Characterization of Freisa Wines from Piedmont (Italy) by Aroma and Element Profile
Abstract
:1. Introduction
2. Materials and Methods
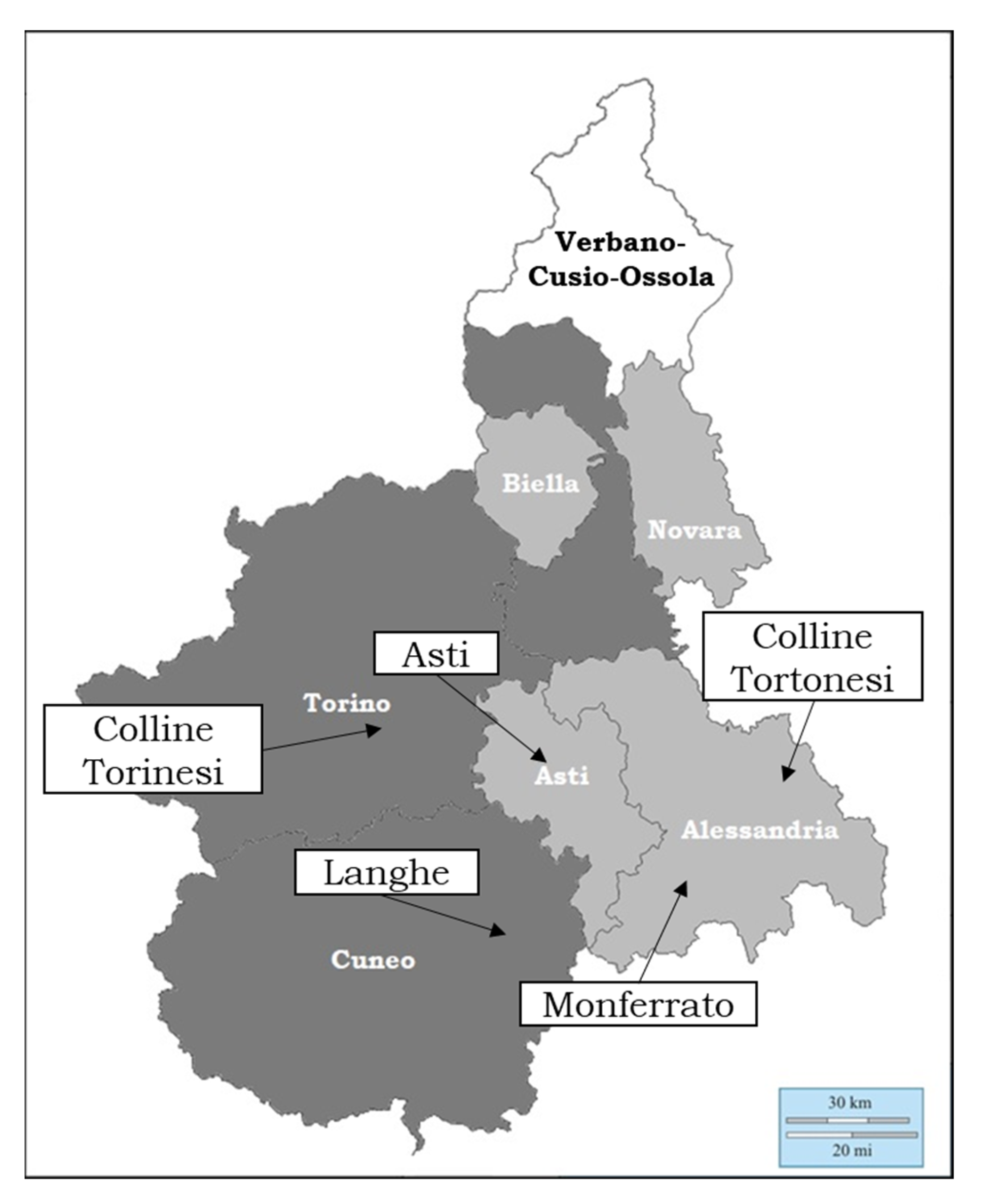
2.1. Samples and Chemicals
2.2. Solid-Phase Microextraction
2.3. GC-MS
2.4. Acid Digestion
2.5. ICP-OES
2.6. Statistical Data Treatment
3. Results and Discussion
3.1. Volatile Compounds
3.2. Major, Minor and Trace Elements
3.3. Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerbi, V.; Rolle, L.; Zeppa, G.; Guidoni, S.; Schneider, A. Indagine sul profilo antocianico di vitigni autoctoni piemontesi. Ind. Delle Bevande 2005, 34, 23–27. [Google Scholar]
- Rolle, L.; Caudana, A.; Gerbi, V. Tecniche di vinificazione per la valorizzazione del vitigno Freisa. In Proceedings of the 31st Congresso Mondiale Della Vite e Del Vino—VI Assemblea Generale dell’OIV, Verona, Italy, 15–20 June 2008. [Google Scholar]
- Rolle, L.; Guidoni, S. Color and anthocyanin evaluation of red winegrapes by CIE L*, a*, b* parameters. J. Int. Sci. Vigne Vin. 2007, 41, 193–201. [Google Scholar] [CrossRef]
- Schneider, A.; Boccacci, P.; Torello Marinoni, D.; Botta, R.; Akkak, A.; Vouillamoz, J. The genetic variability and unexpected parentage of “Nebbiolo”. In Proceedings of the First International Conference on “Nebbiolo” Grapes, Sondrio, Italy, 24 January 2004. [Google Scholar]
- Lacombe, T.; Boursiquot, J.M.; Laucou, V.; Di Vecchi-Staraz, M.; Péros, J.P.; This, P. Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef]
- Ossola, C.; Giacosa, S.; Rìo Segade, S.; Gerbi, V.; Rolle, L. Investigation on ‘Freisa’ red grape variety: Physico-chemical properties of grapes from five Piedmont growing areas and of the produced wines. Ital. J. Food Sci. 2019, 31, 685–704. [Google Scholar] [CrossRef]
- Pawliszyn, J. Applications of Solid Phase Microextraction; The Royal Society of Chemistry: Cambridge, UK, 1999; 655p. [Google Scholar]
- Pohl, P. What do metals tell us about wine? TrAC 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Einax, W.; Zwanziger, H.W.; Gei, S. Chemometrics in Environmental Analysis; Wiley-VHC: Weinhem, Germany, 1997; 404p. [Google Scholar]
- Massart, D.L.; Vandenginste, B.G.M.; Buydens, L.M.C.; De Jono, S.; Leqi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics, Parts A and B; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agr. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Avellone, G.; Salvo, A.; Costa, R.; Saija, E.; Bongiorno, D.; Di Stefano, V.; Calabrese, G.; Dugo, G. Investigation on the influence of spray-drying technology on the quality of Sicilian Nero d’Avola wines. Food Chem. 2018, 240, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Bonikowski, R.; Switakowska, P.; Kula, J. Synthesis, odour evaluation and antimicrobial activity of some geranyl acetone and nerolidol analogues. Flavour Fragr. J. 2015, 30, 238–244. [Google Scholar] [CrossRef]
- Guarrera, N.; Sperlinga, E.; Passerini, A.; Maccarone, E. Evaluation of the aromatic and polyphenolic composition of cola and cola gelato apples grown in the area of the Etna volcano. Ital. J. Food Sci. 2008, 20, 351–364. [Google Scholar]
- Tat, L.; Comuzzo, P.; Battistutta, F.; Zironi, R. Sweet-like off-flavor in Aglianico del Vulture wine: Ethyl phenylacetate as the mainly involved compound. J. Agric. Food Chem. 2007, 55, 5205–5212. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, W.; Yu, H.; Yuan, J.; Tian, H. Evaluation of the perceptual interactions among aldehydes in a Cheddar cheese matrix according to odor threshold and aroma intensity. Molecules 2020, 25, 4308–4323. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, Q.; Xiao, L.; Li, R.; Peng, C.; Liao, X.; Yan, J.; Liu, H.; Xie, G.; Ho, C.-T.; et al. Characterization of aroma compounds of Pu-erh ripen tea using solvent assisted flavor evaporation coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Sci. Hum. Wellness 2022, 11, 618–626. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor potency of aroma compounds in Riesling and Vidal blanc table wines and icewines by gas chromatog-raphy−olfactometry−mass spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Voce, S.; Škrab, D.; Vrhovsek, U.; Battistutta, F.; Comuzzo, P.; Sivilotti, P. Compositional characterization of commercial sparkling wines from cv. Ribolla Gialla produced in Friuli Venezia Giulia. Eur. Food Res. Technol. 2019, 245, 2279–2292. [Google Scholar] [CrossRef]
- Marais, J. Wine aroma composition. Food Rev. 1991, Dec90/Jan91, 18–21. [Google Scholar]
- Barbera, D.; Avellone, G.; Filizzola, F.; Monte, L.G.; Catanzaro, P.; Agozzino, P. Determination of terpene alcohols in Sicilian Muscat wines by HS-SPME-GC-MS. Nat. Prod. Res. 2013, 27, 541–547. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and other volatile compounds. In Handbook of Enology: The Chemistry of Wine. Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 51–64. [Google Scholar]
- Panighel, A.; Flamini, R. Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine R. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Piggott, J.R.; Findlay, A.J.F. Detection thresholds of ester mixtures. In Proceedings of the Flavour Research of Alcoholic Beverages (Foundation for Biotechnical and Industrial Fermentation Research: Helsinki), Helsinki, Finland, 13–15 June 1984; pp. 189–197. [Google Scholar]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds in wine. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, DC, USA, 18–19 June 1991; pp. 156–164. [Google Scholar]
- Liu, J.; Zhao, W.; Li, S.; Zhang, A.; Zhang, Y.; Liu, S. Characterization of the Key Aroma Compounds in Proso MilletWine Using Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Molecules 2018, 23, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumes, R. Wine Aroma Precursors. In Wine Chemistry and Biochemistry; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer Science + Business Media, LLC.: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]
- Winterhalter, P.; Rouseff, R.L. Carotenoid-Derived Aroma Compounds; ACS Symposium Series 802; American Chemical Society: Washington, DC, USA, 2002. [Google Scholar]
- Marengo, E.; Aceto, M. Statistical investigation of the differences in the distribution of metals in Nebbiolo-based wines. Food Chem. 2003, 81, 621–630. [Google Scholar] [CrossRef]
- Bonino, M.; Schellino, R.; Rizzi, C.; Aigotti, R.; Delfini, C.; Baiocchi, C. Aroma compounds of an Italian wine (Ruche’) by HS–SPME analysis coupled with GC–ITMS. Food Chem. 2003, 80, 125–133. [Google Scholar] [CrossRef]
- Kment, P.; Mihaljevic, M.; Ettler, V.; Sebek, O.; Strnad, L.; Rohlova, L. Differentiation of Czech wines using multielement composition—A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Lara, R.; Cerutti, S.; Salonia, J.A.; Olsina, R.A.; Martinez, L.D. Trace element determination of Argentine wines using ETAAS and USN-ICP-OES. Food Chem. Toxicol. 2005, 43, 293–297. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of wine phenolics: A critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Italian Republic. Limiti di Alcuni Componenti Contenuti Nei Vini, in Applicazione Dell’articolo 25 Della legge 12 Dicembre 2016, No. 238; Italian Official Gazette: Roma, Italy, 2017. [Google Scholar]
- Bonifacio, E.; Falsone, G.; Piazza, S. Linking Ni and Cr concentrations to soil mineralogy: Does it help to assess metal contamination when the natural background is high? J. Soils Sediments 2010, 10, 1475–1486. [Google Scholar] [CrossRef]
- Padoan, E.; Malandrino, M.; Giacomino, A.; Grosa, M.; Lollobrigida, F.; Martini, S.; Abollino, O. Spatial distribution and potential sources of trace elements in PM10 monitored in urban and rural sites of Piedmont Region. Chemosphere 2016, 145, 495–507. [Google Scholar] [CrossRef]
- Ziegler, D.; Malandrino, M.; Barolo, C.; Adami, G.; Sacco, M.; Pitasi, F.; Abollino, O.; Giacomino, A. Influence of start-up phase of an incinerator on inorganic composition and lead isotope ratios of the atmospheric PM10. Chemosphere 2021, 266, 129091. [Google Scholar] [CrossRef]
- Bronzi, B.; Brilli, C.; Beone, G.M.; Fontanella, M.C.; Ballabio, D.; Todeschini, R.; Consonni, V.; Grisoni, F.; Parri, F.; Buscema, M. Geographical identification of Chianti red wine based on ICP-MS element composition. Food Chem. 2020, 315, 126248. [Google Scholar] [CrossRef] [PubMed]
- Korenovska, M.; Suhaj, M. Identification of some Slovakian and European wines origin by the use of factor analysis of elemental data. Eur. Food Res. Technol. 2005, 221, 550–558. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Caruso, M.; Scarpa, T.; Palma, A. Analysis of trace elements in southern Italian wines and their classification according to provenance. LWT Food Sci. Technol. 2008, 41, 1808–1815. [Google Scholar] [CrossRef]
- Diaz, C.; Conde, J.E.; Estevez, D.; Perez Olivero, S.J.; Perez Trujillo, J.P. Application of Multivariate Analysis and Artificial Neural Networks for the Differentiation of Red Wines from the Canary Islands According to the Island of Origin. J. Agric. Food Chem. 2003, 51, 4303–4307. [Google Scholar] [CrossRef] [PubMed]




| Compound (μg/L) | Odor Threshold Value | Concentration OAV | ||||
|---|---|---|---|---|---|---|
| Asti (FA) | Chieri (FC) | Colli Tortonesi (CTF) | Langhe (LF) | Monferrato (MF) | ||
| Varietal Compounds | ||||||
| Terpenes | ||||||
| Citronellol | 100 b | 1.97 ± 3.16 0.0197 | 3.06 ± 2.11 0.0306 | 3.11 ± 1.00 0.0311 | 3.55 ± 1.20 0.0355 | 3.74 ± 1.80 0.0374 |
| Limonene | 200 c | 4.93 ± 6.10 0.0246 | 7.95 ± 11.8 0.0398 | 2.16 ± 3.36 0.0108 | 5.70 ± 7.41 0.0285 | 0.72 ± 2.16 0.0036 |
| Linalool | 25 c | 6.84 ± 6.22 0.274 | 5.20 ± 6.15 0.208 | 3.93 ± 4.34 0.157 | 7.92 ± 4.15 0.317 | 7.67 ± 4.93 0.307 |
| Nerolidol | 0.1 d | 0.28 ± 0.83 2.8 | 1.47 ± 2.07 14.7 | 0.67 ± 1.37 6.7 | 0.13 ± 0.33 1.3 | 0.33 ± 0.98 3.3 |
| β-Farnesene | 0.16 e | 0.10 ± 0.42 0.625 | 0.26 ± 0.85 1.625 | 0.66 ± 1.62 4.125 | 0.11 ± 0.41 0.688 | 0.24 ± 0.72 1.5 |
| Total concentration | 14.12 | 17.94 | 10.53 | 17.41 | 12.7 | |
| C6 compounds | ||||||
| 1-hexanol | 8000 c | 0 | 7.90 ± 15.3 0.000988 | 9.14 ± 8.38 0.00114 | 4.56 ± 8.79 0.00057 | 11.0 ± 22.6 0.00138 |
| Fermentative compounds | ||||||
| Esters | ||||||
| 1-Butanol, 3-methyl-, acetate | 30 c | 87.2 ± 81.8 2.91 | 96.5 ± 64.1 3.22 | 45.3 ± 17.5 1.51 | 55.7 ± 31.1 1.86 | 35.2 ± 16.4 1.17 |
| Acetic acid, 2-phenylethyl ester | 250 c | 37.6 ± 55.7 0.15 | 24.2 ± 17.1 0.0968 | 21.3 ± 6.73 0.0852 | 19.5 ± 8.33 0.078 | 24.8 ± 5.20 0.0992 |
| Acetic acid, hexyl ester | 1500 c | 7.33 ± 14.1 0.00489 | 3.67 ± 8.01 0.00245 | 0.22 ± 0.55 0.000147 | 0.52 ± 1.02 0.000347 | 0.27 ± 0.82 0.00018 |
| Benzeneacetic acid, ethyl ester | 73 f | 7.88 ± 6.28 0.108 | 7.95 ± 8.97 0.109 | 7.52 ± 0.84 0.103 | 7.95 ± 2.39 0.109 | 7.92 ± 2.41 0.108 |
| Butanedioic acid, diethyl ester | 200,000 c | 518 ± 321 0.00259 | 639 ± 322 0.00320 | 513 ± 238 0.00256 | 591 ± 194 0.00296 | 584 ± 211 0.00292 |
| Butanoic acid, ethyl ester | 1600 c | 0.80 ± 0.99 0.0005 | 1.35 ± 1.84 0.000844 | 1.42 ± 1.30 0.000888 | 0.97 ± 0.91 0.000606 | 0.33 ± 0.50 0.000206 |
| Decanoic acid, ethyl ester | 200 c | 47.3 ± 37.7 0.236 | 76.3 ± 65.1 0.382 | 73.0 ± 46.4 0.365 | 36.7 ± 23.4 0.184 | 64.3 ± 32.3 0.322 |
| Dodecanoic acid, ethyl ester | 1500 c | 3.02 ± 2.04 0.00201 | 4.36 ± 3.90 0.00291 | 3.92 ± 3.29 0.00261 | 2.92 ± 1.97 0.00195 | 8.74 ± 10.1 0.00583 |
| Ethyl 9-decanoate | 100 c | 0 | 0.99 ± 1.99 0.0099 | 2.36 ± 2.89 0.0236 | 1.17 ± 2.85 0.0117 | 1.16 ± 3.48 0.0116 |
| Ethyl acetate | 7500 c | 39.4 ± 24.2 0.00525 | 23.2 ± 34.2 0.00309 | 46.3 ± 16 0.00617 | 52.3 ± 17.7 0.00697 | 42.2 ± 13.3 0.00563 |
| Hexadecanoic acid, ethyl ester (ethyl palmitate) | 1500 c | 1.91 ± 1.36 0.00127 | 2.07 ± 1.70 0.00138 | 1.44 ± 0.81 0.00096 | 2.65 ± 2.03 0.00177 | 4.25 ± 3.41 0.00283 |
| Nonanoic acid, ethyl ester | N/A | 1.19 ± 2.03 | 1.82 ± 2.42 | 6.09 ± 4.50 | 2.20 ± 2.68 | 7.67 ± 4.08 |
| Octanoic acid, 3-methylbutyl ester (isoamyl octanoate) | N/A | 2.8 ± 10.6 | 0.54 ± 1.79 | 0.72 ± 1.75 | 1.06 ± 2.14 | 0.59 ± 1.77 |
| Octanoic acid, ethyl ester | 5 c | 313 ± 194 62.6 | 400 ± 278 80 | 292 ± 162 58.4 | 220 ± 101 44 | 310 ± 114 62 |
| Pentadecanoic acid, 3-methylbutyl ester (isoamyl pentadecanoate) | N/A | 0 | 0.044 ± 0.14 | 0 | 0.12 ± 0.30 | 0.37 ± 1.11 |
| Tetradecanoic acid, ethyl ester (ethyl myristate) | 2000 c | 1.39 ± 1.25 0.000695 | 1.56 ± 1.90 0.00078 | 1.04 ± 0.96 0.00052 | 1.42 ± 1.15 0.00071 | 5.03 ± 8.10 0.00252 |
| Total concentration | 1068 | 1283 | 1015 | 996 | 1097 | |
| Fusel and higher alcohol | ||||||
| 1-Dodecanol | 1000 c | 5.00 ± 5.14 0.005 | 3.35 ± 4.16 0.00335 | 2.70 ± 1.41 0.0027 | 4.86 ± 3.69 0.00486 | 9.10 ± 9.16 0.0091 |
| 1-Heptanol | 300 c | 1.08 ± 2.36 0.0036 | 3.04 ± 2.44 0.0101 | 2.40 ± 2.15 0.008 | 1.48 ± 0.97 0.00493 | 1.70 ± 0.92 0.00567 |
| 1-Octanol | N/A | 9.56 ± 5.83 | 17 ± 6.73 | 13.7 ± 4.97 | 14.6 ± 6.6 | 14.1 ± 9.05 |
| 3-Heptanol, 2,6-dimethyl- | N/A | 4.28 ± 4.11 | 2.31 ± 4.18 | 4.86 ± 3.98 | 3.84 ± 3.79 | 7.16 ± 5.80 |
| Benzyl alcohol | 200,000 c | 2.97 ± 4.37 0.0000148 | 6.49 ± 6.72 0.0000324 | 4.20 ± 3.94 0.000021 | 2.43 ± 3.56 0.0000122 | 3.38 ± 4.89 0.0000169 |
| Cyclohexanol, 4-(1,1-dimethylethyl)-, cis- | N/A | 1.73 ± 3.13 | 0.46 ± 1.53 | 0.44 ± 1.08 | 0 | 0 |
| Cyclohexanol, 4-(1,1-dimethylethyl)-, trans- | N/A | 1.31 ± 2.21 | 0.25 ± 0.82 | 0 | 0.13 ± 0.48 | 0 |
| isoamyl alcohol | 30,000 c | 573 ± 230 0.0191 | 695 ± 241 0.0232 | 611 ± 193 0.0204 | 585 ± 119 0.0195 | 543 ± 107 0.0181 |
| Phenylethyl Alcohol | 14,000 c | 684 ± 263 0.0489 | 866 ± 409 0.0619 | 824 ± 250 0.0589 | 751 ± 126 0.0536 | 813 ± 189 0.0581 |
| Total concentration | 1283 | 1594 | 1463 | 1363 | 1391 | |
| Aldehydes | ||||||
| Benzaldehyde | 350 g | 1.92 ± 5.28 0.00549 | 0.71 ± 1.66 0.00203 | 0 | 0.86 ± 3.10 0.00246 | 0 |
| Benzeneacetaldehyde | 4 h | 0.63 ± 0.97 0.158 | 1.50 ± 2.45 0.375 | 0.50 ± 0.40 0.125 | 0.46 ± 0.53 0.115 | 0.093 ± 0.28 0.0233 |
| Decanal | 2 i | 0 | 0.86 ± 2.49 0.43 | 0.20 ± 0.48 0.10 | 0.13 ± 0.47 0.065 | 1.03 ± 1.32 0.515 |
| Total concentration | 2.55 | 3.07 | 0.70 | 1.45 | 1.12 | |
| Ketones | ||||||
| Cyclohexanone, 4-(1,1-dimethylethyl)- | N/A | 2.36 ± 4.48 | 0.56 ± 1.87 | 0 | 0 | 0 |
| Fatty acids | ||||||
| Decanoic acid | 1000 c | 99.8 ± 171 0.0998 | 32.1 ± 23.2 0.0321 | 44.7 ± 16 0.0447 | 29.8 ± 14.8 0.0298 | 56.3 ± 23.9 0.0563 |
| Dodecanoic acid | 10,000 c | 3.12 ± 3.84 0.000312 | 0.71 ± 1.96 0.000071 | 1.36 ± 1.21 0.000136 | 1.20 ± 0.96 0.00012 | 2.02 ± 2.04 0.000202 |
| Nonanoic acid | 3000 c | 15.2 ± 15.6 0.00507 | 12.8 ± 12.6 0.00427 | 24.6 ± 7.85 0.0082 | 16.8 ± 7.69 0.0056 | 25.7 ± 12.5 0.00857 |
| Tetradecanoic acid (myristic acid) | 10,000 c | 0.20 ± 0.55 0.00002 | 0.25 ± 0.82 0.000025 | 0.25 ± 0.42 0.000025 | 0.095 ± 0.23 0.0000095 | 0.34 ± 0.74 0.000034 |
| Total concentration | 118 | 45.9 | 70.9 | 47.9 | 84.4 | |
| Hydrocarbons | ||||||
| Benzene, 1-methyl-4-(1-methylethenyl)- | N/A | 2.89 ± 1.79 | 1.64 ± 1.66 | 2.47 ± 1.38 | 2.94 ± 0.64 | 3.24 ± 1.93 |
| o-Xylene | N/A | 0 | 0 | 0.30 ± 0.72 | 0 | 0 |
| Undecane, 2,6-dimethyl- | N/A | 0.16 ± 0.46 | 0.30 ± 0.72 | 0 | 0.059 ± 0.21 | 0 |
| Total concentration | 3.05 | 1.94 | 2.77 | 3.0 | 3.24 | |
| Phenols | ||||||
| Phenol, 4-ethyl- | 440 c | 7.95 ± 26.2 0.0181 | 55.9 ± 87.8 0.127 | 86.9 ± 208 0.198 | 23.4 ± 25.8 0.0532 | 23.9 ± 47.3 0.0543 |
| Phenol, 4-ethyl-2-methoxy- (4-ethyl guaiacol) | 33 j | 6.67 ± 15.8 0.202 | 34.4 ± 43.4 1.04 | 25.0 ± 46.8 0.758 | 12.3 ± 16.2 0.373 | 21.5 ± 34.1 0.652 |
| Total concentration | 14.6 | 90.3 | 112 | 35.7 | 45.4 | |
| Aging volatile compounds | ||||||
| C13-norisoprenoids | ||||||
| α-Ionone | 0.09 c | 33.5 ± 36.2 372 | 25.4 ± 37.9 282 | 17.8 ± 14.3 198 | 19.7 ± 8.61 219 | 16.6 ± 12.3 184 |
| Lactones | ||||||
| Butyrolactone | 1000 k | 0.22 ± 0.44 0.00022 | 0.52 ± 1.33 0.00052 | 0.30 ± 0.48 0.0003 | 0.035 ± 0.13 0.000035 | 0.028 ± 0.083 0.000028 |
| OAV range | 0.0000148–62.6 | 0.000025–80 | 0.000025–58.4 | 0.0000095–44 | 0.000028–62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabezzana, R.; Malandrino, M.; Abollino, O.; Bonometti, E.; Giordana, A.; Turco, F.; Volpi, G.; Operti, L. Characterization of Freisa Wines from Piedmont (Italy) by Aroma and Element Profile. Appl. Sci. 2023, 13, 7425. https://doi.org/10.3390/app13137425
Rabezzana R, Malandrino M, Abollino O, Bonometti E, Giordana A, Turco F, Volpi G, Operti L. Characterization of Freisa Wines from Piedmont (Italy) by Aroma and Element Profile. Applied Sciences. 2023; 13(13):7425. https://doi.org/10.3390/app13137425
Chicago/Turabian StyleRabezzana, Roberto, Mery Malandrino, Ornella Abollino, Elisabetta Bonometti, Alessia Giordana, Francesca Turco, Giorgio Volpi, and Lorenza Operti. 2023. "Characterization of Freisa Wines from Piedmont (Italy) by Aroma and Element Profile" Applied Sciences 13, no. 13: 7425. https://doi.org/10.3390/app13137425
APA StyleRabezzana, R., Malandrino, M., Abollino, O., Bonometti, E., Giordana, A., Turco, F., Volpi, G., & Operti, L. (2023). Characterization of Freisa Wines from Piedmont (Italy) by Aroma and Element Profile. Applied Sciences, 13(13), 7425. https://doi.org/10.3390/app13137425









