Wheat Transformation with ScTPS1-TPS2 Bifunctional Enzyme for Trehalose Biosynthesis Protects Photosynthesis during Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. PCR Analysis
2.3. Drought Stress Treatment
2.4. Relative Water Content
2.5. Gas Exchange Measurements
2.6. Enzymatic Extract Preparation
2.7. Enzyme Activity Assays
2.8. Protein Quantification
2.9. Sugar Content Analysis
2.10. Statistical Analysis
3. Results
3.1. Plant Transformation and Selection
3.2. Relative Water Content during Drought
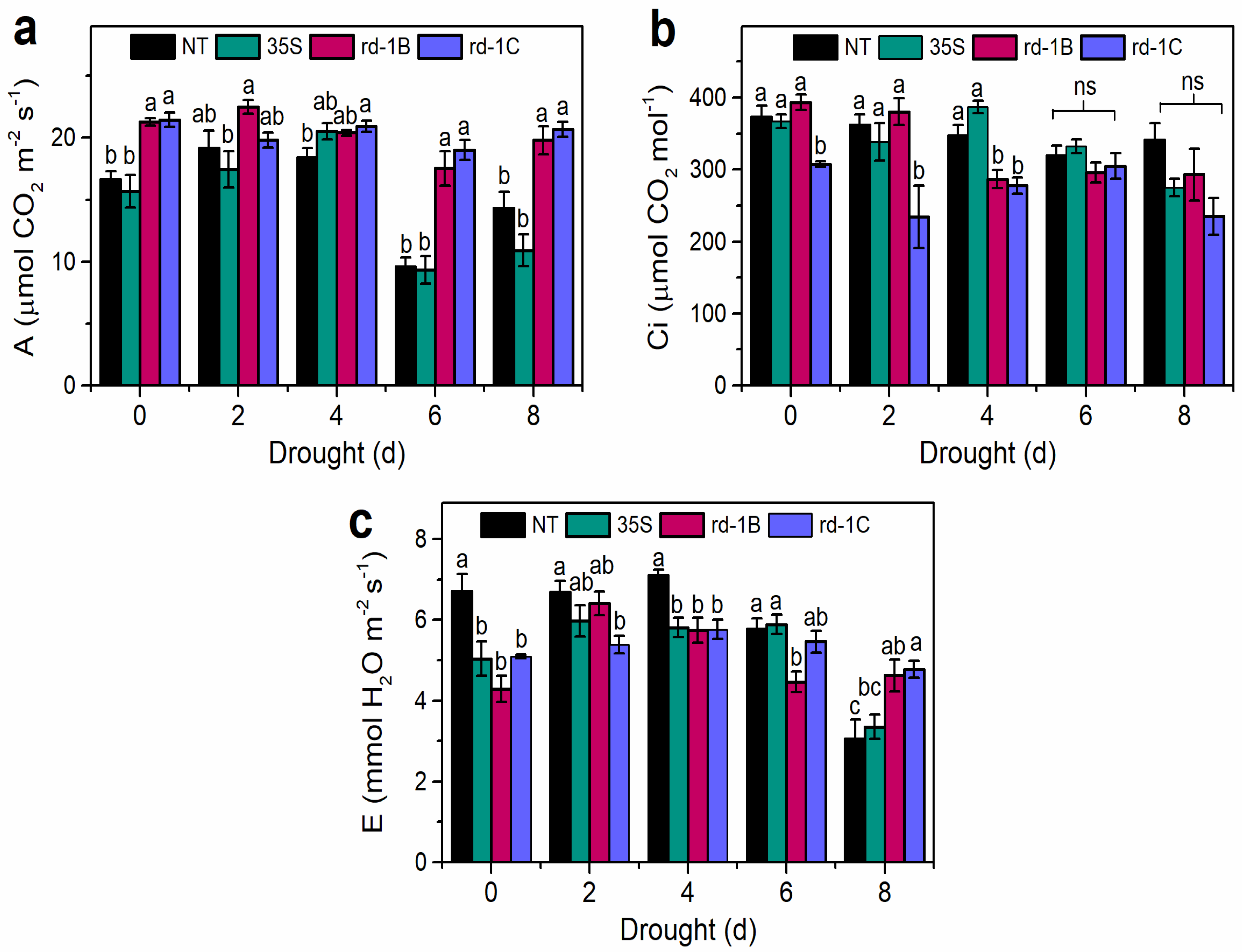
3.3. Photosynthetic Parameters
3.4. Enzyme Activity
3.5. Sugar Content
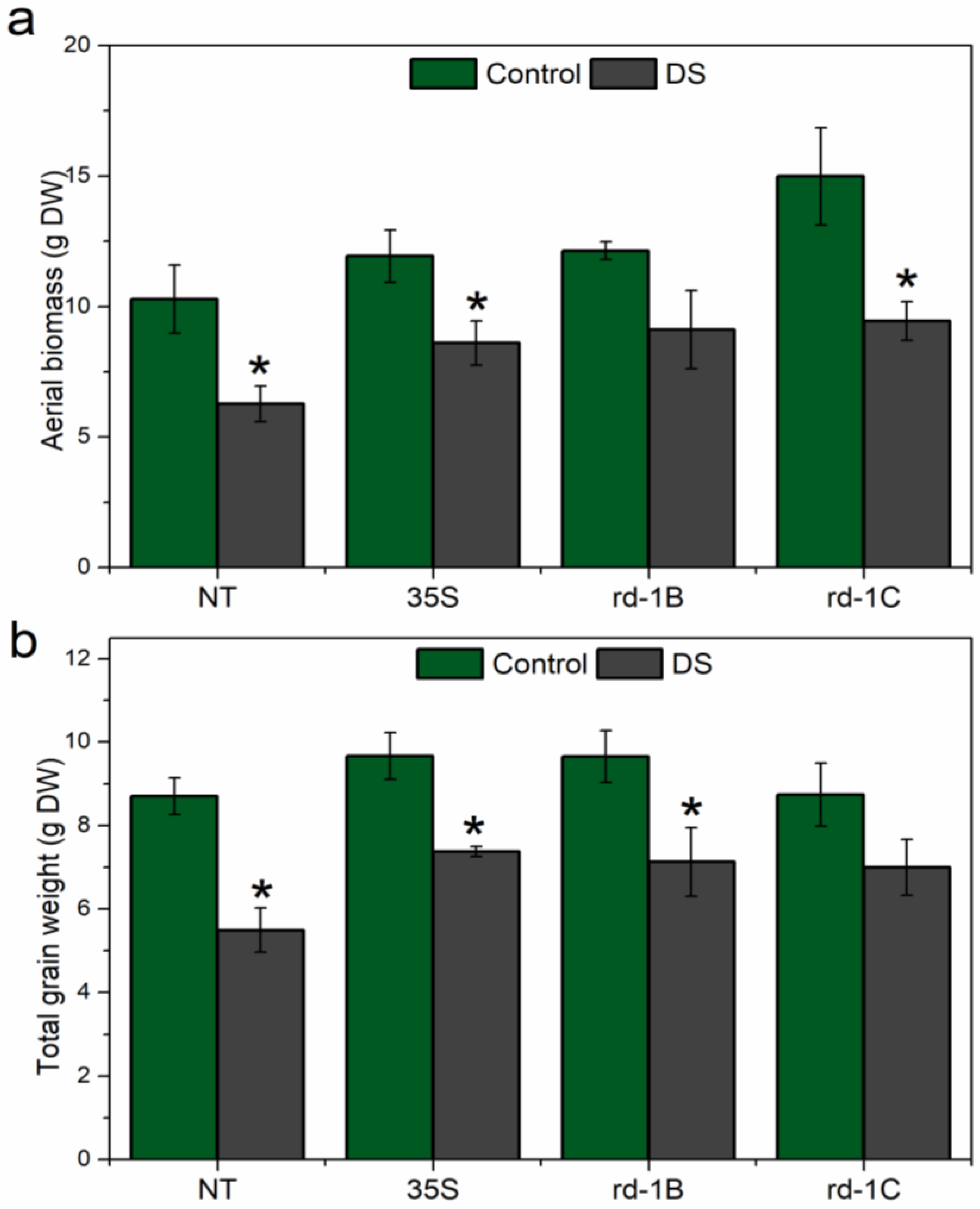
3.6. Plant Biomass after Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety: Wheat yield and food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Lai, C.; Wang, R.Y.; Chen, X.; Lian, Y. Drying tendency dominating the global grain production area. Glob. Food Secur. 2018, 16, 138–149. [Google Scholar] [CrossRef]
- Wingler, A. The function of trehalose biosynthesis in plants. Phytochemistry 2002, 60, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef]
- Phan, L.C.H.B.T.; Van Dijck, P. Biosynthesis and degradation of trehalose and its potential to control plant growth; development; and abiotic stress tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 175–199. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 12–17. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The yin and yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.V. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- Luo, Y.; Li, F.; Wang, G.P.; Yang, X.H.; Wang, W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 2010, 54, 495–501. [Google Scholar] [CrossRef]
- Lyu, J.I.; Min, S.R.; Lee, J.H.; Lim, Y.H.; Kim, J.-K.; Bae, C.-H.; Liu, J.R. Overexpression of a trehalose-6-phosphate synthase/phosphatase fusion gene enhances tolerance and photosynthesis during drought and salt stress without growth aberrations in tomato. Plant Cell Tissue Organ Cult. 2013, 112, 257–262. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, Y.M.; Wang, W.; Zou, C.J. Application of trehalose ameliorates heat stress and promotes recovery of winter wheat seedlings. Biol. Plant. 2014, 58, 395–398. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Bartels, D. Enzymes and metabolites in carbohydrate metabolism of desiccation tolerant plants. Proteomes 2016, 4, 40. [Google Scholar] [CrossRef]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109–114. [Google Scholar] [CrossRef]
- Mu, M.; Lu, X.K.; Wang, J.J.; Wang, D.L.; Yin, Z.J.; Wang, S.; Fan, W.L.; Ye, W.W. Genome-wide identification and analysis of the stress-resistance function of the TPS (Trehalose-6-Phosphate Synthase) gene family in cotton. BMC Genom. 2016, 17, 54. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, M.M.; Rahman, M.M.; Jin, X.; Sun, L.; Zhao, K.; Wang, S.; Sikdar, A.; Noor, H.; Jeon, J.S.; et al. In silico and transcription analysis of trehalose-6-phosphate phosphatase gene family of wheat: Trehalose synthesis genes contribute to salinity, drought stress and leaf senescence. Genes 2021, 12, 1652. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.-C.; Oh, S.-J.; Seo, J.-S.; Choi, W.-B.; Song, S.-I.; Kim, C.-H.; Kim, Y.-S.; Seo, H.-S.; Choi, Y.-D.; Nahm, B.-H.; et al. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol. 2003, 131, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Cortina, C.; Culiáñez-Macià, F.A. Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci. 2005, 169, 75–82. [Google Scholar] [CrossRef]
- Lee, S.-B.; Kwon, H.-B.; Kwon, S.-J.; Park, S.-C.; Jeong, M.-J.; Han, S.-E.; Byun, M.-O.; Daniell, H. Accumulation of trehalose within transgenic chloroplasts confers drought tolerance. Mol. Breed. 2003, 11, 1–13. [Google Scholar] [CrossRef]
- Yeo, E.T.; Kwon, H.B.; Han, S.E.; Lee, J.T.; Ryu, J.C.; Byun, M.O. Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol. Cells 2000, 10, 263–268. [Google Scholar]
- Lawlor, D.W.; Paul, M.J. Source/sink interactions underpin crop yield: The case for trehalose 6-phosphate/SnRK1 in improvement of wheat. Front. Plant Sci. 2014, 5, 418. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef]
- Miranda, J.A.; Avonce, N.; Suárez, R.; Thevelein, J.M.; Van Dijck, P.; Iturriaga, G. A bifunctional TPS–TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 2007, 226, 1411–1421. [Google Scholar] [CrossRef]
- Pellny, T.K.; Ghannoum, O.; Conroy, J.P.; Schluepmann, H.; Smeekens, S.; Andralojc, J.; Krause, K.P.; Goddijn, O.; Paul, M.J. Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnol. J. 2004, 2, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A Nove1 cis-acting element in an Arabidopsis gene 1s involved in responsiveness to drought, low temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [PubMed]
- Hellens, R.P.; Edwards, E.A.; Leyland, N.R.; Bean, B.; Mullineaux, P.M. pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 2000, 42, 819–832. [Google Scholar] [CrossRef]
- Slavík, B. Methods of Studying Plant Water Relations; Springer: Berlin/Heidelberg, Germany; Prague, Czech Republic, 1974; p. 146. [Google Scholar]
- Li, H.; Ahammed, G.J.; Zhou, G.; Xia, X.; Zhou, J.; Shi, K. Unraveling main limiting sites of photosynthesis under below- and above-ground heat stress in cucumber and the alleviatory role of Luffa rootstock. Front. Plant Sci. 2016, 7, 746. [Google Scholar] [CrossRef]
- Zimmermann, G.; Kelly, G.; Latzko, E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-biphosphatase. Eur. J. Biochem. 1976, 70, 361–367. [Google Scholar] [CrossRef]
- Huber, S.C. Interspecific variation in activity and regulation of leaf sucrose phosphate synthetase. Z. Pflanzenphysiol. 1981, 102, 441–450. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Balla, K.; Bencze, S.; Bónis, P.; Árendás, T.; Veisz, O. Changes in the photosynthetic efficiency of winter wheat in response to abiotic stress. Open Life Sci. 2014, 9, 519–530. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Singh, A.K.; Anwar, K.; Pundir, P.; Gautam, R.K.; Krishnamurthy, S.L.; Sopory, S.K.; Pareek, A.; Singla-Pareek, S.L. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J. Exp. Bot. 2020, 71, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Yang, L.; Cong, W.; Zu, Y.; Tang, Z. The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol. Biochem. 2014, 77, 140–148. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Li, T.T.; Hao, Z.J.; Cheng, M.L.; Tao, J. Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperones 2019, 24, 247–257. [Google Scholar] [CrossRef]
- Kahraman, M.; Sevim, G.; Bor, M. The Role of proline, glycinebetaine, and trehalose in stress-responsive gene expression. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 241–256. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.Y.; Fan, Y.Z.; Wang, W.; Zhao, Y.Y. Comparative chloroplast proteome analysis of exogenously supplied trehalose to wheat seedlings under heat stress. Photosynthetica 2018, 56, 1123–1133. [Google Scholar] [CrossRef]
- Nemat Alla, M.; Badran, E.; Mohammed, F. Exogenous trehalose alleviates the adverse effects of salinity stress in wheat. Turk. J. Bot. 2019, 43, 48–57. [Google Scholar] [CrossRef]
- Van Houtte, H.; Vandesteene, L.; López-Galvis, L.; Lemmens, L.; Kissel, E.; Carpentier, S.; Feil, R.; Avonce, N.; Beeckman, T.; Lunn, J.E.; et al. Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol. 2013, 161, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, O.; Verwoerd, T.C.; Voogd, E.; Krutwagen, R.W.H.H.; de Graaf, P.T.H.M.; Poels, J.; van Dunn, K.; Ponstein, A.S.; Damn, B.; Pen, J. Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol. 1997, 113, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
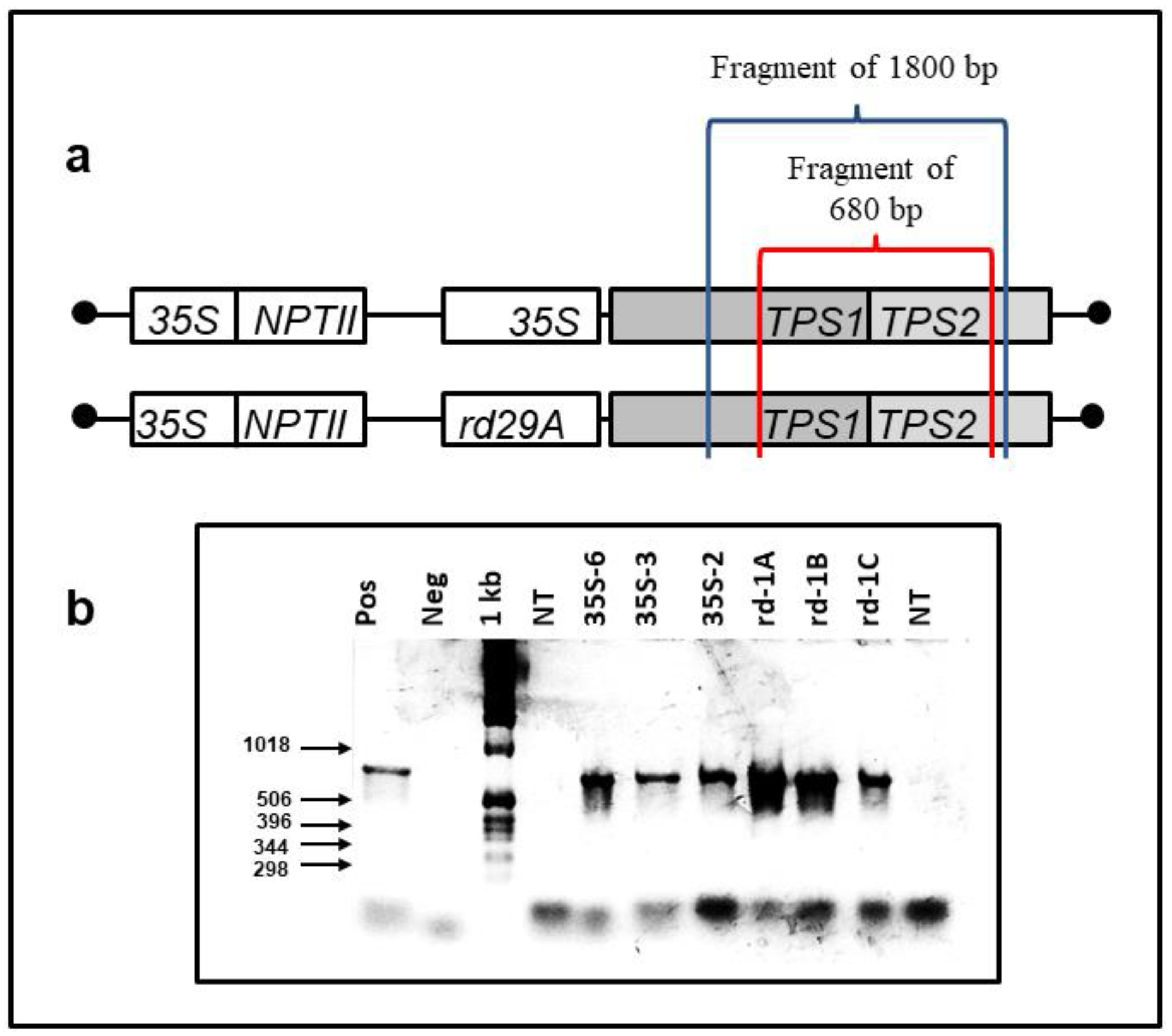


| Relative Water Content (%) | |||||
|---|---|---|---|---|---|
| Drought (d) | 0 | 2 | 4 | 6 | 8 |
| NT | 87.7 ± 2.3 a | 77.3 ± 2.4 bc | 81.9 ± 2.3 ab | 70.9 ± 3.0 bc | 63.1 ± 3.4 c |
| 35S | 92.4 ± 2.7 a | 81.9 ± 2.7 b | 81.7 ± 2.7 b | 77.6 ± 3.5 bc | 70.7 ± 3.5 c |
| rd-1B | 84.1 ± 1.7 a | 86.5 ± 1.7 a | 74.2 ± 2.1 b | 74.3 ± 3.0 b | 67.9 ± 2.7 b |
| rd-1C | 84.9 ± 1.8 a | 80.6 ± 1.7 a | 79.2 ± 1.9 a | 66.3 ± 3.0 b | 65.6 ± 2.7 b |
| Sugar | Drought (d) | ||||
|---|---|---|---|---|---|
| Sucrose (μg g−1 DW) | 0 | 2 | 4 | 6 | 8 |
| NT | 19.3 ± 1.0 d | 21.3 ± 0.8 d | 32.9 ± 0.4 c | 61.6 ± 1.1 a | 37.3 ± 0.5 b |
| 35S | 31.4 ± 0.5 a | 11.2 ± 0.1 c | 6.7 ± 0.04 e | 8.4 ± 0.1 d | 14.0 ± 0.2 b |
| rd-1B | 22.0 ± 0.5 b | 23.9 ± 0.1 a | 23.7 ± 0.3 a | 13.7 ± 0.3 c | 13.7 ± 0.01 c |
| rd-1C | 10.8 ± 0.04 e | 27.3 ± 0.5 b | 18.6 ± 0.01 c | 30.9 ± 0.5 a | 16.6 ± 0.02 d |
| Trehalose (µg g−1 DW) | |||||
| NT | 56.8 ± 0.4 e | 88.4 ± 1.9 d | 146.2 ± 5.2 b | 163.9 ± 1.2 a | 106.5 ± 0.1 c |
| 35S | 108.5 ± 1.6 b | 75.2 ± 0.3 c | 47.9 ± 0.3 e | 115.5 ± 0.7 a | 60.7 ± 1.4 d |
| rd-1B | 60.9 ± 1.3 d | 74.9 ± 0.7 c | 92.2 ± 1.0 a | 82.8 ± 0.1 b | 92.2 ± 0.6 a |
| rd-1C | 76.4 ± 1.5 b | 66.8 ± 1.5 c | 69.9 ± 0.1 c | 76.1 ± 0.8 b | 97.9 ± 0.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Reyes, A.; Valenzuela-Avendaño, J.P.; Figueroa-Soto, C.G.; Mascorro-Gallardo, J.O.; Iturriaga, G.; Castellanos-Villegas, A.; Rivera-Domínguez, M.; Valenzuela-Soto, E.M. Wheat Transformation with ScTPS1-TPS2 Bifunctional Enzyme for Trehalose Biosynthesis Protects Photosynthesis during Drought Stress. Appl. Sci. 2023, 13, 7267. https://doi.org/10.3390/app13127267
Romero-Reyes A, Valenzuela-Avendaño JP, Figueroa-Soto CG, Mascorro-Gallardo JO, Iturriaga G, Castellanos-Villegas A, Rivera-Domínguez M, Valenzuela-Soto EM. Wheat Transformation with ScTPS1-TPS2 Bifunctional Enzyme for Trehalose Biosynthesis Protects Photosynthesis during Drought Stress. Applied Sciences. 2023; 13(12):7267. https://doi.org/10.3390/app13127267
Chicago/Turabian StyleRomero-Reyes, Andrea, Juan Pablo Valenzuela-Avendaño, Ciria G. Figueroa-Soto, José O. Mascorro-Gallardo, Gabriel Iturriaga, Alejandro Castellanos-Villegas, Marisela Rivera-Domínguez, and Elisa M. Valenzuela-Soto. 2023. "Wheat Transformation with ScTPS1-TPS2 Bifunctional Enzyme for Trehalose Biosynthesis Protects Photosynthesis during Drought Stress" Applied Sciences 13, no. 12: 7267. https://doi.org/10.3390/app13127267
APA StyleRomero-Reyes, A., Valenzuela-Avendaño, J. P., Figueroa-Soto, C. G., Mascorro-Gallardo, J. O., Iturriaga, G., Castellanos-Villegas, A., Rivera-Domínguez, M., & Valenzuela-Soto, E. M. (2023). Wheat Transformation with ScTPS1-TPS2 Bifunctional Enzyme for Trehalose Biosynthesis Protects Photosynthesis during Drought Stress. Applied Sciences, 13(12), 7267. https://doi.org/10.3390/app13127267








