A Novel Approach for Gut Ecosystem Resilience: Evaluating Lacti-plantibacillus plantarum-12INH as a Promising Natural Antibacterial Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Characterization
2.2. Molecular Identification and Bioinformatic Analysis
2.3. Bacteriocin Production and Antimicrobial Activity Test
2.4. Bacteriocin Activity Test: pH Resistance
2.5. Bacteriocin Purification and Protein Molecular Weight Analysis
3. Results
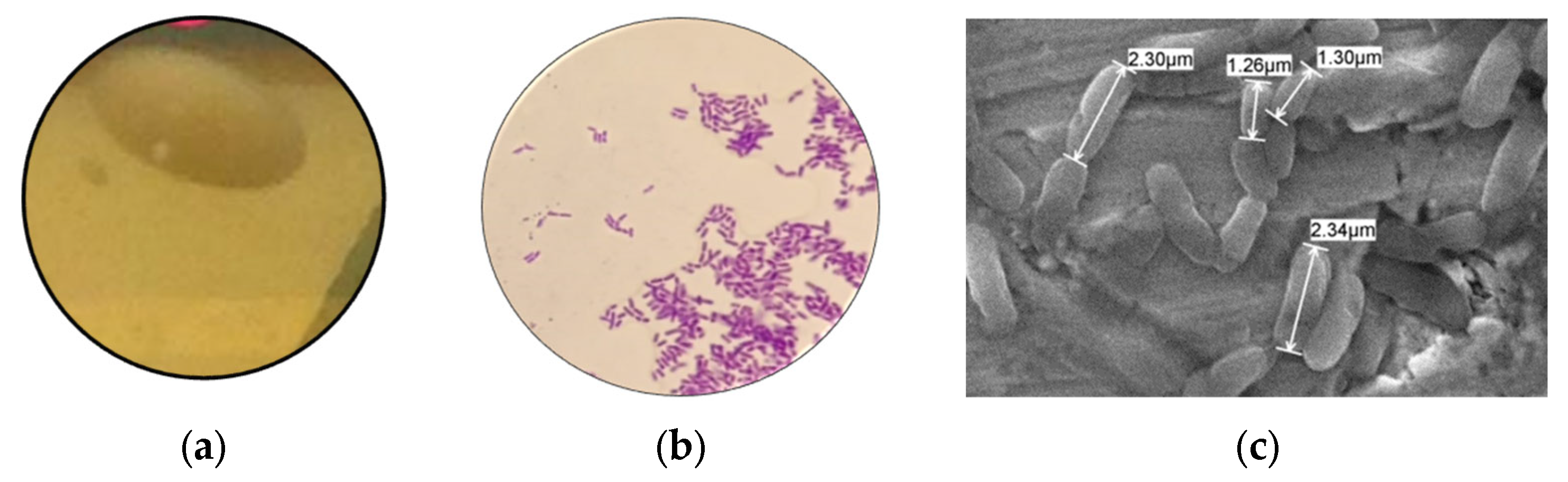
3.1. Characterization Probiotic
3.2. DNA Molecular Identification and Phylogenic Trees
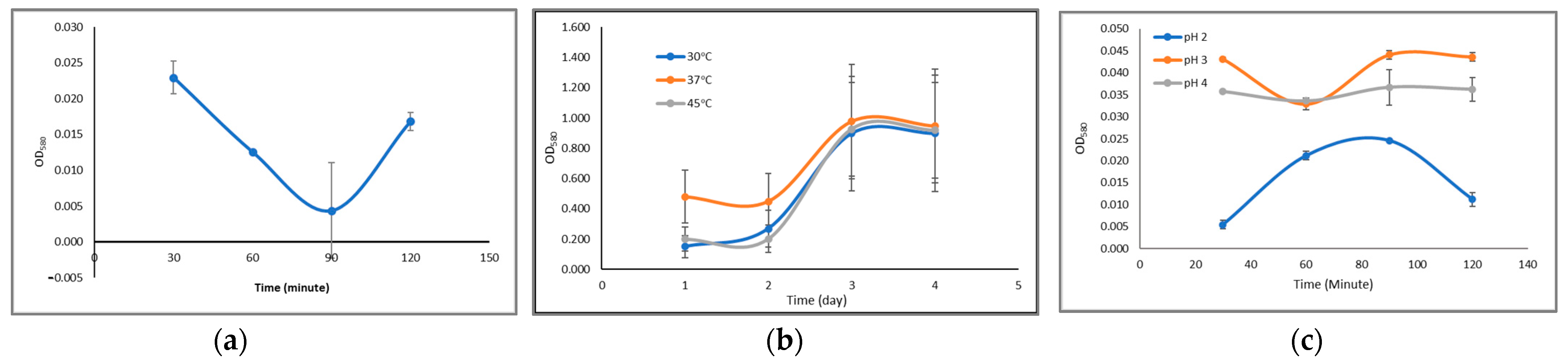
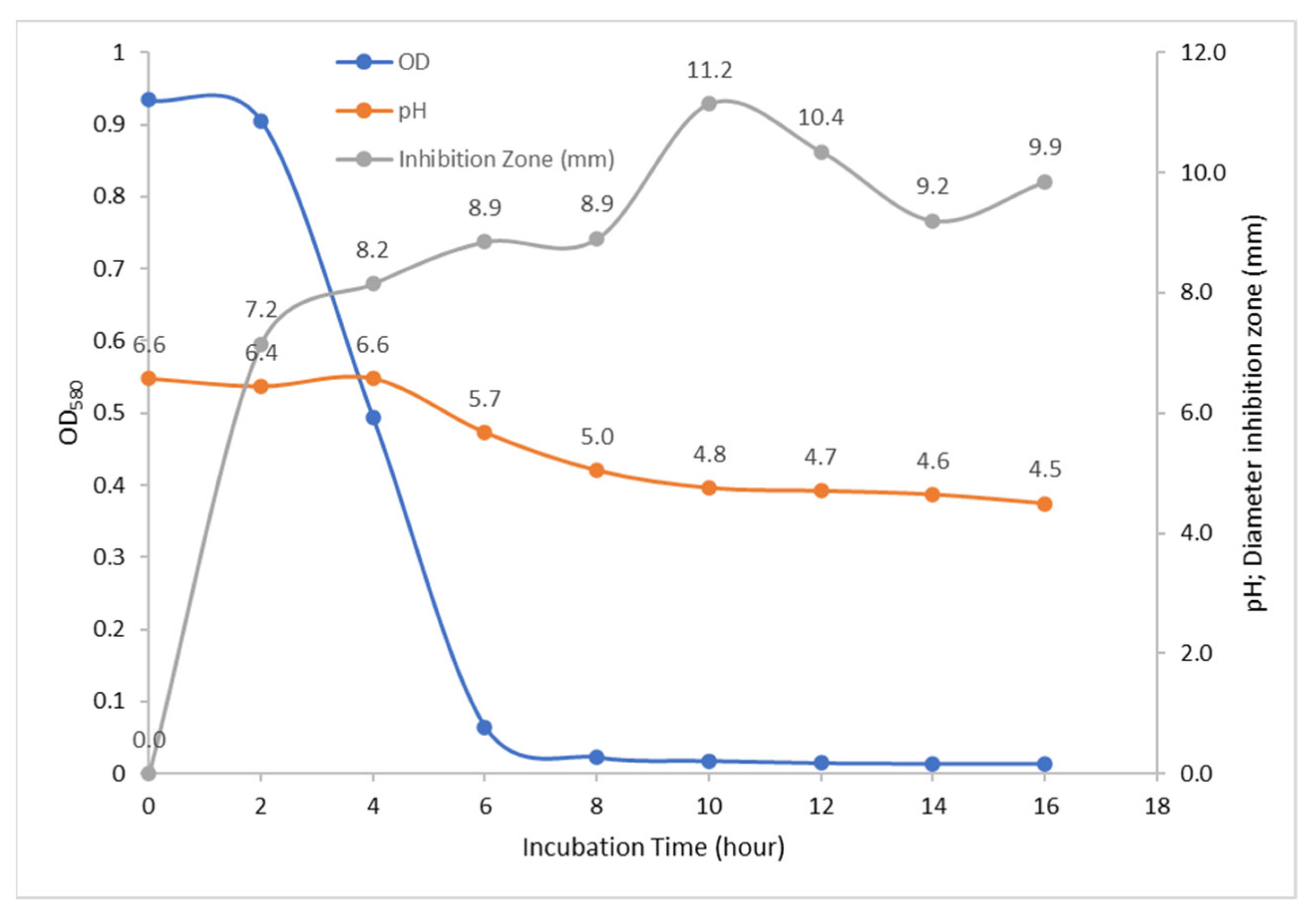
3.3. Bacteriocin Production and Antimicrobial Activity
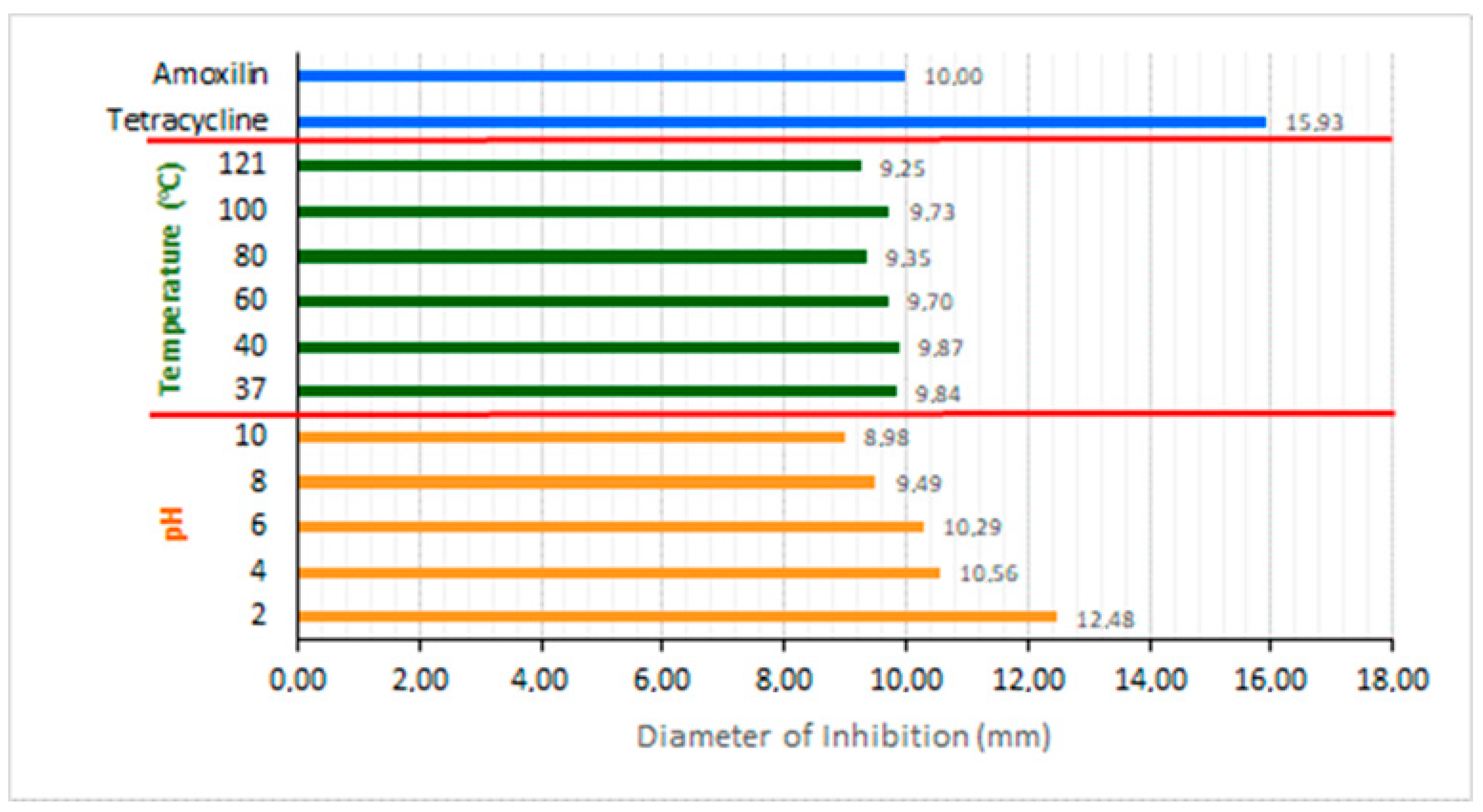
3.4. Bacteriocin Activity against pH Resistance and Temperature Resistance
3.5. Bacteriocin Molecular Weight
4. Discussion
5. Conclusions
- Rod-shaped, catalase negative, measuring 1.26–2.34 m, able to survive at low pH (2, 3, and 4), 3% bile salts, and temperatures of 30, 37, and 45 °C;
- Can produce bacteriocin secondary metabolites with a molecular weight of 13.59 kDa which have the ability to be an anti-microbial against bacterial growth E. coli under conditions of pH 2, 4, 6, 8, and 10 and temperatures 40 °C, 60, 80, 100, and 120 °C similar to amoxicillin and tetracycline antibiotics;
- Has the potential to be used as a functional food and as an alternative to standard natural antibacterial agents for assisting digestion while maintaining a healthy gut microbiome.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erick, M. Breast milk is conditionally perfect. Med. Hypotheses 2017, 111, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.W.; Hsu, K.; Chao, C.C.; Yang, S.H.; Lin, Z.H.; Chen, Y.J.; Cao, C.C.; Huang, Y.M.; Chang, H.S.; Tsai, C.H.; et al. Bacterial Composition and Diversity in Breast Milk Samples from Mothers Living in Taiwan and Mainland China. Front. Microbiol. 2017, 8, 965. [Google Scholar] [CrossRef]
- Martín, R.; Hans, G.H.J.H.; Erwin, E.G.; Esther, J.; Leonides, F.; Hauke, S.; Tuan, M.R. Cultivation-Independent Assessment of The Bacterial Diversity of Breast Milk among Healthy Women. Res. Microbiol. 2007, 158, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Rajoka, R.; Siddiq, M.; Haobin, Z.; Zhu, J.; Yan, L.; Shao, D.; Xu, X.; Shi, J. Identification, Characterization, and Probiotic Potential of Lactobacillus rhamnosus Isolated from Human Milk. LWT-Food Sci. Technol. 2017, 84, 271–280. [Google Scholar]
- Nordstrom, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V): Three Decades of Research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pang, H.; Wang, L.; Ma, C.; Wu, G.; Liu, Y.; Guan, Y.; Zhang, M.; Qin, G.; Tan, Z. Bacteriocin-Producing Lactic Acid Bacteria Strains with Antimicrobial Activity Screened from Bamei Pig Feces. Foods 2022, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Chang, Y.H.; Yune, J.H.; Jeong, C.H.; Shin, D.M.; Kwon, H.C.; Kim, D.H.; Hong, S.W.; Hwang, H.; Jeong, J.Y.; et al. Probiotic Properties of Lactiplantibacillus plantarum LB5 Isolated from Kimchi Based on Nitrate Reducing Capability. Foods 2020, 9, 1777. [Google Scholar] [CrossRef]
- Pelczar, M.J.; Chan, E.C.S.; Krieng, N.R. Microbiology: Concepts and Applications; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Goh, H.F.; Philip, K. Purification and Characterization of Bacteriocin Produced by Weissella confusa A3 of Dairy Origin. PLoS ONE 2015, 10, e0140434. [Google Scholar] [CrossRef]
- Kunchala, R.; Banerjee, R.; Mazumdar, S.D.; Durgalla, P. Characterization of potential probiotic bacteria isolated from sorghum and pearl millet of the semi-arid tropics. Arican J. Biotechnol. 2016, 15, 613–621. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C. Brief Communication (Revised) MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Morello, J.A.; Granato, P.A.; Mizer, H.E. Laboratory Manual and Workbook in Microbiology, 7th ed.; The McGraw−Hill Companies: New York, NY, USA, 2003. [Google Scholar]
- Hata, T.; Tanaka, R.; Ohmomo, S. Isolation and Characterization of Plantaricin ASM1: A new Bacteriocin Produced by Lactobacillus plantarum A-1. Int. J. Food Microbiol. 2010, 137, 94–99. [Google Scholar] [CrossRef]
- Abo-Amer, A.E. Characterization of a Bacteriocin-Like Inhibitory Substance Produced by Lactobacillus plantarum Isolated from Egyptian Home-Made Yogurt. ScienceAsia 2007, 33, 313–319. [Google Scholar] [CrossRef]
- Ogunbanwo, S.; Sanni, A.; Onilude, A.A. Characterization of Bacteriocin Produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr. J. Biotechnol. 2003, 2, 219–227. [Google Scholar] [CrossRef]
- Yavuzdurmaz, H. Isolation, Characterization, Determination of Probiotic Properties of Lactic Acid Bacteria from Human Milk. Master’s Thesis, Izmir Institute of Technology, Izmir, Turkey, 17 October 2007. [Google Scholar]
- Boland, M.J. Human Digestion—A Processing Perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef]
- Thi, D.; Thuy, B.; Nguyen, T. Isolation, Screening, Identification and Optimization of Culture Parameters to Produce γ -aminobutyric Acid by Lactiplantibacillus pentosus R13, an Isolate from Ruoc (Fermented Shrimp Paste). Appl. Food Biotechnol. 2022, 9, 1–8. [Google Scholar]
- Siegumfeldt, H.; Rechinger, K.B.; Jakobsen, M. Dynamic Changes of Intracellular pH in Individual Lactic Acid Bacterium Cells in Response to a Rapid Drop in Extracellular pH. Appl. Environ. Microbiol. 2000, 66, 2330–2335. [Google Scholar] [CrossRef]
- Siswara, H.N.; Arief, I.I.; Wulandari, Z. Plantarisin Asal Lactobacillus plantarum IIA-1A5 sebagai Pengawet Alami Daging Ayam Bagian Paha pada Suhu Refrigerator. J. Ilmu Produksi dan Teknol. Has. Peternak. 2019, 7, 123–130. [Google Scholar] [CrossRef]
- Amina, Z.; Noureddine, S.; Venkatesan, A.; Hicham, B.; Yamina, B.; Leila, B.; Mebrouk, K. Purification and Molecular Characterization of Two-Antimicrobial Peptides Produced by Lactobacillus plantarum DU10. Int. J. Biol. Chem. 2015, 9, 46–58. [Google Scholar] [CrossRef]
- Serrano-Niño, J.C.; Solís-Pacheco, J.R.; Gutierrez-Padilla, J.A.; Cobián-García, A.; Cavazos-Garduño, A.; González-Reynoso, O.; Aguilar-Uscanga, B.R. Isolation and Identification of Lactic Acid Bacteria from Human Milk with Potential Probiotic Role. J. Food Nutr. 2016, 4, 170–177. [Google Scholar]
- Afrin, S.; Hoque, M.A.; Sarker, A.K.; Satter, M.A.; Bhuiyan, M.N.I. Characterization and Profiling of Bacteriocin-like Substances Produced by Lactic Acid Bacteria from Cheese Samples. Access Microbiol. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Younas, S.; Mazhar, B.; Liaqat, I.; Ali, S.; Tahir, H.M.; Ali, N.M. Bacteriocin Production by Lactobacilli and Their Role as Antibacterial Tool against Common Pathogens. J. Oleo Sci. 2022, 71, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Salleh, F.; Lani, M.N.; Chilek, T.Z.T.; Kamaruding, N.A.; Ismail, N. Lactic Acid Bacteria Producing Sorbic Acid and Benzoic Acid Compounds from Fermented Durian Flesh (Tempoyak) and Their Antibacterial Activities against Foodborne Pathogenic Bacteria. Appl. Food Biotechnol. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef]



| Description | Results |
|---|---|
| Species | Lactiplantibacillus plantarum strain NCIMB 8826 |
| Maximum score | 8851 |
| Total score | 1770 |
| Query coverage | 100% |
| e-Value | 0.0 |
| Percent identity | 100% |
| Accession number | CP037961.1 |
| Bacteria | Time Produce Bacteriocin (h) | Zone Inhibition against E. coli (mm) | pH Resistance | Temperature Resistance (°C) | Originated | References |
|---|---|---|---|---|---|---|
| L. plantarum-12INH | 10 | 9.25–12.48 | 2–10 | 37–121 | Breast milk native Indonesia | Our research |
| L. plantarum IIA-1A5 | 24 | 8.94 | 6.35–6.48 | 3–5 | - | [20] |
| L. plantarum DU10 | 16 | 5–10 | 6.5 | 37 | Raw camel milk | [21] |
| L. fermentum | 24 | 10 | 6.5 | 37 | Human milk | [22] |
| L. plantarum | 24 | 6–9 | 4–8 | 30–90 | Cheese | [23] |
| L. plantarum H1 | 18–24 | 4 | - | 37 | Pickle | [24] |
| L. plantarum V1 | 18–24 | 18 | - | 37 | Fermented durian flesh | [25] |
| L. plantarum QP19 | 24 | 12–15 | 5–5.5 | 37 | Pig feces | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanidah, I.-I.; Kamal, G.R.; Nurhadi, B.; Lani, M.N.; Andriyono, S.; Moody, S.D.; Harlina, P.W. A Novel Approach for Gut Ecosystem Resilience: Evaluating Lacti-plantibacillus plantarum-12INH as a Promising Natural Antibacterial Agent. Appl. Sci. 2023, 13, 7378. https://doi.org/10.3390/app13137378
Hanidah I-I, Kamal GR, Nurhadi B, Lani MN, Andriyono S, Moody SD, Harlina PW. A Novel Approach for Gut Ecosystem Resilience: Evaluating Lacti-plantibacillus plantarum-12INH as a Promising Natural Antibacterial Agent. Applied Sciences. 2023; 13(13):7378. https://doi.org/10.3390/app13137378
Chicago/Turabian StyleHanidah, In-In, Ghea Raihan Kamal, Bambang Nurhadi, Mohd Nizam Lani, Sapto Andriyono, Sumanti Debby Moody, and Putri Widyanti Harlina. 2023. "A Novel Approach for Gut Ecosystem Resilience: Evaluating Lacti-plantibacillus plantarum-12INH as a Promising Natural Antibacterial Agent" Applied Sciences 13, no. 13: 7378. https://doi.org/10.3390/app13137378
APA StyleHanidah, I.-I., Kamal, G. R., Nurhadi, B., Lani, M. N., Andriyono, S., Moody, S. D., & Harlina, P. W. (2023). A Novel Approach for Gut Ecosystem Resilience: Evaluating Lacti-plantibacillus plantarum-12INH as a Promising Natural Antibacterial Agent. Applied Sciences, 13(13), 7378. https://doi.org/10.3390/app13137378







