al. [
32], there are two types of risks: internal ones within a firm’s supply chain and external ones in its environment.
Regarding safety and quality, IFM is an essential product for infants. Hence, it is linked to public health safety and community well-being [
33]. Risks imposed by crucial products are higher and must be assessed and managed more meticulously. The dimensions of IFMSC risk factors were identified from the literature and classified into three main points, environmental, operational, and quality, based on the model proposed by Liu et al. [
34], as illustrated in
Figure 2. Explicit and legible correlations between environmental, operational, and product quality information can be used to achieve supply chain risk management (SCRM) [
35].
2.3.1. Environmental Risks
Environmental risks connect the environment to human health. These risks comprise those impacting the whole supply chain network or those affecting a specific supply chain stage. In addition to pollution, radiation, noise, land use patterns, and uncontrollable external factors, climate change also falls under this category [
36]. Ultimately, the environmental efficiency of any food in the supply chain is influenced dramatically by the downstream processes, including the distribution of products by various channels. Choosing efficient environmental strategies and adopting appropriate approaches are critical to improving food quality systems [
37]. Therefore, identifying environmental risk factors is crucial to public health decision-making [
38,
39,
40,
41,
42,
43].
2.3.2. Operational Risks
Operational risk is the risk of losses resulting from flawed processes, policies, systems, or events that disrupt business operations. Several factors can trigger operational risk, including employee errors, criminal activity, and physical events [
44]. It is crucial to understand and monitor the underlying issues that can affect the performance of any process in the supply chain as they affect the continuity of a business process. This vital performance indicator contributes to the perceived quality of service delivery. Thus, identifying and reducing operational risks in a supply chain can ensure business success, improving the product’s operational efficiency [
45]. Operational risks can also impede the monitoring of flawed product recalls due to quality standards breaches [
46]. Azizsafaei et al. [
37] stated that operational risks arise from day-to-day activities, systems, processes, and people, resulting in damaged products or delivery delays. It is even more dangerous if no traceability technology tracks the product [
47,
48].
2.3.3. Quality Risks
Identifying and analyzing quality information is important for certifying a product’s compliance with its quality conditions. Furthermore, all products have quality information that can be used directly to identify and assess risks [
35]. Information on quality involves time series conditions and dynamic risk analysis, which can cumulatively affect the entire supply chain [
47].
As shown in
Table 1, 160 risks were identified from the literature, repeated risks were removed, risks with the same meaning were merged, and then categorized into predefined dimensions. For the final analysis, 50 risks were selected.
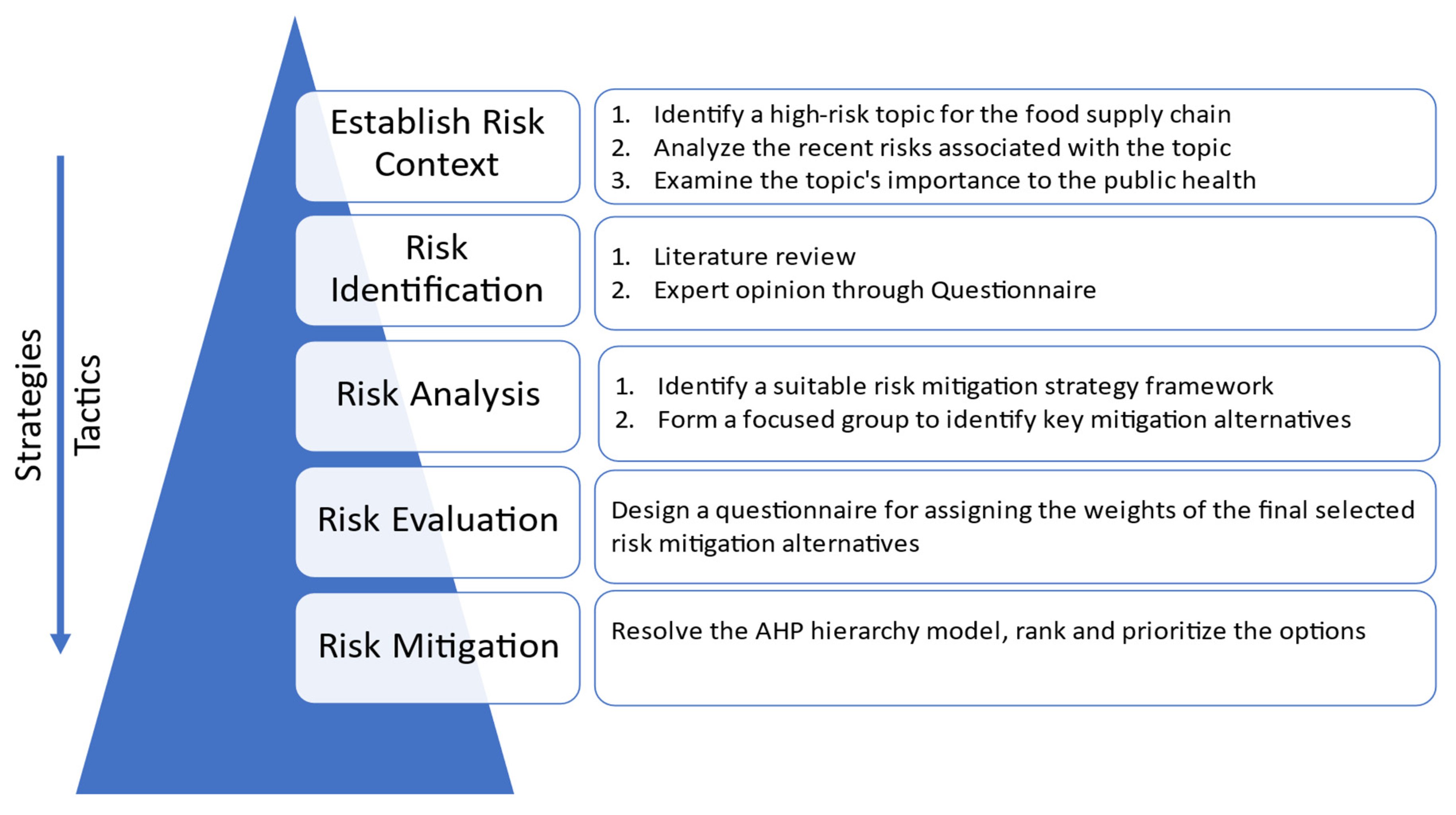
2.4. SCRM Process
Properly understanding SCRM begins with understanding the different processes that must be followed. Even though different studies propose different frameworks for the processes [
54,
55,
56,
57,
58], most agree with the steps illustrated in
Figure 3.
2.4.1. Risk Identification
For SCRM, identifying risks is the first and most critical step to understanding and addressing the threat level appropriately. The process involves identifying risk types, factors, or combinations [
26].
2.4.2. Risk Analysis
A risk analysis is one aspect of the overall risk management plan, built on risk assessment. The probability that an event will occur, and the significance of the consequences are related to this process. In this step, the likelihood and importance of each risk are to be determined, and then a score is assigned to each risk [
59].
2.4.3. Risk Evaluation
An evaluation of risk determines how serious a risk is. There are qualitative and quantitative ways to assess risks. The best way to evaluate risk is to investigate each activity related to a specific risk thoroughly, check out the activities’ efficiency, and discover the flaws in their implementation [
60]. This process allows a systematic analysis of the whole SCR to be conducted. Risk assessment and analysis are prerequisites to evaluating risk.
2.4.4. Risk Mitigation
An essential goal of risk mitigation is to minimize the impact of potential risks by implementing a plan to manage, eliminate, or limit setbacks [
61]. Risk mitigation involves reducing risks to a level the supply chain organization can tolerate or accept [
62]. In addition, the risk can also undergo treatment by implementing risk modification measures.
2.5. Mitigation Criteria for IFM
A supply chain risk management tool focuses on managing products, services, and their lifecycles effectively [
63]. A major part of this act entails assessing and mitigating risks to ensure that users are safe. Risk mitigation or optimization aims to reduce the severity or likelihood of a loss [
64]. It is nevertheless essential to implement comprehensive risk management, including identifying, assessing, treating, and monitoring supply chain risks. The objectives are to reduce vulnerability, ensure continuity, and provide profitability, resulting in competitive advantage, by implementing internal tools, techniques, and strategies and coordinating and collaborating externally with supply chain members [
65]. Having gained a better understanding of how other supply chain elements affect risk susceptibility, organizations have begun to focus on managing supply chain risk [
62]. Various uncertainties make risk reduction critical for ensuring effective supply chain operations. The risk must exist and be identified first to implement mitigation strategies effectively. From the literature, a framework of key mitigation strategy factors was identified. The framework was formulated based on Brown’s [
66] five mitigation strategies.
Figure 4 depicts the key strategies for risk mitigation.
The following is a detailed explanation of each key mitigation strategy factor used in the framework.
Accepting the risk: To accept the risk, supply chain members and stakeholders collaborate to analyze every risk, so all members know the risks associated with the critical product in the supply chain and their implications beforehand. After that, each risk is defined in terms of its consequences so the members can determine which risks are acceptable. Cost, schedule, and performance risks are the major ones.
Avoiding the risk: It is possible to apply this strategy to the accepted risk by taking preventative measures to prevent the risk from occurring in the first place.
Controlling the risk: Whenever the accepted risks cannot be avoided, supply chain members can devise an action plan to minimize or remove their impact.
Transferring the risk: This involves sharing or handing over some of the responsibility for the risk and its consequences to another party to eliminate their effects.
Monitoring the risk: The risks may have changed since they were identified, so observing them and keeping an eye out for any new risks that might come up is necessary to avoid unexpected consequences.
5. Conclusions
Nowadays, food quality is increasingly a focus of our society due to the evolution of science and advancements in modern technology. Several food quality incidents have recently occurred, negatively impacting suppliers’ reputations and consumers’ interests and significantly impacting public health. Taking special care regarding infant formula milk (IFM) is crucial because we are dealing with newborns and babies. Infants are more likely to become infected with foodborne pathogenic bacteria due to their immature immune systems and the permeability of their digestive tracts.
The IFM has been a target of smugglers and those looking to make illegal high profits, like the Chinese scandal in 2008 when melamine was added to deceive consumers by increasing the white color to look like concentrated protein. In addition, Abbott Laboratories issued a massive recall in February of 2022 after acknowledging that some versions of Similac, Alimentum, and EleCare baby formula contained Salmonella Newport and Cronobacter Sakazakii bacteria. A lab report confirmed that IFMs were contaminated, and the production facility failed to maintain sanitary conditions.
Food safety involves several links between the actors of the infant formula milk supply chains (IFMSCs), including but not limited to the suppliers of raw materials, manufacturers, transporters and distributors, and standards of storage facilities to ensure adherence to quality [
74]. The authors of the current research identified a gap in the literature on how to overcome the concerns and challenges in the IFMSCs to ensure the quality of IFM. Therefore, this study aimed to evaluate the performance measurement of IFM in the supply chain by identifying the most critical risks that can affect IFM quality, emphasizing the essential criteria, and mitigating them appropriately to eliminate the rate of adulteration and contamination. As part of mitigating risks, specific steps were taken to understand the different processes for supply chain risk mitigation (SCRM), starting with identifying the risk, analyzing it, evaluating it, and finally, eliminating or limiting setbacks. The research employed the triangulation paradigm (literature analysis, surveys, and interviews) to ensure that qualitative and quantitative tools were combined effectively, thus improving the reliability of data collection and verification. A predefined environmental, operational, and product quality information dimension was used to identify IFMSC risk factors. The predefined dimensions were also used to categorize 50 of 160 risks.
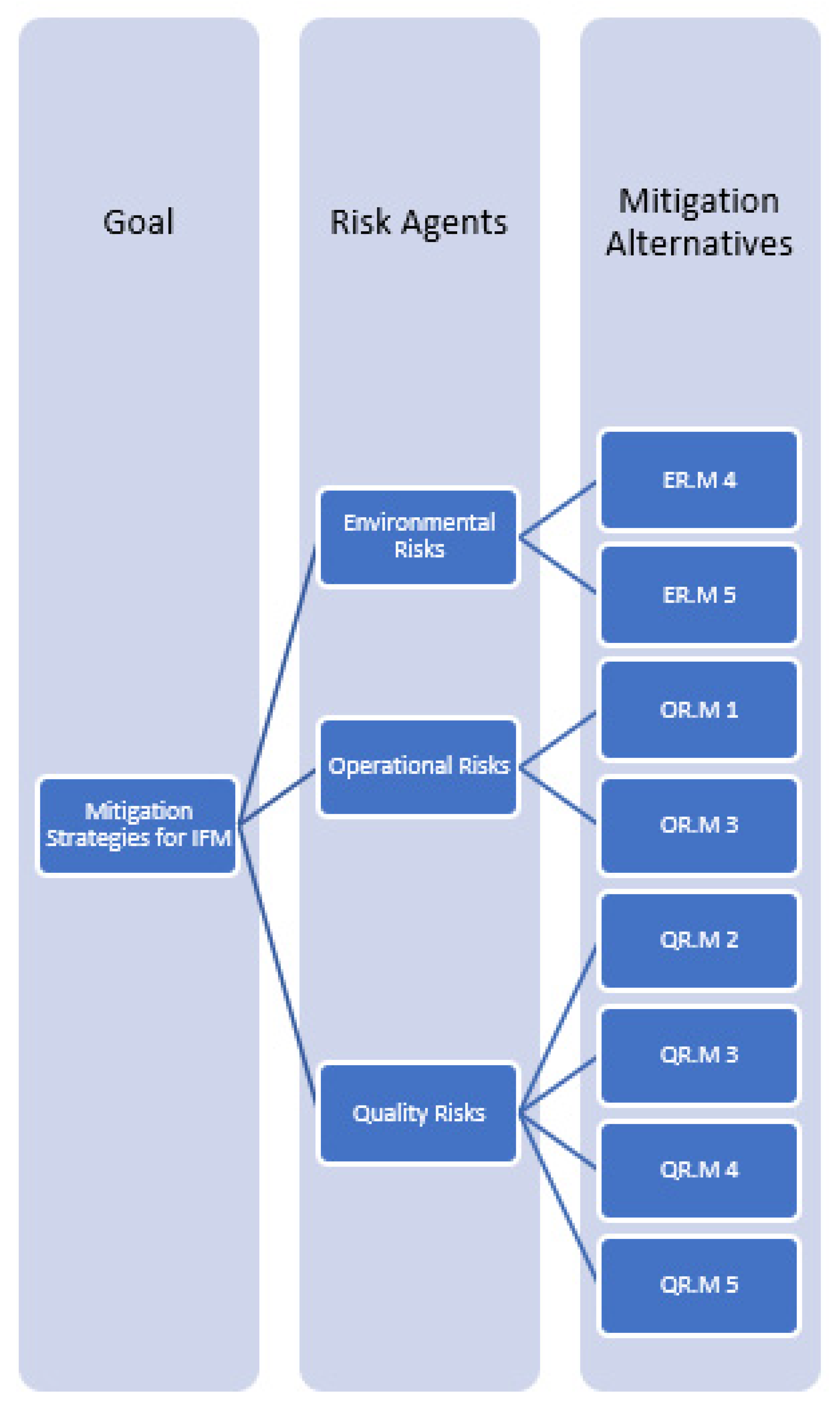
According to the survey questionnaire results, three risks were classified as crisis issues and selected, including “Lack of assessment of environmental and natural disasters”, “Lack of visibility and potential traceability of products”, and “Contamination (biochemical/microbial)”. For risk analysis, expert feedback was required on the mitigation strategies for each of the five major mitigation strategy factors (acceptance, avoidance, controlling, transference, and monitoring).
An analytic hierarchy process (AHP) model was developed to prioritize mitigation strategies for the IFMSC based on expert feedback to maximize safety. To maximize the quality of the IFM, QR.M2 was ranked highest and most important, based on an avoidance strategy from the five key mitigation strategies. It makes sense, particularly when we are discussing a product for infants. Accepting, controlling, transferring, or monitoring our risks is inappropriate in this situation. Public health, especially infant health, depends on avoiding risks, and IFM must undergo precise testing and quality checks at every stage of the supply chain to ensure its quality. Although IFMs require extensive quality control throughout the food supply chain (FSC) before they are delivered to consumers, finding an equilibrium point between the centralized and the decentralized models without giving a single authority point to any part of the supply chain is imperative. The decentralized supply chain model distributes the supply chain’s activities from a central authority and gives equal control to all stakeholders. As a result, a semi-decentralized model could be enhanced by using traceability technology as a decentralized control and avoiding giving one authority point to a specific stakeholder or part of the chain, thus, allowing them to collaborate and work effectively even in the absence of trust [
48,
51].
The causes and impacts of each risk could be studied in future research systematically for each phase of the IFSC. Further analysis of the costs and benefits of implementing technologies in the IFMSC might provide insight into the importance of the support gained through their implementation. It is possible to apply other risk identification methods to see if the same results can be achieved and to compare the differences.