Abstract
The utilization of enzyme-induced calcium carbonate precipitation (EICP) to consolidate aeolian sand has received significant attention in recent years. However, urease activity is directly affected by temperature, which varies greatly from day to night, especially in desert areas. To investigate the effect of alternating temperature on aeolian sand cementation by EICP, three experimental groups were designed to simulate the sunrise-to-sunset cycle in a natural desert environment: T1 (a process from heating to constant temperature to cooling), T2 (a process from cooling to constant temperature to heating), and T3 (a process of constant temperature throughout) as a control group. The differences in calcium carbonate content, precipitation rate of calcium carbonate, permeability coefficients, and shear wave velocity were compared and analyzed. Meanwhile, scanning electron microscopy (SEM) was conducted to observe the external cementation states by mineralization. The results showed that T2 had the highest calcium carbonate content, followed by T3 and, finally, T1, which were also confirmed by permeability coefficient and shear wave velocity tests. In addition, different alternating temperature processes would affect the survival time of the urease, and T2 showed the longest reaction time as the urease stayed active for the longest time in this process. The results provide a scientific reference for the selection of construction periods in which EICP can be optimally applied for the on-site aeolian sand cementation.
1. Introduction
In recent years, with the development of the economy and the demand for material transportation, the desert highway has gradually become the link of cross-regional economic exchange. However, the natural environment in desert areas is harsh, and sand particles under the effect of wind transport will be deposited near the road surface due to the increase in friction, resulting in sand burial disasters and affecting traffic safety. Microbially induced carbonate precipitation (MICP), a new technology in the field of aeolian sand reinforcement, has received extensive attention from scholars [1,2]. The basic principle is to use urease from urease-producing bacteria such as Sporosarcina pasteurii to decompose urea and produce carbonate ions. Carbonate ions and calcium ions in the environment use urease bacteria as nucleation sites to produce calcium carbonate crystals, thus filling sand pores and leading to cementation between sand particles [3,4]. The improved method of using urease to directly decompose urea based on MICP is called enzyme-induced calcite precipitation (EICP) [5].
There are many studies of MICP and EICP in the application of sand defense and solidification. It has previously been observed that calcium carbonate can be cemented on the surface of the sand to form a solid crust layer, which improves the erosion resistance of the sand surface [6,7]. Similarly, evidence from a number of experimental studies employing wind tunnel tests has established that calcium carbonate is a cementing material capable of solidifying sand and exhibits good resistance to weathering [8,9,10]. In a desert area, a field experiment of biomineralization crust was conducted, which formed a cured layer of approximately 2–2.5 cm on the aeolian sand surface, indicating that the calcium carbonate cemented layer can also exhibit good strength performance and stability in a desert field environment [11].
However, both the survival and metabolism of microorganisms in MICP as well as the breakdown of urea by urease in EICP are susceptible to temperature variations [12,13,14,15]. When the temperature exceeds 60 °C, the microorganisms in MICP usually die due to the high temperature and cannot continue to produce calcium carbonate [16]. In terms of EICP, within the temperature range from 15 °C to 70 °C, the activity of urease increased with increasing temperature and reached its optimum at 70 °C, after which further increases in temperature decreased urease activity, and at 100 °C, urease activity was almost completely inhibited [17,18,19]. In desert areas where rainfall is low, the long hours of sunshine and the low thermal conductivity of the sand lead to high surface desert sand temperatures, which can often reach 50 °C to 60 °C in summer [20,21]. The microorganisms in MICP are susceptible to high desert temperatures, which may lead to a reduction in the activity of microorganisms, whereas the urease in EICP is better adapted to temperature variations [22]. Based on solidifying desert sand field tests, Miao et al. found that EICP could be a useful method to improve the resistance of aeolian sand to strong wind erosion [23,24]. Similar experiments regarding the utilization of EICP to consolidate desert sand can be found in Almajed et al. [25].
In addition, the reaction process of the EICP consolidation of sand can last more than three days [26], and the diurnal temperature variation of the desert sand surface can reach more than 20 °C [21,27]. Therefore, when EICP is used to consolidate aeolian sand in a desert environment, the influence of diurnal temperature variation must be considered. However, few studies have focused on the influence of alternating temperature on the effectiveness of EICP consolidation, especially in desert areas, where the diurnal temperature variations are significant.
To study the influence of alternating temperature on the effectiveness of EICP in consolidating aeolian sand, aeolian sand in the Taklamakan Desert (Xinjiang Uyghur Autonomous Region, China) was collected for research material, and three experimental groups (T1, T2, and T3) of temperature processes were designed. Based on the testing of the calcium carbonate content, precipitation rate of calcium carbonate, permeability coefficients, and shear velocity wave of the three experimental groups at different maintenance periods, the effectiveness of EICP consolidation of the three temperature groups was compared and analyzed. Meanwhile, SEM was conducted to observe the external cementation states by mineralization.
2. Materials and Methods
2.1. Materials and Sample Preparation
The sand used for the experiments was natural wind-deposited sand from the Taklamakan Desert in Xinjiang Uyghur Autonomous Region, China (located next to the G217 highway, coordinates E 78.65, N 39.87), as shown in Figure 1. To reduce the contamination of aeolian sand components by human activities, all aeolian sand was harvested from an open area 1000 m away from the highway. The gradation curves obtained from the particle analysis experiments are shown in Figure 2. The results showed that 96% of the sand grains had particle sizes between 0.075 mm and 0.25 mm, the curvature coefficient (Cc) was 1.06, and the inhomogeneity coefficient (Cu) was 2.22 (less than 5). These values indicate that the aeolian sand was poorly graded. At the same time, the natural aeolian sand permeability coefficient was 2.37 × 10−3 cm·s−1.
Figure 1.
Sampling location of aeolian sand, Taklamakan Desert, Xinjiang Uyghur, China.
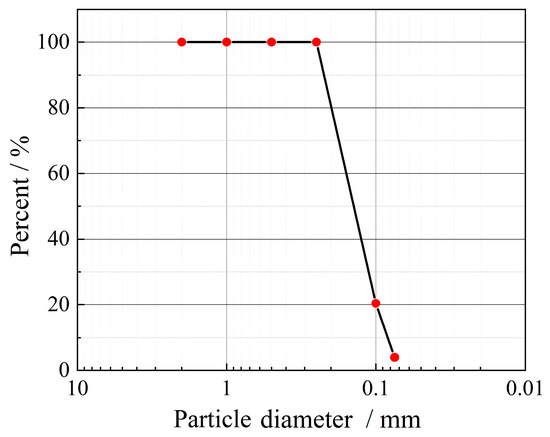
Figure 2.
Particle size distribution of aeolian sand sampled from the Taklamakan Desert, Xinjiang Uyghur.
The cementing materials used for this experiment to consolidate the aeolian sand included anhydrous calcium chloride, urea, urease, and distilled water. Anhydrous calcium chloride and urea were purchased from Tianjin Hengxing Chemical Preparation Co., Ltd. (Tianjin, China), with the purity of CaCl2 being more than 98.0% and that of CO(NH2)2 being more than 99.0%, respectively. Urease, purchased from Kamaishu (Shanghai) Biotechnology Co., Ltd. (Shanghai, China), with a purity of more than 99.0%, was extracted from giant beans and exhibited a specific activity of 200 U/mg at room temperature. In addition, the urease needs to be maintained at −20 °C to ensure a dormant state, and the urease activity achieves its peak state at 45 °C. Under the temperature range of this experiment (20–40 °C), the urease activity showed an increasing trend with the temperature. The specific chemical composition of urease and anhydrous calcium chloride is shown in Table 1.

Table 1.
The chemical composition of urease and anhydrous calcium chloride.
Based on the orthogonal experimental design, the solidification effectiveness of different cementing material proportions was compared, and the material mass proportions with the highest final calcium carbonate content were obtained for use in the mixing method of EICP-reinforced aeolian sand [28]. The material mass proportions are quoted in this study, and the mass of each material added in this experiment is shown in Table 2.

Table 2.
The mass of each experimental material added.
Five sand column samples were prepared for each alternating temperature group. Three temperature groups with a total of 15 sand column samples were used. The mold for holding the aeolian sand was cylindrical in shape and made of high-density polyethylene, with an inner diameter of 50 mm and a height of 100 mm (shown in Figure 3).

Figure 3.
The mold for holding the aeolian sand: (a) schematic of the mold (inner diameter: 50 mm, height: 100 mm, and material: high-density polyethylene); (b) schematic of the aeolian sand column sample (diameter: 50 mm and height: 100 mm).
The mixing method was used in this study, which refers to pouring the cementing material into the aeolian sand at one time point and mixing it well. This method can avoid the need for frequent removal of the samples from the chamber when using the multiple injection method, which has a poor influence on the control of the sample temperature. In addition, the influence of sand particle size on temperature needs to be considered, and in order to minimize the influence of this factor, the aeolian sand needs to be mixed as uniformly as possible before the sample preparation.
The sample preparation steps were as follows: According to the results in Table 2, the corresponding mass of each material was weighed, mixed, and dissolved thoroughly in a beaker. The cementing solution was immediately poured into disinfected aeolian sand (aeolian sand was heated in a 100 °C baking oven for 24 h to eliminate the effect of microorganisms) and mixed well using a sand blender. The mixed aeolian sand was loaded into the cylindrical mold within 15 min by using the layered tamping method [29]: 5 layers total and 25 hits per layer with a hammer weighing 2.5 kg.
2.2. Method
2.2.1. Alternating Temperature Setting and Sample Maintenance
In this study, three groups of alternating temperature processes were designed: T1 is a cyclic process of heating to constant temperature to cooling, which simulates the sunrise-to-sunset process in a natural desert environment. T2 is a cyclic process of cooling to constant temperature to heating, which simulates the sunset-to-sunrise process in a natural desert environment. Meanwhile, T3 is a process of constant temperature throughout as a control group.
The range of the alternating temperature was set to the highest temperature of 40 °C and the lowest temperature of 20 °C. The constant temperature was set to 30 °C throughout the whole process. The alternating temperature was set as a cycle of 12 h, and there were 8 cycles for a total of 96 h. The temperature variation of the three experimental groups during a cycle is shown in Figure 4.
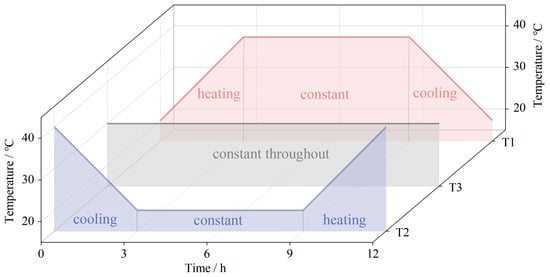
Figure 4.
Temperature variations of the three experimental groups during a cycle (12 h).
The cementing material and aeolian sand were preheated, and the temperature was controlled during the sample preparation process. This ensures that the initial reaction temperature was achieved as soon as possible after the material was mixed, and the effect of the temperature difference from the outer to the inner layer of the aeolian sand column sample was minimized as much as possible. Since urease must be at a temperature of −20 °C to maintain activity without loss and the mass of urease added was extremely small (2.5 mg), the temperature of added urease will have little influence on the temperature of all materials; therefore, the urease was not preheated.
The preheating steps were as follows: Before the materials were mixed, anhydrous calcium chloride, urea, distilled water, and aeolian sand from each temperature experimental group were placed in a temperature maintenance chamber (YH-60B, produced by Shanghai Meiyu Instrument Co., Shanghai, China) with a constant temperature of 20 °C (T1), 40 °C (T2), and 30 °C (T3), all with 20% humidity, for 24 h.
The temperature maintenance steps were as follows: After the preheated steps were conducted for each material (except urease), the samples were prepared according to the description in Section 2.1 and immediately placed in the temperature maintenance chamber, which had been adjusted to the starting temperature (T1: 20 °C, T2: 40 °C, and T3: 30 °C). The samples were maintained according to the temperature curves shown in Figure 4.
2.2.2. Calcium Carbonate Content Test and Calculation of the Calcium Carbonate
Precipitation Rate
The calcium carbonate content of aeolian sand column samples was tested using the acid-washing method [30]. The steps were as follows: The aeolian sand column samples were removed from the temperature maintenance chamber every 12 h, and the aeolian sand in the mold was poured out and mixed well. Approximately 30 g of aeolian sand was weighed and placed in a Petri dish, and the remaining aeolian sand continued to complete the preparation and maintenance according to the method described in Section 2.2.1. The mass of the Petri dish was weighed and recorded as . The Petri dish was filled with distilled water, mixed thoroughly, and allowed to stand for 30 min, and then the upper clear layer was removed using a pipette. This process was repeated three times to wash away the dissolvable salts from the aeolian sand. The aeolian sand that was washed away from the dissolvable salt was dried in a baking oven at 100 °C for 12 h, and the mass of the Petri dish with dry aeolian sand was weighed at this time and recorded as . An excess of 2 mol/L hydrochloric acid solution was added dropwise to the dry aeolian sand sample and mixed until there were no more bubbles, and the process of washing away the dissolvable salt was repeated three times. The aeolian sand washed away from calcium carbonate and dissolvable salts was dried in an oven at 100 °C for 12 h, and the mass of the Petri dish with dry aeolian sand was weighed at this time and recorded as .
Each experimental group was tested five times in parallel, and the calcium carbonate content per unit quantity of dry matter was calculated according to Equation (1).
The rate of calcium carbonate precipitation is the proportion of the actual calcium carbonate production to the theoretical calcium carbonate production. According to the comparison of the calcium carbonate precipitation rate at different maintenance periods (12 h, 24 h, 36 h, 48 h, and 60 h), the consolidation effectiveness of EICP by the mixing method was evaluated, and the optimal reaction duration was predicted.
2.2.3. Permeability Coefficient Test
The permeability coefficient was tested in accordance with the specifications of GB/T 50123-2019 [29], and the steps were as follows: The aeolian sand column samples were removed from the temperature maintenance chamber every 12 h, and the aeolian sand in the mold was poured out and mixed well. The appropriate amount of aeolian sand was loaded into the falling head permeameter (height: 40 mm, cross-sectional area: 2.998 × 104 mm2) with a thickness of approximately 10 mm per layer, and the density of sand in the falling head permeameter was consistent with the sand column sample after layered tamping. The head of the permeameter was connected to a water head pipe, water was supplied to the pipe, and the head height was controlled to within 2 m. After opening the outlet drain valve to expel air from the falling head permeameter and saturating the desert sand, the time, outlet temperature, and heights of the initial and final water heads were tested and recorded, with each parameter accurate to 0.1.
Each experimental group was tested five times in parallel, and the permeability coefficient was calculated according to Equation (2).
where is the permeability coefficient at °C (cm/s), is the cross-sectional area of the water head pipe (cm2), is the permeation time (s), is the initial water head height (cm), and is the final water head height (cm).
2.2.4. Shear Wave Velocity Test
In this regard, shear velocity wave values were measured by an ultrasonic detector (Proceq Pundit PL-200, Proceq Trading Shanghai Co. Ltd., Shanghai, China) with an input signal at 250 kHz frequency. To ensure that the samples were as unaffected by room temperature, each test was controlled to be completed within 15 min, and the steps were as follows: Fifteen sand column samples were removed from the temperature maintenance chamber every 12 h. The length of the sand column in each mold was measured and recorded as . The ultrasonic transducers were evenly covered with coupling and closely attached to both ends of the sand column. The transmission time of the shear waves through the waveform graph on the ultrasonic detector was obtained and recorded as .
The length of the sand column () and transmission time () were accurate to 0.1, and the tests were repeated five times to take the average value. The shear wave velocity was calculated according to Equation (3).
2.2.5. Scanning Electron Microscopy (SEM) Observation
To further investigate the mechanism of EICP in cured aeolian sand, SEM was conducted after temperature maintenance of these specimens to observe the external cementation states by mineralization. After 96 h, the aeolian sand column samples were removed from the temperature maintenance chamber, three temperature groups were sampled, respectively, and the sizes and attachment patterns of the calcium carbonate were observed by ZEISS crossbeam 540.
3. Results
3.1. Calcium Carbonate Content
The calcium carbonate content per unit mass of dry matter for different maintenance periods (from 12 h to 96 h) is shown in Figure 5. The variation in calcium carbonate content in the three experimental groups (T1, T2, and T3) was different in terms of calcium carbonate production rate, reaction duration, and final calcium carbonate content.
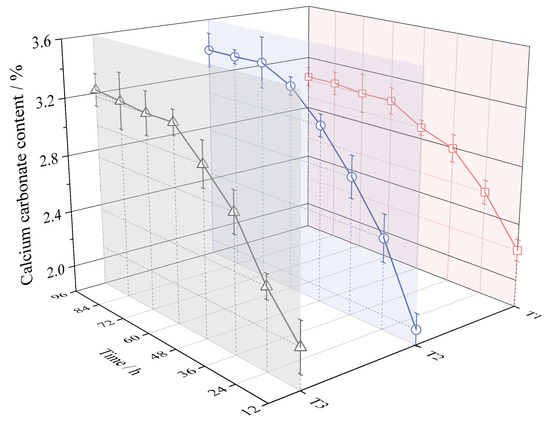
Figure 5.
Calcium carbonate content in different reaction time periods.
In the initial period of the reaction, the process of T1 would increase the rate of calcium carbonate production, while T2 would decrease the rate of calcium carbonate production. At the 12th hour, the calcium carbonate content of T1 was the highest at 2.21%, while the calcium carbonate content of T2 was only 1.92%, which was 13.12% less than that of T1. At 36 h, the calcium carbonate content of the three groups was almost the same. From 36 h to 96 h, the calcium carbonate content of T2 gradually exceeded that of T1.
The reaction duration of T2 was longer. The calcium carbonate content in T1 and T3 remained almost constant at 60 h and thereafter. While the calcium carbonate content in T2 increased in the period from 60 h to 72 h, the reaction process was gradually exhausted at 72 h and thereafter.
The T1 treatment decreased the final calcium carbonate production, while the T2 treatment increased the final calcium carbonate production. The final calcium carbonate content of T1 was the lowest at 3.05%. However, the final calcium carbonate content of T2 was the highest at 3.38%, which was 10.82% higher than that of T1.
3.2. Calculation of the Calcium Carbonate Precipitation Rates
Figure 6 shows the rate of calcium carbonate precipitation for different reaction periods. It can be seen that the calcium carbonate precipitation rate increased rapidly in the initial reaction period (before 12 h), while the rate of increase continued to decline, and then the reaction gradually exhausted after 60 h. The increasing trend of the calcium carbonate precipitation rate was similar to that of the calcium carbonate content (Figure 5). T2 produced the highest final calcium carbonate precipitation rate, which was 10.27% higher than that of T1. The final calcium carbonate precipitation rates of T1, T2, and T3 at 96 h were 73.45%, 81.43%, and 77.69%, respectively.
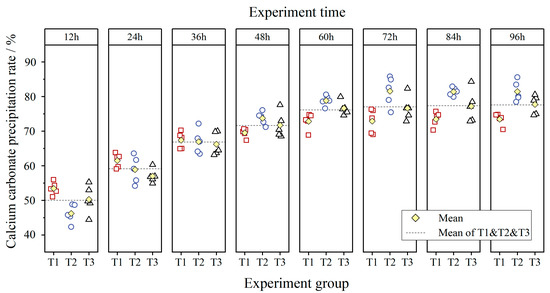
Figure 6.
The rate of calcium carbonate precipitation in different reaction periods (T1: red square; T2: blue circle; T3: black triangle).
Moreover, the mean of T1, T2, and T3 (Figure 6) shows the final calcium carbonate precipitation rate using the mixing method was 77.53% at 96 h. In comparison, the calcium carbonate precipitation rate at 60 h reached 76.10%, which reached 98.16% of that at 96 h.
3.3. Permeability Coefficients
Figure 7 shows the permeability coefficients in different reaction time periods. Some observations can be made from these results. They are as follows:
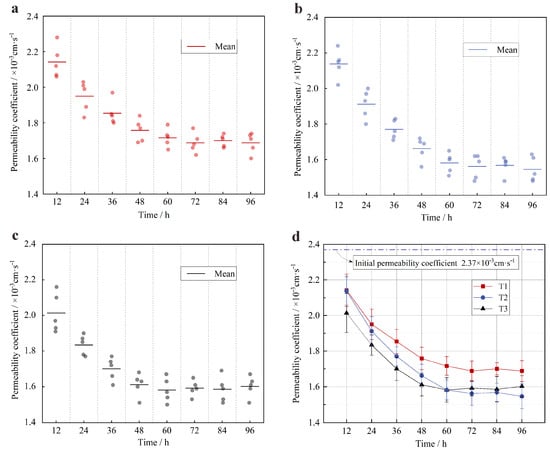
Figure 7.
Permeability coefficient in different reaction periods: (a) variation of permeability coefficient in the process of T1 (red circle); (b) variation of permeability coefficient in the process of T2 (blue circle); (c) variation of permeability coefficient in the process of T3 (black circle); and (d) comparison of the permeability coefficient between T1, T2, and T3.
The permeability coefficient curve showed a continuous decreasing trend until 60 h, after which the decreasing rate gradually slowed down. Among the three experimental groups, the fastest decreasing rate was in the process of T2, followed by T3, and finally, T1. The decreasing rate of T1 was similar to that of T2 and T3 in the initial period, which slowed down significantly after 48 h. The permeability coefficient curves of the three experimental groups all generally stabilized at and after 60 h, and the permeability coefficients remained almost constant.
As can be seen by comparing Figure 6 and Figure 7, the calcium carbonate precipitation rate at 60 h can reach approximately 98% of that at 96 h, and the permeability coefficients also varied very little from 60 h to 96 h; the arithmetic mean of the permeability coefficients of 15 samples in four time periods (60 h, 72 h, 84 h, and 96 h) was taken as the final permeability coefficients to reduce the error. The results were as follows: 1.70 × 10−3 cm·s−1 (T1), 1.56 × 10−3 cm·s−1 (T2), and 1.59 × 10−3 cm·s−1 (T3). The final permeability coefficient of T2 decreased the most, with a decrease of 34.2%. T3 was similar to T2, with a decrease of 32.9%. The final permeability coefficient of T1 decreased the least, with only 28.3%.
3.4. Shear Wave Velocity
The data of shear wave velocity for the three experimental groups at different maintenance periods are shown in Figure 8. Inspections show the highest increase in shear wave velocity for T2, followed by T1, and finally, T3. Specifically, the shear wave velocity shows a continuous increase until 60 h, while the increase rate decreases gradually. The increase rates of T1 and T2 are significantly higher than those of T3, and the increase in shear wave velocity ranges from 100 m·s−1 to 150 m·s−1. After 60 h, the shear wave velocity gradually stabilized and remained constant. At 96 h, the shear wave velocities of T1 and T3 were close, but that of T2 were significantly higher than those of T1 and T3, exceeding 100 m·s−1. Meanwhile, the increasing trend of shear wave velocity is consistent with the changing trend of calcium carbonate content in Figure 5.
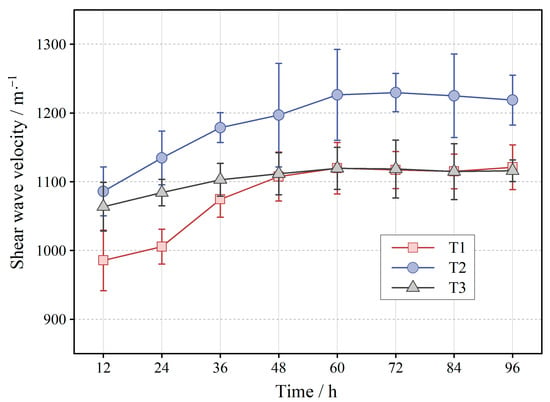
Figure 8.
Shear wave velocity in different reaction periods.
3.5. SEM Images
Figure 9 shows the bonding of precipitated calcium carbonate on the surface of aeolian sand particles.
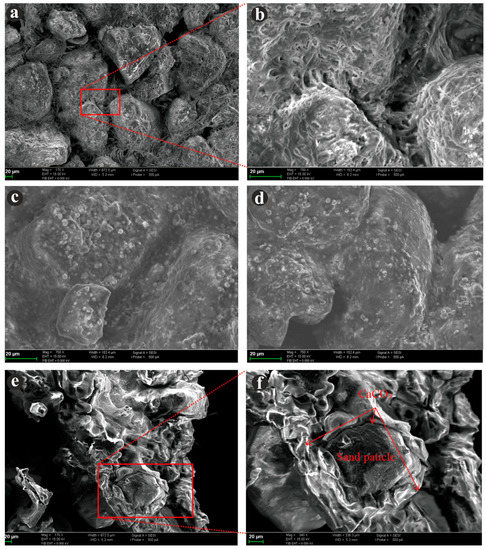
Figure 9.
SEM images of the cemented aeolian sand specimens. (a,b) The connectivity of the sand particles treated by EICP; (c,d) spherical material on the surface of sand particles and the pores between the sand particles; and (e,f) calcium carbonate was generated around sand particles.
Figure 9a,b show that the calcium carbonate precipitate formed a film that blocked the pores between the sand particles. However, some pores still exist between the sand particles, indicating that the generated calcium carbonates are not enough to fill all the pores. Furthermore, it could be found that the spherical material with an irregular and disordered structure was mainly distributed on the surface of sand particles and the pores between the sand particles. Additionally, the spherical material was probably vaterite, which was clearly observed for all test cases after magnifying the SEM images as in Figure 9c,d regardless of T1, T2, and T3; this appeared in the form of spherical or spherical aggregates. Figure 9e,f show that the thicknesses of the calcium carbonate were approximately 10–25 µm inside the samples, and calcium carbonate was generated around sand particles, which served as nucleation sites, and attached to these particles.
4. Discussion
4.1. Influence of Alternating Temperature on the Calcium Carbonate Content
The process of T1 increased the rate of calcium carbonate production in the initial reaction period, the reaction duration was relatively shorter, and the final calcium carbonate content was the lowest. In contrast, the process of T2 decreased the rate of calcium carbonate production in the initial reaction period but allowed for a longer reaction duration, and the final calcium carbonate content was the highest. The process of T3 produces results that are between those of T1 and T2.
The increase in temperature increased the urease activity, which also accelerated the rate of calcium carbonate production [31,32]. Similar observations were found in Wang et al., where the urease activity in sand consolidation by EICP gradually increased with increasing temperature (from 25 to 45 °C) [33]. This is also evidenced by the result of calcium carbonate production (T2 > T3 > T1) at 12 h, as shown in Figure 5. However, the increase in urease activity was limited. Urease is a protein, and its activity will decay over time [34,35,36]. Cuccurullo et al. found that when crude soybean extract samples were exposed to laboratory air (temperature 25 °C and relative humidity 40%), urease was largely inactivated after 72 h and showed a decrease in urease activity within the first few hours [26]. Based on the measurement of the urease activity of crude extracts from watermelon seeds at various temperatures (from 25 to 70 °C), Dilrukshi et al. claimed that the urease activity decreased with time and that the decay rate was more pronounced at high temperatures [12]. Similar observations regarding the effect of time on urease activity can also be found in Shu et al. [37]. Therefore, the survival duration of urease will also directly affect the final calcium carbonate production.
Only the calcium carbonate content of T2 maintained a significant increasing trend after 60 h, while that of T1 and T3 remained almost constant. The calcium carbonate precipitation rate reached approximately 75% at 60 h; the reactants were not yet completely consumed, but the reaction stopped. This indicates that the urease in the process of T1 and T3 was mostly inactivated, while the urease in T2 maintained a certain activity after 60 h, and the reaction continued until 72 h, which eventually produced the highest calcium carbonate content.
The process of T2 corresponds to the process from sunset to sunrise in natural desert environments. From the above results, it is recommended the construction step of mixing the cementing solution into the aeolian sand be scheduled at sunset when using the EICP and mixing methods for curing aeolian sand.
4.2. Influence of Alternating Temperature on the Calcium Carbonate Precipitation Rate
The final calcium carbonate precipitation rate was highest in T2, followed by T3, and lowest in T1. Since the survival duration of urease directly affects the final calcium carbonate production, the process of T2 was able to keep urease alive for a longer period of time, which led to the highest final calcium carbonate production. The 15 samples had the same proportions of material mass, and theoretically, the calcium carbonate production of each sample would be the same. The highest final calcium carbonate content was found in the process of T2, which corresponded to the highest rate of calcium carbonate precipitation.
As the mixing method is not subsequently supplemented with cementing solution, the initial cementing solution was used at a high concentration. The initial concentration of CaCl2 is 3 mol/L, and the concentration of the cementing solution increases further under the influence of evaporation. However, the final calcium carbonate precipitation rate reached 81.43% in the process of T2. The average calcium carbonate precipitation rate in the three experimental groups (T1, T2, and T3) also reached approximately 75%. The literature found that the final calcium carbonate precipitation rate could be more than 80% or even 100% based on the multiple injection method and using a low concentration of cementing solution to consolidate aeolian sand [33,38]. Reducing the concentration of the cementing solution has been confirmed as one of the methods to improve the calcium carbonate precipitation rate [39,40].
However, for the mixing method, a low concentration of the cementing solution cannot provide sufficient calcium ions to ensure the continuing production of calcium carbonate. For injection and grout methods, the high concentration of calcium carbonate can lead to the blockage of pore channels due to the high rate of calcium carbonate production in the initial period, which results in a low calcium carbonate precipitation rate [41,42,43]. In this study, the aeolian sand and the cementing material were mixed well before the reaction, there is no risk of the injection or grout channels being blocked, and a higher concentration of cementing solution could be used to ensure the continuous production of calcium carbonate. The literature found the inhibitory effect of a high calcium ion concentration will be weakened in the highly active urease solution, and the production rates of calcium carbonate can be maintained at a high level [44]. Of course, a commercial urease with high urease activity was used in this experiment, which was extracted from giant beans and exhibited a specific activity of 200 U/mg at room temperature. The initial concentration of urease activity and CaCl2 in the cementing solution is approximately 17,000 U/L and 3 mol/L, respectively. The final calcium carbonate precipitation rate can be up to 81.43%. This suggests that the use of high calcium ion concentration and highly active urease solution is feasible based on the mixing method for sand consolidation by EICP.
4.3. Influence of Alternating Temperature on the Permeability Coefficient
T2 exhibited the greatest influence on the permeability coefficient, followed by T3 and then T1. The increase in calcium carbonate content can decrease the permeability coefficient of the samples to a certain extent [45,46,47]. The final calcium carbonate content of T2 is higher than that of T1, and theoretically, the influence on the permeability coefficient of T2 will also be greater than T1, which is also demonstrated in Figure 7. It can be seen by comparing Figure 5 and Figure 7 that the higher the amount of calcium carbonate content is, the greater the decrease in the permeability coefficient, and both can be verified.
4.4. Influence of Alternating Temperature on the Shear Wave Velocity
The highest shear wave velocity increase and final shear wave velocity values were found for T2, followed by T1, and finally, T3. The literature confirmed that with the increase in the calcium carbonate content in the sand matrix, the shear wave velocity of the cemented specimen increased [48,49]. This was also confirmed in this experiment, where the trend of shear wave velocity in Figure 8 is consistent with the trend of calcium carbonate content in Figure 5. The shear wave velocities of all three experimental groups showed a steady increase until 60 h, and did not increase after 60 h. Combined with the analysis of calcium carbonate content, it is clear that the reaction process of EICP is gradually completed, and the calcium carbonate content progressively stabilized after 60 h. This indicates that the shear wave velocity is strongly influenced by the calcium carbonate content of the cemented specimen after 60 h, regardless of the temperature alteration process. This also suggests that the shear wave velocity can be used as a non-destructive evaluation indicator of calcium carbonate precipitation [39].
At 96 h, the shear wave velocities of T1 and T3 were close, but T2 was significantly higher than those of T1 and T3, exceeding 100 m·s−1. Since the absolute values of shear wave velocity increase in all three experimental groups are within 200 m·s−1, it can be seen that the shear wave velocity increase in T2 is more advantageous.
4.5. Influence of Alternating Temperature on the SEM Images
Crystal morphology was commonly used as one of the important markers to distinguish mineral types, and its differences ultimately affect the effectiveness of calcium carbonate crystal in solidification [50,51]. There were usually three different amorphous calcium carbonate forms, namely vaterite, aragonite, and calcite, which were transformed from amorphous calcium carbonate [52].
However, similar microscopic morphology of calcium carbonates was observed regardless of T1, T2, and T3. It could be found that vaterites were clearly observed for all test cases after magnifying the SEM images, as in Figure 9c,d; this appeared in the form of spherical or spherical aggregates. It has been shown that the growth kinetics of vaterite was superior to calcite at high urease activity in the calcium carbonate precipitation process [53]. In this regard, a commercial urease with high urease activity was used in this experiment. Similar observations regarding the dominant precipitation morphology of vaterite can also be found in the sand consolidation experiments of EICP by Nafisi et al. [50].
Furthermore, it could also be found that the calcium carbonate with irregular and disordered structure was generated around sand particles (Figure 9e,f). The sand particles served as nucleation sites, and calcium carbonates were attached to these particles. The thicknesses of the calcium carbonate were approximately 10–25 µm, while the calcium carbonate connected the surrounding sand particles into an aggregate. This finding suggests that sand particles can be used as nucleation sites to separate calcium carbonate from solutions [25,39,54,55], but this adsorption is not strong. Some pores still exist between the sand particles; the generated calcium carbonates are not enough to fill all the pores.
5. Conclusions
Based on the mixing method and EICP to consolidate aeolian sand, the differences in calcium carbonate content, calcium carbonate precipitation rate, permeability coefficients, and shear wave velocity of the three alternating temperature experimental groups (T1, T2, and T3) at different maintenance periods were tested and analyzed. Meanwhile, the mineralized external cementation state was observed by SEM. The following conclusions can be drawn.
- T2 had the highest calcium carbonate content and the greatest alteration on the permeability coefficient and shear wave velocity, followed by T3 and, finally, T1. The different alternating temperatures would affect the urease survival time, and T2 showed the longest reaction time as the urease stayed active for the longest time;
- At a high urease activity, vaterites could be clearly observed in the form of spherical or spherical aggregates. Moreover, sand particles could be used as nucleation sites to separate calcium carbonate from solutions, but this adsorption is not strong;
- T2 reflected the process from sunset to sunrise in a natural desert environment. Utilizing the alternating temperature process, the construction step of mixing the cementing solution into the aeolian sand can be scheduled at sunset to obtain better on-site cementation effectiveness.
Author Contributions
Z.Z., Q.Z., Y.Z. and D.L. conceived of the study, designed this study, and carried out the field investigation. Q.Z. and Y.Z. carried out the lab work and analyzed data. Q.Z. and Y.Z. wrote the original manuscript. Z.Z. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Projects of the Financial Science and Technology Plan of Xinjiang Production and Construction Corps, grant number 2020AA002.
Institutional Review Board Statement
Not Application.
Informed Consent Statement
Not Application.
Data Availability Statement
Some or all data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no competing interests.
References
- DeJong, J.T.; Soga, K.; Banwart, S.A.; Whalley, W.R.; Ginn, T.R.; Nelson, D.C.; Mortensen, B.M.; Martinez, B.C.; Barkouki, T. Soil Engineering In Vivo: Harnessing natural biogeochemical systems for sustainable, multi-functional engineering solutions. J. R. Soc. Interface 2011, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Montoya, B.M.; DeJong, J.T. Stress-Strain Behavior of Sands Cemented by Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2015, 141, 04015019. [Google Scholar] [CrossRef]
- Pan, X.; Chu, J.; Yang, Y.; Cheng, L. A new biogrouting method for fine to coarse sand. Acta Geotech. 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Tiwari, N.; Satyam, N.; Sharma, M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci. Rep. 2021, 11, 10324. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Tirkolaei, H.K.; Kavazanjian, E. Enhancing the strength of granular material with a modified enzyme-induced carbonate precipitation (EICP) treatment solution. Constr. Build. Mater. 2020, 271, 121529. [Google Scholar] [CrossRef]
- Meyer, F.D.; Bang, S.; Min, S.; Stetler, L.D.; Bang, S.S. Microbiologically-Induced Soil Stabilization: Application of Sporosarcina pasteurii for Fugitive Dust Control. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; 2011; pp. 4002–4011. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; Devlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. Eng. Ground Improv. 2014, 168, 206–216. [Google Scholar] [CrossRef]
- Zhan, Q.W.; Qian, C.X.; Yi, H.H. Microbial-induced mineralization and cementation of fugitive dust and engineering appli-cation. Constr. Build. Mater. 2016, 121, 437–444. [Google Scholar] [CrossRef]
- Naeimi, M.; Chu, J. Comparison of conventional and bio-treated methods as dust suppressants. Environ. Sci. Pollut. Res. 2017, 24, 23341–23350. [Google Scholar] [CrossRef]
- Arab, M.; Alsodi, R.; Shanablah, A.; Kavazanjian, E.; Zeiada, W. Evaluation of microstructural strength of bio-cemented sand crust using rheometry. Geotech. Lett. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, Y.X.; Gao, Y.; Siriguleng, B. Fieldexperimental study on stability of bio-mineralizationcrust in Ulan Buh Desert, Inner Mongolia of China. Rock Soil Mech. 2019, 40, 1291–1298. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Nakashima, K.; Kawasaki, S. Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found. 2018, 58, 894–910. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef]
- Tang, C.-S.; Yin, L.-Y.; Jiang, N.-J.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.L.; Zhang, Y.; Jiang, X.; Li, C.; Chu, J.; Xiao, Y. Dynamic strength of temperature-controlled MICP-treated cal-careous sand. Chin. J. Geotech. Eng. 2021, 43, 511–519. [Google Scholar] [CrossRef]
- Rebata-Landa, V. Microbial Activity in Sediments: Effects on Soil Behavior. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2007. [Google Scholar]
- Sahrawat, K.L. Effects of temperature and moisture on urease activity in semi-arid tropical soils. Plant Soil 1984, 78, 401–408. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2004. [Google Scholar]
- Wu, L.Y.; Miao, L.C.; Sun, X.H.; Chen, R.F.; Wang, C.C. Experimental study on sand solidification using plant-derived urease-induced calcium carbonate precipitation. Chin. J. Geotech. Eng. 2020, 349, 120–126. (In Chinese) [Google Scholar] [CrossRef]
- Li, L.; Li, X.-Y.; Xu, X.-W.; Lin, L.-S.; Zeng, F.-J. Effects of high temperature on the chlorophyll a fluorescence of Alhagi sparsifolia at the southern Taklamakan Desert. Acta Physiol. Plant. 2014, 36, 243–249. [Google Scholar] [CrossRef]
- Wu, S.; An, G.; Wang, L.; Zhang, C. A thermochemical heat and cold control strategy for reducing diurnal temperature variation in the desert. Sol. Energy Mater. Sol. Cells 2022, 235, 111460. [Google Scholar] [CrossRef]
- Nemati, M.; Greene, E.A.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process. Biochem. 2004, 40, 925–933. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2020, 31, 1317–1324. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X. Enzyme-catalysed mineralisation experiment study to solidify desert sands. Sci. Rep. 2020, 10, 10611. [Google Scholar] [CrossRef] [PubMed]
- Almajed, A.; Lemboye, K.; Arab, M.G.; Alnuaim, A. Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found. 2020, 60, 356–371. [Google Scholar] [CrossRef]
- Cuccurullo, A.; Gallipoli, D.; Bruno, A.W.; Augarde, C. Soil stabilization against water erosion via calcite precipitation by plant-derived urease. In Geotechnical Research for Land Protection and Development; Lecture Notes in Civil Engineering Book Series; Springer: Cham, Switzerland, 2020; Volume 40, pp. 753–762. [Google Scholar] [CrossRef]
- Jin, L.L.; He, Q.; Li, Z.J.; Liu, Q.; Huang, J. Temperature Features of Sand Dune in Hinterland of Taklimakan Desert. J. Arid Meteor. 2010, 28, 134–141. [Google Scholar] [CrossRef]
- Zhang, Y. Study on the Ratio and Effect of Enzyme Induced Calcite Precipitation Reinforced Desert Sand under Alternating Temperature. Master′s Thesis, China University of Geosciences Beijing, Beijing, China, 2022. [Google Scholar]
- GB/T50123-2019; Standard for Geotechnical testing method, The Ministry of Housing and Urban-Rural Development of the People’s Republic of China 2019. Ministry of Water Resources of the People’s Republic of China: Beijing, China, 2019.
- Lee, S.; Kim, J. An experimental study on enzymatic-induced carbonate precipitation using yellow soybeans for soil stabilization. KSCE J. Civ. Eng. 2020, 24, 2026–2037. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.; Karim, R.; Beecham, S.; Saint, C. A Review of Enzyme Induced Carbonate Precipitation (EICP): The Role of Enzyme Kinetics. Sustain. Chem. 2021, 2, 92–114. [Google Scholar] [CrossRef]
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2023, 12, 1562. [Google Scholar] [CrossRef]
- Wang, H.X.; Miao, L.C.; Sun, X.H.; Wu, L.Y.; Fan, G.C. Experimental study on sand solidification and optimization of EICP in different temperature environments. J. Southeast Univ. Nat. Sci. Ed. 2022, 52, 712–719. [Google Scholar] [CrossRef]
- Sumner, J.B. The isolation and crystallization of the enzyme urease preliminary. J. Biol. Chem. 1926, 69, 435–441. [Google Scholar] [CrossRef]
- Pettit, N.; Smith, A.; Freedman, R.; Burns, R. Soil urease: Activity, stability and kinetic properties. Soil Biol. Biochem. 1976, 8, 479–484. [Google Scholar] [CrossRef]
- Marzadori, C.; Miletti, S.; Gessa, C.; Ciurli, S. Immobilization of jack bean urease on hydroxyapatite: Urease immobilization in alkaline soils. Soil Biol. Biochem. 1998, 30, 1485–1490. [Google Scholar] [CrossRef]
- Shu, S.; Yan, B.; Ge, B.; Li, S.; Meng, H. Factors Affecting Soybean Crude Urease Extraction and Biocementation via Enzyme-Induced Carbonate Precipitation (EICP) for Soil Improvement. Energies 2022, 15, 5566. [Google Scholar] [CrossRef]
- Wu, L.; Miao, L.; Kawasaki, S.; Wang, H. Effects of Reaction Conditions on EICP-Treated Desert Aeolian Sand. KSCE J. Civ. Eng. 2022, 26, 2662–2674. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, W.; Liu, Z.; Wang, Q.; Chen, Y. Influence of injection methods on calcareous sand cementation by EICP technique. Constr. Build. Mater. 2022, 363, 129724. [Google Scholar] [CrossRef]
- Shu, S.; Yan, B.; Meng, H.; Bian, X. Comparative study of EICP treatment methods on the mechanical properties of sandy soil. Soils Found. 2022, 62, 101246. [Google Scholar] [CrossRef]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Hommel, J.; Lauchnor, E.; Gerlach, R.; Cunningham, A.B.; Ebigbo, A.; Helmig, R.; Class, H. Investigating the Influence of the Initial Biomass Distribution and Injection Strategies on Biofilm-Mediated Calcite Precipitation in Porous Media. Transp. Porous Media 2016, 114, 557–579. [Google Scholar] [CrossRef]
- Tang, C.S.; Pan, X.H.; Lyu, C.; Dong, Z.H.; Liu, B.; Wang, D.L.; Li, H.; Cheng, Y.J.; Shi, B. Bio-geoengineering Technology and the Applications. Geol. J. China Univ. 2021, 27, 625–654. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Shi, W.; Song, D.; Yu, L.; Shi, L.; Han, Z. Experimental study on the calcium carbonate production rates and crystal size of EICP under multi-factor coupling. Case Stud. Constr. Mater. 2023, 18, e01802. [Google Scholar] [CrossRef]
- Hommel, J.; Gehring, L.; Weinhardt, F.; Ruf, M.; Steeb, H. Effects of Enzymatically Induced Carbonate Precipitation on Capillary Pressure–Saturation Relations. Minerals 2022, 12, 1186. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Guo, H. Bio-improved hydraulic properties of sand treated by soybean urease induced carbonate precipitation and its application Part 2: Sand-geotextile capillary barrier effect. Transp. Geotech. 2021, 27, 100484. [Google Scholar] [CrossRef]
- Mo, Y.; Yue, S.; Zhou, Q.; Liu, X. Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation. Materials 2021, 14, 5140. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Al Qabany, A.; Mortensen, B.; Martinez, B.; Soga, K.; DeJong, J. Microbial Carbonate Precipitation: Correlation of S-Wave Velocity with Calcite Precipitation. Geo-Front. Congr. 2011, 3993–4001. [Google Scholar] [CrossRef]
- Nafisi, A.; Safavizadeh, S.; Montoya, B.M. Influence of Microbe and Enzyme-Induced Treatments on Cemented Sand Shear Response. J. Geotech. Geoenviron. Eng. 2019, 145, 06019008. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.-H.; Kim, S.-G.; Park, J.-Y.; Kang, C.-H.; Jeong, J.-H.; So, J.-S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Zehner, J.; Røyne, A.; Sikorski, P. Calcite seed-assisted microbial induced carbonate precipitation (MICP). PLoS ONE 2021, 16, e0240763. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Géotechnique 2016, 66, 546–555. [Google Scholar] [CrossRef]
- Phua, Y.; Røyne, A. Bio-cementation through controlled dissolution and recrystallization of calcium carbonate. Constr. Build. Mater. 2018, 167, 657–668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).