Thermal Properties of Ultrasound-Extracted Okra Mucilage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
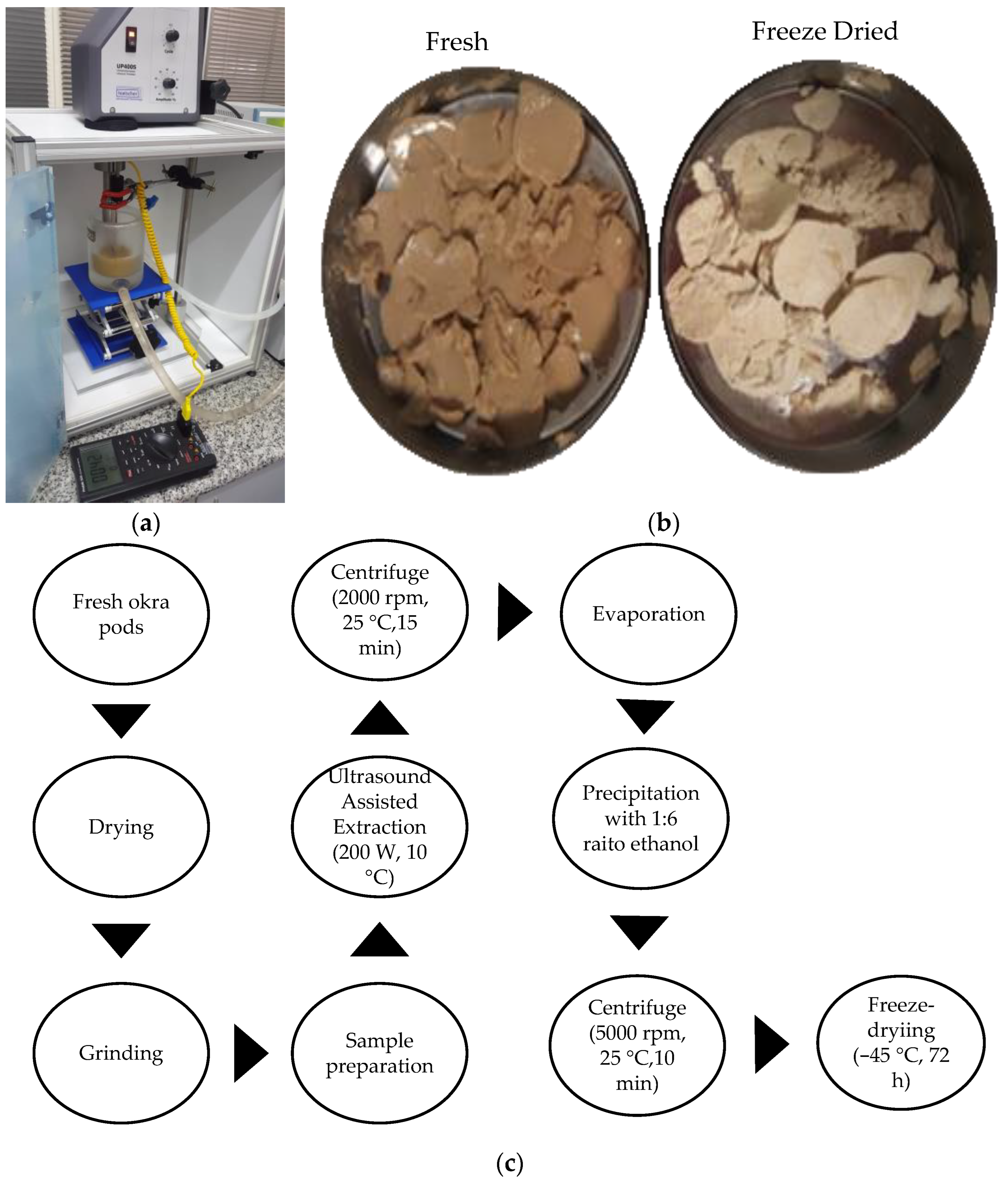
2.2.1. Ultrasound-Assisted Extraction Method
2.2.2. Ultrasound-Assisted Extraction Yield
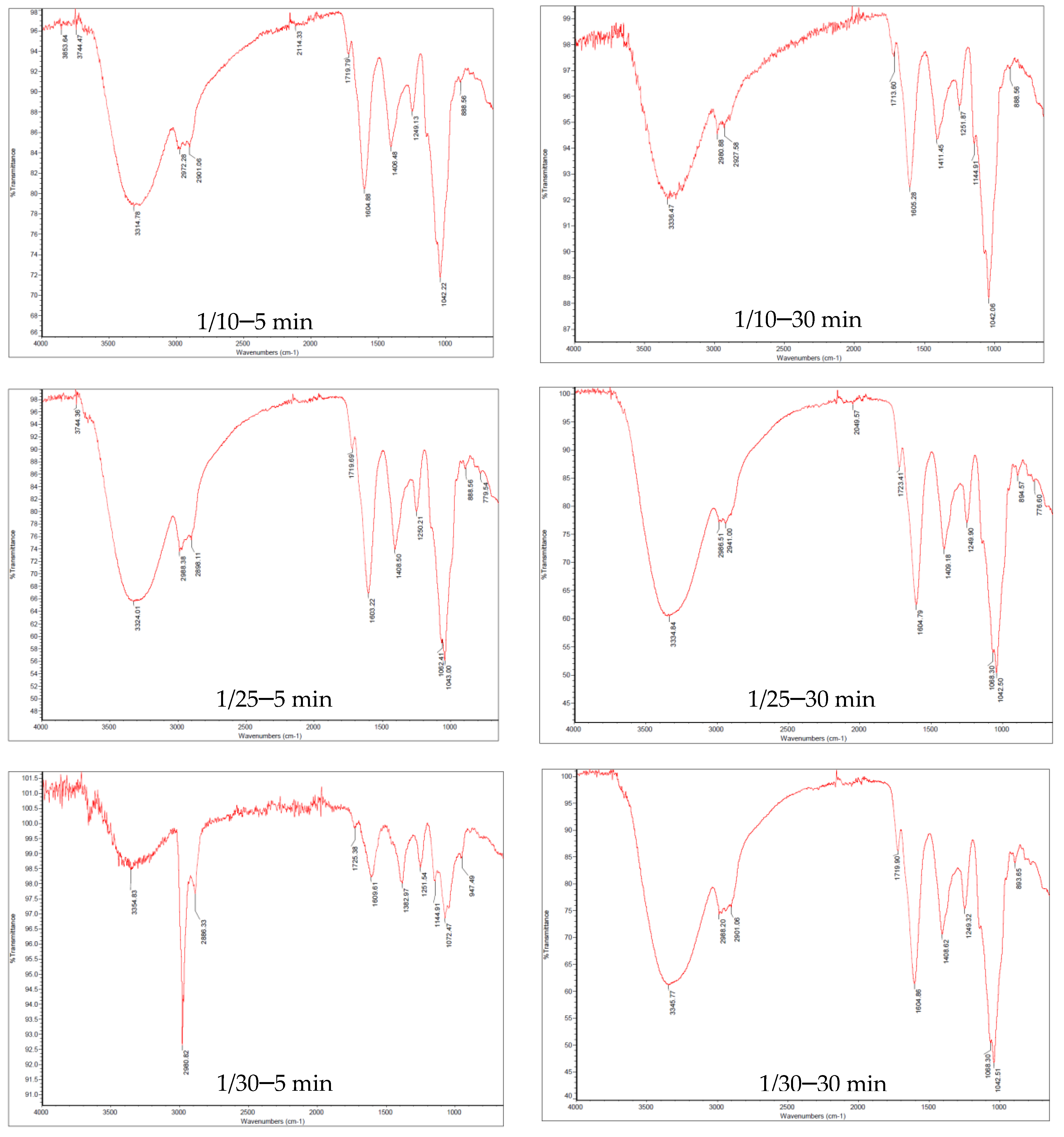
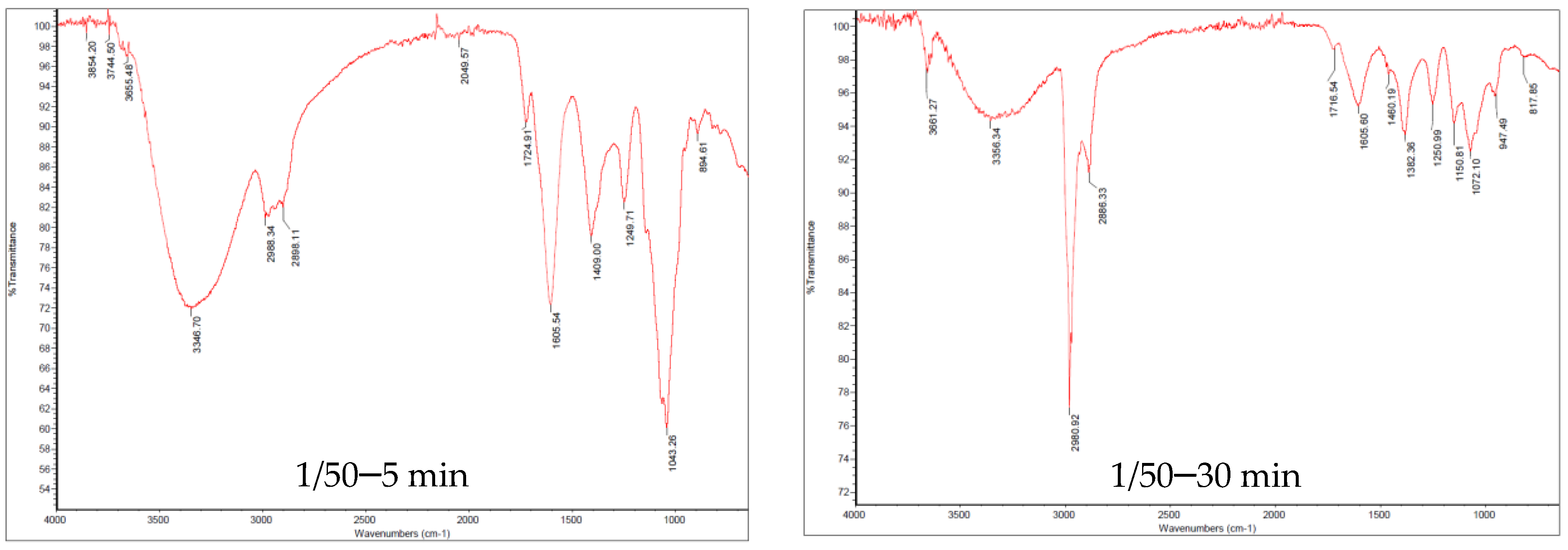
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Thermal Characterization
Differential Scanning Calorimetry (DSC)
Thermogravimetric Analysis (TGA)
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Yield of the Ultrasound-Assisted Extraction of Okra Polysaccharides (UAEOP)
3.2. Molecular Characterization of the UAEOP
3.3. Characterization of Thermal Properties
3.3.1. Differential Scanning Calorimetry (DSC)
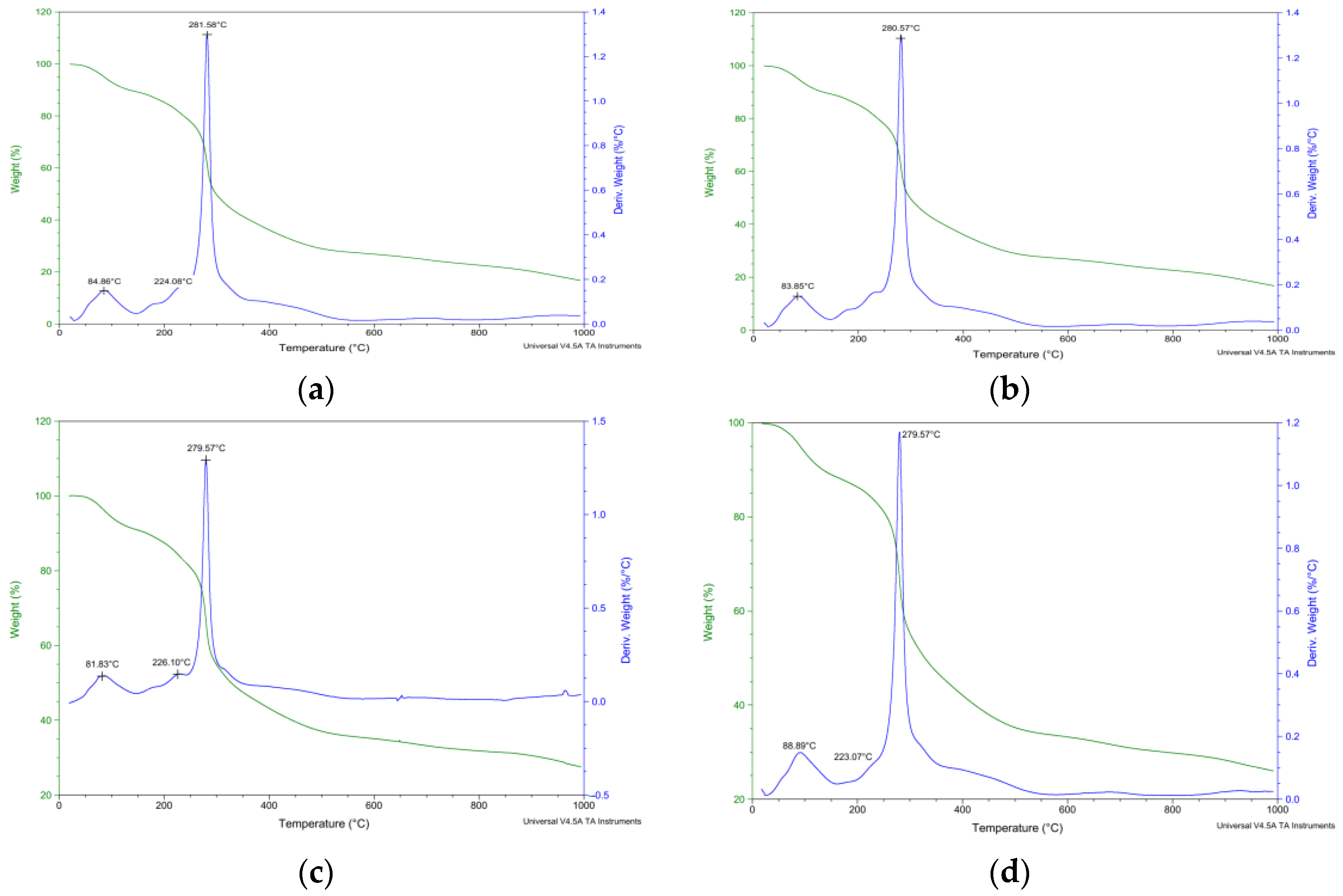
3.3.2. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira Filho, J.G.; Lira, M.M.; de Sousa, T.L.; Campos, S.B.; Lemes, A.C.; Egea, M.B. Plant-Based Mucilage with Healing and Anti-Inflammatory Actions for Topical Application: A Review. Food Hydrocoll. Health 2021, 1, 100012. [Google Scholar] [CrossRef]
- Zhu, W.; Obara, H. The Pre-Shearing Effect on the Rheological Properties of Okra Mucilage. Colloids Surf. A Phys. Eng. Asp. 2022, 648, 129257. [Google Scholar] [CrossRef]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and Characterization of Mucilage Polysaccharide for Biomedical Applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Tang, L.; Liu, X.; Gao, X.; Jia, J. Ultrasound-Assisted Extraction Process of Polysaccharides from Okra Assisted by Response Surface Methodology. In Proceedings of the International Conference on Advanced Manufacturing and Industrial Application, Paris, France, 1 December 2015; Atlantis Press: Paris, France, 2015; pp. 149–152. [Google Scholar]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus Esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Wang, C.; Ma, H.; Ma, Y.; Yan, J.-K. Physicochemical and Functional Characteristics of Polysaccharides from Okra Extracted by Using Ultrasound at Different Frequencies. Food Chem. 2021, 361, 130138. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wu, L.X.; Cai, W.D.; Xiao, G.S.; Duan, Y.; Zhang, H. Subcritical Water Extraction-Based Methods Affect the Physicochemical and Functional Properties of Soluble Dietary Fibers from Wheat Bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of Combined Enzyme-Ultrasonic Extraction on the Physicochemical Characteristics and Properties of Okra Polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra Extracts in Pharmaceutical and Food Applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-Assisted Extraction and Structural Characterization by NMR of Alginates and Carrageenans from Seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound Modified Polysaccharides: A Review of Structure, Physicochemical Properties, Biological Activities and Food Applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Hedayati, S.; Niakousari, M.; Babajafari, S.; Mazloomi, S.M. Ultrasound-Assisted Extraction of Mucilaginous Seed Hydrocolloids: Physicochemical Properties and Food Applications. Trends Food Sci. Technol. 2021, 118, 356–361. [Google Scholar] [CrossRef]
- Rao, M.; Rizvi, S. Engineering Properties of Foods; Marcel Decker. Inc.: New York, NY, USA, 1995. [Google Scholar]
- Chen, G.; Fang, C.; Ran, C.X.; Tan, Y.; Yu, Q.; Kan, J. Comparison of Different Extraction Methods for Polysaccharides from Bamboo Shoots (Chimonobambusa Quadrangularis) Processing by-Products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and Characterization of Acetylated LM-Pectins Extracted from Okra Pods. Food Hydrocoll. 2015, 43, 726–735. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Kaur, J.; Panayampadan, A.S.; Dar, O.I.; Kothakota, A.; Pandiselvam, R. Understanding the Effects of Ultrasound Processng on Texture and Rheological Properties of Food. J. Texture Stud. 2022, 53, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Mohammad, M.A.; Rudrangi, S.R.S.; Fleming, L.T.; Merchant, H.A.; Smith, A.M.; Conway, B.R. Impact of Purification on Physicochemical, Surface and Functional Properties of Okra Biopolymer. Food Hydrocoll. 2017, 71, 311–320. [Google Scholar] [CrossRef]
- Al-Amoudi, R.H.; Taylan, O.; Kutlu, G.; Can, A.M.; Sagdic, O.; Dertli, E.; Yilmaz, M.T. Characterization of Chemical, Molecular, Thermal and Rheological Properties of Medlar Pectin Extracted at Optimum Conditions as Determined by Box-Behnken and ANFIS Models. Food Chem. 2019, 271, 650–662. [Google Scholar] [CrossRef]
- Zhao, Q.; Kennedy, J.F.; Wang, X.; Yuan, X.; Zhao, B.; Peng, Y.; Huang, Y. Optimization of Ultrasonic Circulating Extraction of Polysaccharides from Asparagus Officinalis Using Response Surface Methodology. Int. J. Biol. Macromol. 2011, 49, 181–187. [Google Scholar] [CrossRef]
- De Alvarenga Pinto Cotrim, M.; Mottin, A.C.; Ayres, E. Preparation and Characterization of Okra Mucilage (Abelmoschus Esculentus) Edible Films. Macromol. Symp. 2016, 367, 90–100. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive Okra Polymer Based Buccal Patches as Platform for Controlled Drug Delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. Optimisation of Extraction and Sludge Dewatering Efficiencies of Bio-Flocculants Extracted from Abelmoschus Esculentus (Okra). J. Environ. Manag. 2015, 157, 320–325. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide Extraction from Abelmoschus Esculentus: Optimization by Response Surface Methodology. Carbohydr. Polyp. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Effects of Extraction Methods on the Physicochemical Characteristics and Biological Activities of Polysaccharides from Okra (Abelmoschus Esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin Isolation and Characterization from Six Okra Genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Ormanli, E.; Bayraktar, O.; Şahar, U.; Tavman, S.; Kumcuoglu, S. Development and Characterization of Films Based on Okra Polysaccharides and Whey Protein Isolate. J. Food Meas. Charact. 2022, 17, 264–277. [Google Scholar] [CrossRef]
- Wang, R.; Chen, P.; Jia, F.; Tang, J.; Ma, F. Optimization of Polysaccharides from Panax Japonicus C.A. Meyer by RSM and Its Anti-Oxidant Activity. Int. J. Biol. Macromol. 2012, 50, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, T.; Feng, W.; Wang, W.; Zou, Y.; Zheng, D.; Takase, M.; Li, Q.; Wu, H.; Yang, L.; et al. Purification, Characterization and Immunomodulating Activity of a Polysaccharide from Flowers of Abelmoschus Esculentus. Carbohydr. Polym. 2014, 106, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, G.; Mishra, A.; Chawra, H.; Singh, S.K.; Pansare, K. Okra Mucilage: Potential Role in Drug Delivery. SGVU J. Pharm. Res. Educ. 2021, 6, 649–661. [Google Scholar]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A Comparison Study on Polysaccharides Extracted from Laminaria Japonica Using Different Methods: Structural Characterization and Bile Acid-Binding Capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Noordin, M.I.; Kadivar, A. The Use of Hibiscus Esculentus (Okra) Gum in Sustaining the Release of Propranolol Hydrochloride in a Solid Oral Dosage Form. BioMed Res. Int. 2014, 2014, 735891. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Ritzoulis, C.; Papastergiadis, E.S.; Panayiotou, C. Composite Materials Based on Okra Hydrocolloids and Hydroxyapatite. Food Hydrocoll. 2014, 42, 348–354. [Google Scholar] [CrossRef]
- Li, M.F.; Fan, Y.M.; Xu, F.; Sun, R.C. Structure and Thermal Stability of Polysaccharide Fractions Extracted from the Ultrasonic Irradiated and Cold Alkali Pretreated Bamboo. J. Appl. Polym. Sci. 2011, 121, 176–185. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.D.O.; Flôres, S.H. Edible Film Production from Chia Seed Mucilage: Effect of Glycerol Concentration on Its Physicochemical and Mechanical Properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, I.M.; Kenny, J.M.; Puglia, D.; Santulli, C.; Sarasini, F. Morphological, Thermal and Mechanical Characterization of Okra (Abelmoschus Esculentus) Fibres as Potential Reinforcement in Polymer Composites. Compos. Sci. Technol. 2010, 70, 116–122. [Google Scholar] [CrossRef]


| Solid/Solvent Ratio | Time (min) | Yield (%) |
|---|---|---|
| 1:10 g/mL * | 5 | 5.33 ± 0.21 d |
| 30 | 2.52 ± 0.13 D | |
| 1:25 g/mL * | 5 | 6.13 ± 0.36 c |
| 30 | 8.15 ± 0.19 C | |
| 1:30 g/mL | 5 | 9.79 ± 0.22 b |
| 30 | 9.02 ± 0.35 B | |
| 1:50 g/mL | 5 | 11.04 ± 0.32 a |
| 30 | 11.63 ± 0.68 A |
| UAEOP Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | |||||||
| UAEOP Ratios | 1/10 | 1/25 | 1/30 | 1/50 | 1/10 | 1/25 | 1/30 | 1/50 |
| ΔH (j/g) * | 185.4 ± 9.90 c† | 185 ± 3.89 c | 224.3 ± 1.20 a | 214.05 ± 3.89 b† | 158.20 ± 1.41 d† | 185 ± 3.25 c | 225.60 ± 3.11 a | 203.50 ± 7.07 b† |
| Tm (°C) * | 167.63 ± 4.36 b | 172.67 ± 1.02 a | 169.92 ± 1.17 ab | 170.26 ± 0.97 ab† | 167.75 ± 2.04 b | 170.29 ± 1.39 a | 170.99 ± 1.17 a | 166.74 ± 0.64 b† |
| T0 (°C) * | 166.42 ± 5.35 a | 169.12 ± 0.34 a† | 168.89 ± 0.80 a | 169.46 ± 0.54 a† | 165.40 ± 1.50 b | 167.51 ± 1.44 a† | 168.22 ± 0.91 a | 163.84 ± 0.99 c† |
| Tg (°C) * | 41.645 ± 2.28 a† | 50.26 ± 1.54 a† | 42.22 ± 4.50 a† | 47.70 ± 1.04 a† | 46.78 ± 0.55 c† | 46.53 ± 0.85 c† | 49.99 ± 0.90 b† | 52.96 ± 1.37 a† |
| Solid/Solvent Ratio | Degradation Stage | Tpeak (°C) | Weight Loss (%) | Residue (%) |
|---|---|---|---|---|
| 1:10 g/mL | I | 84.86 | 14.23 | 27.48 |
| II | 279.57 | 58.29 | ||
| 1:25 g/mL | I | 84.86 | 12.78 | 16.74 |
| II | 281.58 | 70.48 | ||
| 1:30 g/mL | I | 83.85 | 12.9 | 16.81 |
| II | 280.57 | 70.29 | ||
| 1:50 g/mL | I | 89.9 | 13.35 | 26.06 |
| II | 280.57 | 60.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öncü Glaue, Ş.; Akcan, T.; Tavman, Ş. Thermal Properties of Ultrasound-Extracted Okra Mucilage. Appl. Sci. 2023, 13, 6762. https://doi.org/10.3390/app13116762
Öncü Glaue Ş, Akcan T, Tavman Ş. Thermal Properties of Ultrasound-Extracted Okra Mucilage. Applied Sciences. 2023; 13(11):6762. https://doi.org/10.3390/app13116762
Chicago/Turabian StyleÖncü Glaue, Şelale, Tolga Akcan, and Şebnem Tavman. 2023. "Thermal Properties of Ultrasound-Extracted Okra Mucilage" Applied Sciences 13, no. 11: 6762. https://doi.org/10.3390/app13116762
APA StyleÖncü Glaue, Ş., Akcan, T., & Tavman, Ş. (2023). Thermal Properties of Ultrasound-Extracted Okra Mucilage. Applied Sciences, 13(11), 6762. https://doi.org/10.3390/app13116762






