Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus
Abstract
1. Introduction
2. Methods
3. Innate Immunity of the Small Intestine
3.1. Intestinal Epithelial Cells and Innate Immunity
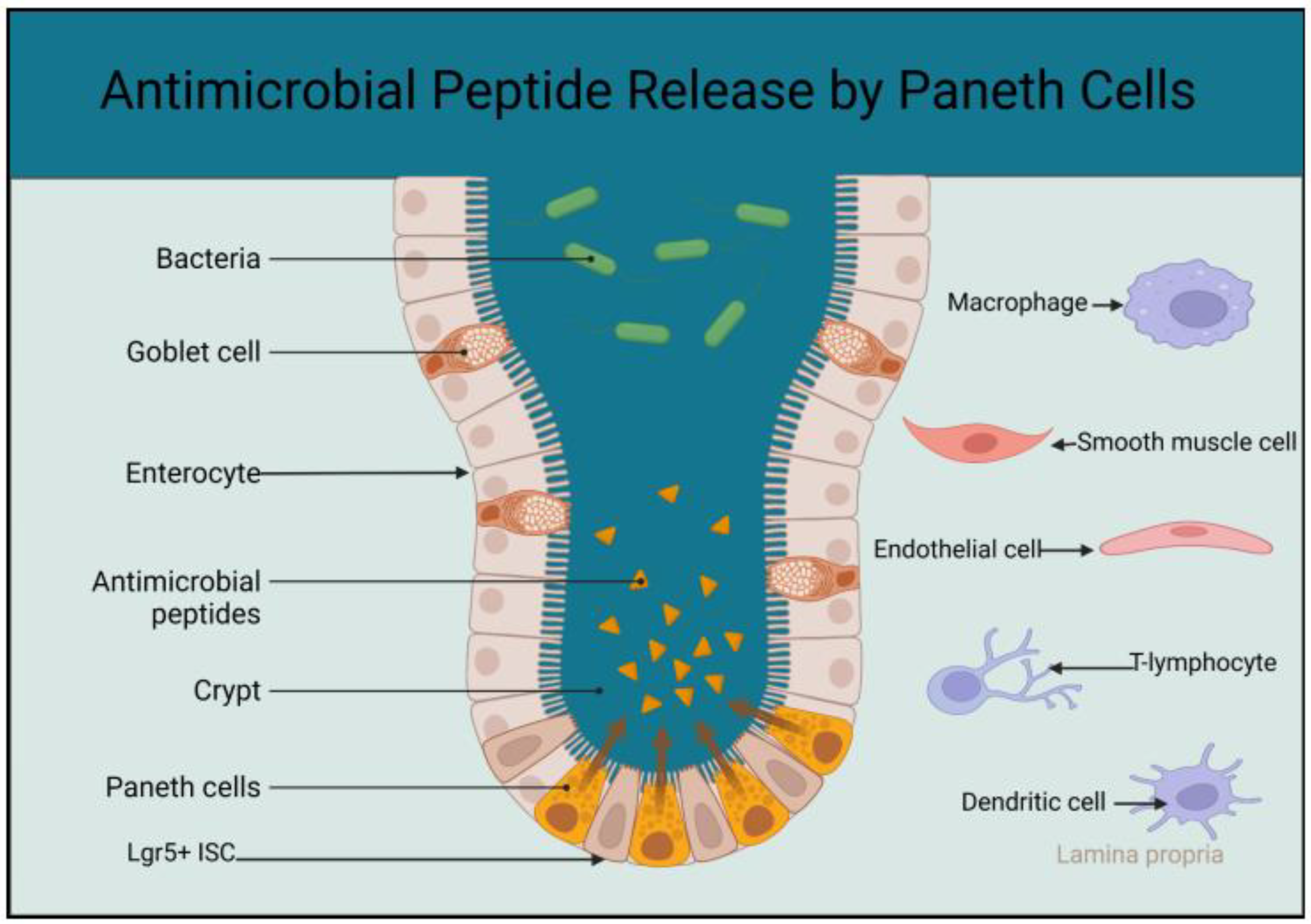
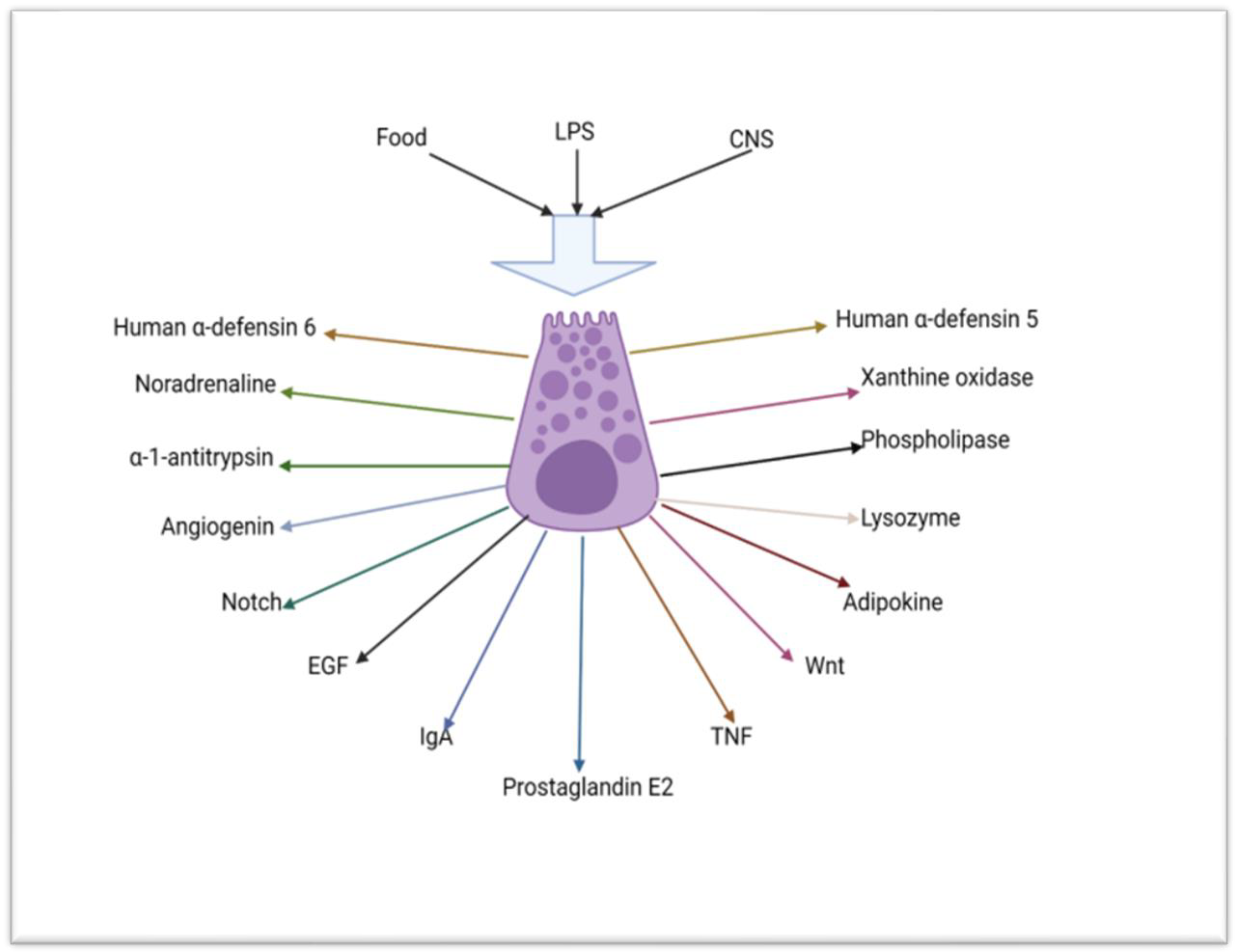
3.1.1. The Role of Paneth Cells in the Innate Immunity of the Small Intestine
3.1.2. Absorptive Enterocytes, Goblet Cells, Tuft Cells, M-Cells and Junctional and Innate Immunity of the Small Intestine
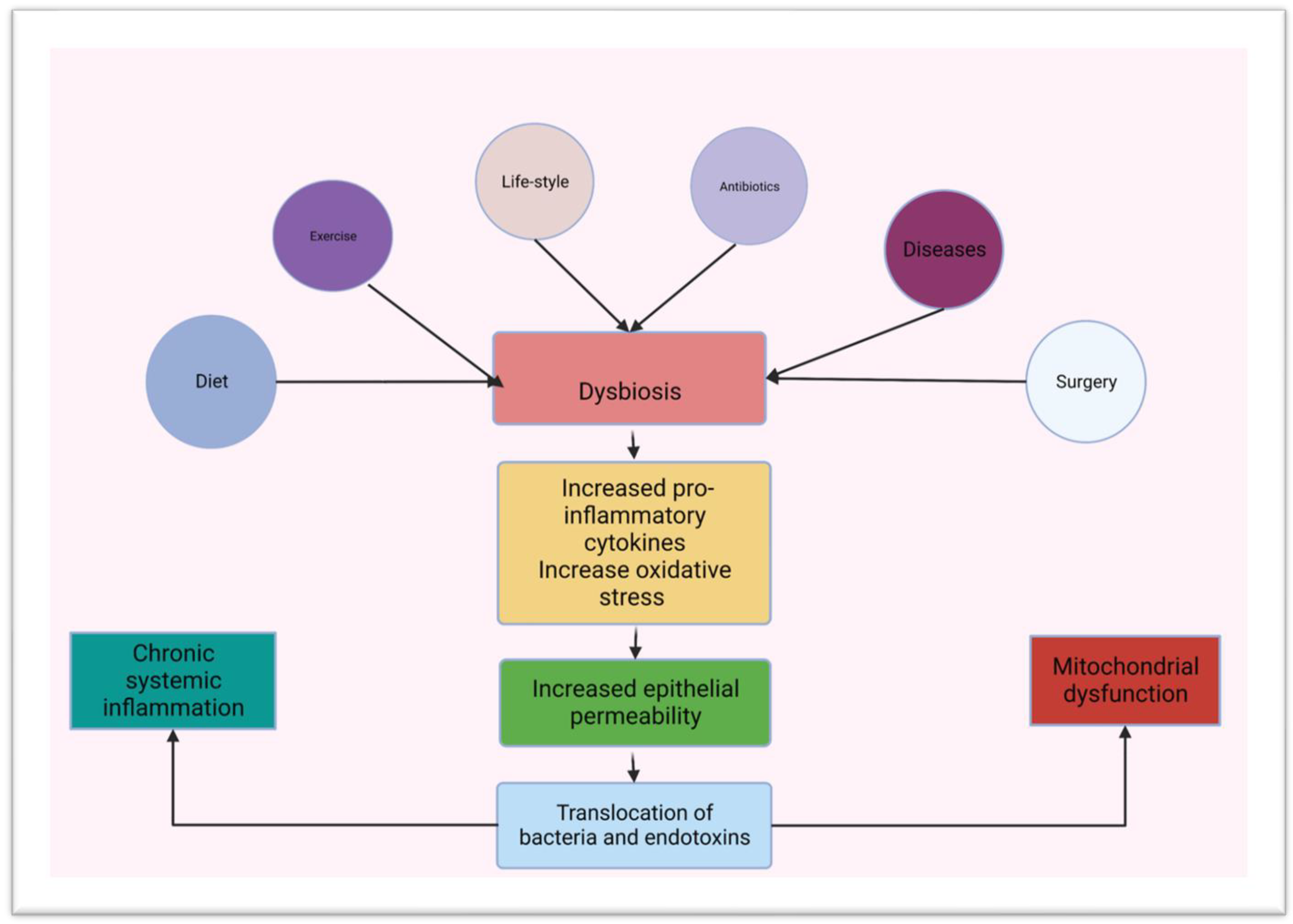
3.2. Gut Microbiota and Innate Immunity in the Small Intestine
4. Diabetes Mellitus
4.1. Pathogenesis of Diabetes Mellitus
4.1.1. Type 2 Diabetes Mellitus
4.1.2. Type 1 Diabetes Mellitus
4.1.3. Pancreatogenic (Type 3c) Diabetes Mellitus
4.1.4. Gestational Diabetes Mellitus
4.1.5. Human Immunodeficiency Virus Infection and Antiretroviral Drug-Associated Diabetes Mellitus
4.1.6. Post-Transplant Diabetes after Solid Organ Transplant
4.1.7. Maturity Onset Diabetes of the Young
4.2. Complications of Diabetes Mellitus
4.3. Management of Diabetes Mellitus
4.3.1. Lifestyle Changes and Management of Diabetes Mellitus
4.3.2. Dietary Management of Diabetes Mellitus
4.3.3. Nutritional Supplements and Management of Diabetes Mellitus
4.3.4. Ketogenic Diet and Management of Diabetes Mellitus
4.3.5. Bariatric Surgery and Management of Diabetes Mellitus
4.3.6. Other Options for the Treatment of Diabetes Mellitus
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.; Kinloch-des-Loes, S.; Granath, A.; Sonnerberg, A.; Goh, L.E.; Anderson, J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS 2007, 21, 565–574. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell. Biol. 2020, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef] [PubMed]
- Cari, L.; Rosati, L.; Leoncini, G.; Lusenti, E.; Gentili, M.; Nocentini, C.; Riccardi, C.; Migliorati, G.; Ronchetti, S. Association of GILZ with MUC2, TLR2 and TLR4 in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2235. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, M.; Baginska, N.; Gorski, A.; Jonczyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martinez, M.S.; Chacin, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C.; et al. Microbiota and Diabetes Mellitus: Role of Lipid Mediators. Nutrients 2020, 12, 3039. [Google Scholar] [CrossRef]
- Donaldson, D.S.; Shih, B.B.; Mabbott, N.A. Aging-Related Impairments to M Cells in Peyers’s Patches Coincide with Disturbances to Paneth Cells. Front. Immunol. 2021, 12, 761949. [Google Scholar] [CrossRef]
- Latorre, E.; Layunta, E.; Grasa, L.; Pardo, J.; Garcia, S.; Alcalde, A.I.; Mesonero, J.E. Toll-like receptots 2 and 4 modulate intestinal IL-10 differently in ileum and colon. United Eur. Gastroenterol. J. 2018, 6, 446–453. [Google Scholar] [CrossRef]
- Gubatan, J.; Holman, D.R.; Puntasecca, C.J.; Polevoi, D.; Rubin, S.J.S.; Rogalla, S. Antimicrobial peptides and the gut microbiome in inflammatory bowel disease. World J. Gastroenterol. 2021, 27, 7402–7422. [Google Scholar] [CrossRef]
- Wehkamp, J.; Stange, E.F. An Update Review on the Paneth Cell as Key to Ileal Crohn’s Disease. Front. Immunol. 2020, 11, 646. [Google Scholar] [CrossRef]
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Sankaran-Walters, S.; Hart, R.; Dills, C. Guardians of the Gut Enteric Defensins. Front. Microbiol. 2017, 8, 647. [Google Scholar] [CrossRef]
- Yang, E.; Shen, J. The Roles and functions of Paneth cells in Crohn’s disease: A critical review. Cell Prolif. 2020, 54, e12958. [Google Scholar] [CrossRef]
- Bevins, C.L. Events at the host-microbial interface of the gastrointestinal tract. V. Paneth cell alpha-defensins in intestinal host defense. Am. J. Physiol. Liver Physiol. 2005, 289, G173–G176. [Google Scholar]
- Hein, M.J.A.; Kvansakul, M.; Lay, F.T.; Phan, T.K.; Hulett, M.D. Defensin-lipid interactions in membrane targeting: Mechanisms of action and opportunities for the development of antimicrobial and anticancer therapeutics. Biochem. Soc. Trans. 2022, 50, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Bevins, C.; Ghosh, D.; Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 2002, 59, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ding, J.; Liao, C.; Xu, J.; Liu, X.; Lu, W. Defensins: The natural peptide antibiotic. Adv. Drug Deliv. Rev. 2021, 179, 114008. [Google Scholar] [CrossRef] [PubMed]
- Hannon, B.A.; Fairfield, W.D.; Adams, B.; Kyle, T.; Crow, M.; Thomas, D.M. Use and abuse of dietary supplements in persons with diabetes. Nutr. Diabetes 2020, 10, 14. [Google Scholar] [CrossRef]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise Immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar] [PubMed]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in Alzheimer’s Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High Fat Diet Alters Gut Microbiota and the Expression of Paneth Cell-Antimicrobial Peptides Preceding Changes of Circulating Inflammatory Cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Liu, C.; Wu, W.K.K.; Wong, S.H.; Jia, W.; Sung, J.J.Y.; Yu, J. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarker. Microbiome 2022, 10, 35. [Google Scholar] [CrossRef]
- Sommer, F.; Backhed, F. The gut microbiota-Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Jaime, R.; Franciso, G.; Luis, B.F.; Aldo, M.; Vera, L.S.; Henry, C. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Ismail, N.A.M.; Razalli, N.H.; Gnanou, J.V.; Ali, R.A.R. Gut Microbia and Gestational Daibetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Nagpal, R.; Newman, T.M.; Wang, S.; Jain, S.; Lovato, J.F.; Yadav, H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J. Diabetes Res. 2018, 2018, 3462092. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β–cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Fatima, G.; Magomedova, A.; Watanabe, S.; Elkilany, G. Why and How the Indo-Mediterranean Diet May Be Superior to Other Diets: The Role of Antioxidants in the Diet. Nutrients 2022, 14, 898. [Google Scholar] [CrossRef]
- Adolph, T.E.; Tomczak, M.F.; Niederreiter, L.; Ko, H.J.; Bock, J.; Martinez-Naves, E.; Glickman, J.N.; Tschurtschenthaler, M.; Hartwig, J.; Hosomi, S.; et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013, 503, 272–276. [Google Scholar] [CrossRef]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut microbiota and obesity. Clin. Nutr. Exp. 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Amoroso, C.; Perillo, F.; Strati, F.; Fantani, M.C.; Caprioli, F.; Facciotti, F. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Taur, Y.; Juluru, K.; Yagan, N.; Zhu, Y.S.; Pamer, E.; Glesby, M.J. Intestinal Dysbiosis and Markers of Systemic Inflammation in Viscerally and Generally Obese Persons Living with HIV. Am. J. Ther. 2020, 83, 81–89. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Sharma, K. Obesity and Diabetic Kidney Disease: Role of Oxidant Stress and Redox Balance. Antioxid. Redox Signal. 2016, 25, 208–216. [Google Scholar] [CrossRef]
- Ansari, D.M.; Harahwa, T.; Abuelgasim, E.; Harky, A. Glycated Haemoglobin Levels and Its Effect on Outcomes in Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2022, 37, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Dinic, S.; Jovanovic, J.A.; Uskokovic, A.; Mihailovic, M.; Grdovic, N.; Tolic, A.; Rajic, J.; Dordevic, M.; Vidakovic, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou-Marketou, N.; Paschou, S.A.; Marketos, N.; Adamidi, S.; Adamidis, S.; Kanaka-Gantenbien, C. Diabetic nephropathy in type 1 diabetes. Minerva Med. 2018, 109, 218–228. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Fa, W.H.; Tian, C.R.; Yuan, C.S.; Jie, N. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging 2022, 14, 2902–2919. [Google Scholar] [CrossRef]
- Vilas-Boas, E.A.; Almeida, D.C.; Roma, L.P.; Ortis, F.; Carpinelli, A.R. Lipotoxicity and β-Cell Failure in Type Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells 2021, 10, 3328. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.I.; Sahebkar, A. Molecular Mechanism Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Brownrigg, J.R.W.; Apelqvist, J.; Bakker, K.; Schaper, N.C.; Hinchliffe, R.J. Evidence-based Management of PAD & the Diabetic Foot. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Uckay, I.; Aragon-Sanchez, J.; Lew, D.; Lipsky, B.A. Diabetic foot infections: What have learned in the last 30 years? Int. J. Infect. Dis. 2015, 40, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Mellbin, L.; Cosentino, F. Diabetes: Prevalence, prognosis and management of a potent cardiovascular risk factor. Eur. J. Prev. Cardiol. 2017, 24 (Suppl. S3), 52–60. [Google Scholar] [CrossRef]
- Quin, J. Diabetes and HIV. Clin. Med. 2014, 14, 667–669. [Google Scholar] [CrossRef]
- Gierynska, M.; Szulc-Dabrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Liu, J.; Williams, B.; Frank, D.; Dillon, S.M.; Wilson, C.C.; Landay, A.L. Inside Out: HIV, the Gut Microbiome, and the Mucosal Immune System. J. Immunol. 2017, 198, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevence to Autoimmune Diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Basak, O.; van de Born, M.; Korving, J.; Beumer, J.; van der Elst, S.; van Es, J.H.; Clevers, H. Mapping early fate determination in Lgr5+ crypt stem cells using a novel ki67-RFP allele. EMBO J. 2014, 3, 2057–2068. [Google Scholar] [CrossRef]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakuragi, N.; Takakuwa, A.; Ayabe, T. Paneth cell α-defensins and enteric microbiota in health and disease. Biosci. Microbiota Food Health 2016, 35, 57–67. [Google Scholar] [CrossRef]
- Zhu, G.; Hu, J.; Xi, R. The cellular niche for intestinal stem cells: A team effort. Cell Regen. 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.D.; Frank, U.; Weiss, F.U.; Sendler, M.; Kacprowski, T.; Ruhlemann, M.; Bang, C.; Franke, A.; Volker, U.; Volzeke, H.; et al. The Gut Microbiome in Patients with Chronic Pancreatitis Is Characterized by Significant Dysbiosis and Overgrowth by Opportunistic Pathogens. Clin. Transl. Gastroenterol. 2020, 11, e00232. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.D.T.M.; Heimesaat, M.M.; Bereswill, S. Changes of the intestinal microbiome-host homeostasis in HIV-infected individuals-a focus on bacterial gut microbiome. Eur. J. Microbiol. Immunol. 2017, 7, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2018, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Al-Rashidi, H.E. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi J. Biol. Sci. 2022, 29, 1628–1643. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalances in the Pathogenesis of Necrotizing Enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 229–238.e1. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Servin, A.L. The frontline of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef]
- Mukerjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28. [Google Scholar] [CrossRef] [PubMed]
- Cray, P.; Sheahan, B.J.; Dekaney, C.M. Secretory Sorcery: Paneth Cell Control of Intestinal Repair and Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, D.; Biles, B. Postnatal changes in the distribution and elemental composition of Paneth cells in normal and corticosteroid-treated rats. Cell Tissue Res. 1986, 246, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef]
- Singh, R.; Balasubramanian, I.; Zhang, L.; Gao, N. Metaplastic Paneth Cells in Extra-Intestinal Mucosal Niche Indicate a Link to Microbiome and Inflammation. Front. Physiol. 2020, 11, 280. [Google Scholar] [CrossRef]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Physiol. 2020, 11, 587. [Google Scholar] [CrossRef]
- Lee, V.H.; Gulati, A.S. Implications of Paneth cell dysfunction on gastrointestinal health and disease. Curr. Opin. 2022, 38, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Grinat, J.; Kosel, F.; Goveas, N.; Kranz, A.; Alexopoulou, D.; Rajewsky, K.; Sigal, M.; Stewart, A.F.; Heuberger, J. Epigenetic modifier balances Mapk and Wnt signalling in differentiation of goblet and Paneth cells. Life Sci. Alliance 2022, 5, e202101187. [Google Scholar] [CrossRef]
- Holly, M.K.; Smith, J.G. Paneth Cells during Viral Infection and Pathogenesis. Viruses 2018, 10, 225. [Google Scholar] [CrossRef]
- Farin, H.F.; Karthaus, W.R.; Kujala, P.; Rakhshandehroo, M.; Schwank, G.; Vries, R.G.J.; Kalkhoven, E.; Nieuwenhuis, E.E.S.; Clevers, H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J. Exp. Med. 2014, 211, 1393–1405. [Google Scholar] [CrossRef]
- Ouellette, A.J. Paneth cell α-defensins in enteric immunity. Cell. Mol. Life Sci. 2011, 68, 2215–2229. [Google Scholar] [CrossRef]
- Gassler, N. Paneth cells in intestinal physiology and pathophysiology. World J. Gastrointest. Pathophysiol. 2017, 8, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Nolan, E.M. Human α-Defensin 6: A Small Peptide that Self-Assembles and Protects the Host by Entangling Microbes. Acc. Chem. Res. 2017, 50, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Dayton, T.L.; Clevers, H. Beyond growth signaling: Paneth cells metabolically support ISCs. Cell Res. 2017, 27, 851–852. [Google Scholar] [CrossRef]
- Jackson, D.; Theiss, A.L. Intestinal Stem Cell Regulation via Glycolytic Activity of Neighboring Paneth Cells. J. Gastroenterol. Hepatol. Endosc. 2017, 2, 1019. [Google Scholar]
- Heneghan, A.F.; Pierre, J.F.; Tandee, K.; Shanmuganayagam, D.; Wang, X.; Reed, J.D.; Steele, J.L.; Kudsk, K.A. Parenteral Nutrition Decreases Paneth cell function and Intestinal Bactericidal Activity while Increasing Susceptibility to Bacterial Enteroinvasion. J. Parenter. Enter. Nutr. 2014, 38, 817–824. [Google Scholar] [CrossRef]
- Hodin, C.M.; Lenaerts, K.; Grootjans, J.; de Haan, J.J.; Hadfoune, M.; Verheyen, F.K.; Kiyama, H.; Heineman, E.; Buurman, W.A. Starvation Compromises Paneth Cells. Am. J. Pathol. 2011, 179, 2885. [Google Scholar] [CrossRef]
- Liu, T.C.; Gurram, B.; Baldridge, M.T.; Head, R.; Lam, V.; Luo, C.; Cao, Y.; Simpson, P.; Hayward, M.; Holtz, M.L.; et al. Paneth cell defects in Crohn’s disease patients promotes dysbiosis. J. Clin. Investig. 2016, 1, e86907. [Google Scholar] [CrossRef]
- Liu, T.C.; Kern, J.T.; VanDussen, K.L.; Xiong, S.; Kaiko, G.E.; Wilen, C.B.; Rajala, M.W.; Caruso, R.; Holtzman, M.J.; Gao, F.; et al. Interaction between smoking and ATG16L1T300A triggers Paneth cell defects in Crohn’s disease. J. Clin. Investig. 2018, 128, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.S.; McGovern, D.P.B. Paneth Cell Alterations in the Development and Phenotype of Crohn’s Disease. Gastroenterology 2017, 152, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Feakins, R.; Domizio, P.; Murphy, J.; Bevins, C.; Wilson, J.; McPhail, G.; Poulsom, R.; Dhaliwal, W. Paneth cell granule depletion in the human intestine under infective and nutrional stress. Clin. Exp. Immunol. 2004, 135, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Chung, H.K.; Xiao, L.; Yu, T.X.; Wang, S.R.; Piao, J.J.; Rao, J.N.; Gorospe, M.; Wang, J.Y. MicroRNA-195 regulates Tuft cell function in the intestinal epithelium by altering translation of DCLK1. Am. J. Physiol. Cell Physiol. 2021, 320, C1042–C1054. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef]
- Billipp, T.E.; Nadjsombati, M.S.; von Moltke, J. Tuning tuft cells: New ligands and effector functions reveal tissue-specific function. Curr. Opin. Immunol. 2021, 68, 98–106. [Google Scholar] [CrossRef]
- O’Leary, C.E.; Schneider, C.; Locksley, R.M. Tuft cells-systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu. Rev. Immuno.l 2019, 37, 47–72. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, M.; D’Agati, V.D.; Lee, H.T. Norepinephrine released by intestinal Paneth cells exacerbates ischemic AKI. Am. J. Physiol. Ren. Physiol. 2020, 318, F260–F272. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Siljander, H.; Honkanen, J.; Knip, M. Micriobiome and Type 1 diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; Naya, I.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; Asunsolo, A.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B.; Hardt, W.D. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 2011, 14, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Tosh, P.K. A clinician’s primer of the role of the microbiome in human health and disease. Mayo Clin. Proc. 2014, 89, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Miller, G.; Li, X.; Saxena, D. Virome and bacteriome: Two sides of the same coin. Curr. Opin. Virol. 2019, 37, 37–43. [Google Scholar] [CrossRef]
- Yang, K.; Niu, J.; Zuo, T.; Sun, Y.; Xu, Z.; Tang, W.; Liu, Q.; Zhang, J.; Ng, E.K.W.; Wong, S.K.H.; et al. Alterations in the Gut Virome in Obesity and Type 2 Diabetes Mellitus. Gastroenterology 2021, 161, 1257–1269. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]
- Yokoi, Y.; Nakamura, K.; Yoneda, T.; Kikuchi, M.; Sugimoto, R.; Shimizu, Y.; Ayabe, T. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci. Rep. 2019, 9, 2710. [Google Scholar] [CrossRef]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing reactive oxygen species generation by rebooting gut microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar] [CrossRef]
- Jimenez-Uribe, A.P.; Hernandez-Cruz, E.Y.; Ramirez-Magana, K.J.; Pedraza-Chaverri, J.P. Involvement of Tricarboxylic Acid Cycle Metabolites in Kidney Diseases. Biomolecules 2021, 11, 1259. [Google Scholar] [CrossRef]
- Berger, N.A. Young Adult Cancer: Influence of the Obesity Pandemic. Obesity 2018, 26, 641–650. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Auteieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Corcionivoschi, N.; Gundogdu, O.; Chifiriuc, M.C.; Marutescu, L.G.; Ispas, B.; Savu, O. Dysbiosis in the Development of Type! Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. Int. J. Mol. Sci. 2021, 22, 2763. [Google Scholar] [CrossRef] [PubMed]
- Ewald, N.; Hardt, P.D. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J. Gastroenterol. 2013, 19, 7276–7281. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Beltrand, J.; Busiah, K.; Vaivre-Douret, L.; Fauret, A.L.; Berdugo, M.; Cavé, H.; Polak, M. Neonatal Diabetes Mellitus. Front. Pediatr. 2020, 8, 540718. [Google Scholar] [CrossRef]
- Chowdhury, A.T. Post-transplant diabetes mellitus. Clin. Med. 2019, 19, 392–395. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathyophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Sarkar, S.; Brown, T.T. Diabetes in People with HIV. Curr. Diabetes Rep. 2022, 21, 13. [Google Scholar] [CrossRef]
- Gueddouri, D.; Cauzac, M.; Fauveau, V.; Benhamed, F.; Charifi, W.; Beaudoin, L.; Rouland, M.; Sicherre, F.; Lehuen, A.; Postic, C.; et al. Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol. Metab. 2022, 57, 101438. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative Stress and the Etiology of Insulin Resistance and Type 2 Diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.M.; Li, F.X.; Yan, Y.; Zhang, Y.; Kong, L.I.; Zhu, P.; Wang, K.F.; Zhang, F.; Liu, B.; Lu, C. Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chin. Med. J. 2019, 132, 78–85. [Google Scholar] [CrossRef]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.; Czaja, K. Diet driven microbiota dysbiosis is associated with vagal remodelling and obesity. Physiol. Behav. 2017, 273, 305–327. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, R.; Han, B.; Wei, H.; Chen, L.; Du, H.; Li, G.; Yang, Y.; Chen, X.; Cui, L.; et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat. Commun. 2022, 13, 6356. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Madhulika, A.; Deepika, G.; Rao, G.V.; Reddy, D.N.; Subramanyam, C.; Sasikala, M.; Talukdar, R. Altered intestinal microbiota in patients with chronic pancreatitis: Implications in diabetes and metabolic abnormalities. Sci. Rep. 2017, 7, 43640. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Chigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Feng, Y.; Jing, F.; Han, Y.; Lyu, N.; Liu, F.; Li, J.; Song, X.; Xie, J.; Qiu, Z.; et al. Association Between Gut Microbiota and CD4 Recovery in HIV-1 Infected Patients. Front. Microbiol. 2018, 9, 1461. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Cardoso, S.; Klatt, N.R.; Reyes-Teran, G. Impact of antiretroviral drugs on the microbiome: Unknown answers to important questions. Curr. Opin. HIV AIDS 2018, 13, 53–60. [Google Scholar] [CrossRef]
- Zevin, A.S.; McKinnon, L.; Burgener, A.; Klatt, N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. 2016, 11, 182–190. [Google Scholar] [CrossRef]
- El-Far, M.; Tremblay, C.L. Gut microbial diversity in HIV infection post combined antiretroviral therapy: A key target for prevention of cardiovascular disease. Curr. Opin. HIV AIDS 2018, 13, 38–44. [Google Scholar] [CrossRef]
- Gootenberg, D.B.; Paer, J.M.; Luevano, J.M.; Kwon, D.S. HIV-associated changes in the enteric microbial community: Potential role in loss of homeostasis and development of systemic inflammation. Curr. Opin. Infect. Dis. 2017, 30, 31–43. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, F.; Zhang, R.; Shen, Y.; Liu, L.; Wang, J.; Yang, J.; Tang, Q.; Xun, J.; Qi, T.; et al. Changes in intestinal microbiota in hIV-1-infected subjects following cART initiation: Influence of CD4++ T cell count. Emerg. Microbes Infect. 2018, 7, 113. [Google Scholar] [CrossRef]
- Rhoades, N.; Mendoza, N.; Jankeel, A.; Sureshchandra, S.; Alvarez, A.D.; Doratt, B.; Heidari, O.; Hagan, R.; Brown, B.; Scheibel, S.; et al. Altered Immunity and Microbial Dysbiosis in Aged Individuals with Long-Term Controlled HIV Infection. Front. Immunol. 2019, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Villaneuva-Millan, M.J.; Perez-Matute, P.; Recio-Fernandez, E.; Rosales, J.M.L.; Oteo, J.A. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J. Int. AIDS Soc. 2017, 20, 21526. [Google Scholar] [CrossRef]
- Rajasuriar, R.; Wright, E.; Lewin, S.R. Impact of antiretroviral therapy (ART) timing on chronic activation/inflammation and end-organ damage. Curr. Opin. HIV AIDS 2015, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Mehraj, V. Potential contribution of gut microbiota and systemic inflammation on HIV vaccine effectiveness and vaccine design. AIDS Res. Ther. 2017, 14, 48. [Google Scholar] [CrossRef]
- Xu, X.; Ling, Q.; He, Z.I.; Gao, F.; Zheng, S.S. Post-transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 465–470. [Google Scholar]
- Brodosi, L.; Petta, S.; Petroni, M.L.; Marchesini, G.; Morelli, M.C. Management of Diabetes in Candidates for Liver Transplantation and in Transplant Recipients. Transplantation 2022, 106, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Thoefner, L.B.; Rostved, A.A.; Pommergaard, H.C.; Rasmussen, A. Risk factors for metabolic syndrome after liver transplantation: A systemati review and meta-analysis. Transplant. Rev. 2018, 32, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Lok, C.E.; Kim, S.J.; Knoll, G.; Shah, B.R.; Naylor, K.; Luo, B.; Vinegar, M.; Dixon, S.N.; Hawley, C.; et al. Impact of Pretransplant and New-Onset Diabetes After Transplantation on the Risk of Major Adverse Cardiovascular Events in Kidney Transplant Recipients: A Population-based Cohort Study. Transplantation 2021, 105, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.; Hornum, M.; Terrones-Campos, C.; Crone, G.C.; Wareham, N.E.; Soeborg, A.; Rasmussen, A.; Gustafsson, F.; Perch, M.; Soerensen, S.S.; et al. Posttransplantation Diabetes Mellitus Among Solid Organ Recipients in a Danish Cohort. Transpl. Int. 2022, 35, 10352. [Google Scholar] [CrossRef]
- Urakami, T. Maturity-onset diabetes of the young (MODY): Current perspectives on diagnosis on diagnosis and treatment. Diabetes Metab. Syndr Obes. 2019, 12, 1047–1056. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.W.; Shao, L.; Sun, S.H.; Wu, J.; Song, Q.H.; Zou, H.S.; Ling, Z.X. Gut microbiota dysbiosis in Chinese children with type 1 diabetes mellitus: An observational study. World J. Gastroenterol. 2021, 27, 2394–2414. [Google Scholar] [CrossRef]
- Arora, A.; Behl, T.; Shgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef]
- Das, T.; Jayasudha, R.; Chakravarthy, S.; Prashanthi, G.S.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Sharma, S.; Shivaji, S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci. Rep. 2021, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Haung, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The role of the microbiome in diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 172, 108645. [Google Scholar] [CrossRef]
- Talukdar, R.; Sarkar, P.; Jakkampudi, A.; Sarkar, S.; Aslam, M.; Jandhyala, M.; Deepika, G.; Unnisa, M.; Reddy, D.N. The gut microbiome in pancreatogenic diabetes differs from that of Type 1 and Type 2 diabetes. Sci. Rep. 2021, 11, 10978. [Google Scholar] [CrossRef]
- Hwang, J.L.; Weiss, R.E. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef]
- Kelli, M.; Steven, F.; Eva, L.; Thomas, W.; Patrice, E.F. New insights into the mechanism of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef]
- Annani-Akollor, M.E.; Addai-Mensah, O.; Fondjo, L.A.; Sallah, L.; Owiredu, E.W.; Acheampong, E.; Akamugri, S. Predominant Complications of Type 2 Diabetes in Kumasi: A 4- Year Retrospective Cross-Sectional Study at a Teaching Hospital in Ghana. Medicina 2019, 55, 125. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Banach, M.; Edmonds, M.; Papanas, N.; Papazoglou, D. Complications of Diabetes. Diabetes Res. 2015, 2015, 189525. [Google Scholar] [CrossRef] [PubMed]
- Ambachew, S.; Assefa, M.; Tegegne, Y.; Zekele, J.A. The Prevalence of Intestinal Parasites and Their Associated Factors among Diabetes Mellitus Patients at the University of Gondar Referaal Hospital, Northwest Ethiopia. J. Parasitol. Res. 2020, 2020, 8855965. [Google Scholar] [CrossRef] [PubMed]
- Lemaignen, A.; Birgand, G.; Ghodhbane, W.; Alkhoder, S.; Lolom, I.; Belorgey, S.; Lescure, F.-X.; Armand-Lefevre, L.; Raffoul, R.; Dilly, M.-P.; et al. Sternal wound infection after cardiac surgery: Incidence and risk factors according to clinical presentation. Clin. Microbiol. Infect. 2015, 21, 674.e11–674.e18. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef]
- Corazzari, C.; Matteucci, M.; Kołodziejczak, M.; Kowalewski, M.; Formenti, A.M.; Giustina, A.; Beghi, C.; Barili, F.; Lorusso, R. Impact of pre-operative glycometabolic status on outcomes in cardiac surgery: Systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 164, 1950–1960.e10. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, A.; Cao, J.; Liu, Y.; Lou, J.; Li, H.; Ma, Y.; Song, Y.; Mi, W.; Liu, J. Association of Diabetes Mellitus With Postoperative Complications and Mortality After Non-Cardiac Surgery: A Meta-Analysis and Systematic Review. Front. Endocrinol. 2022, 13, 841256. [Google Scholar] [CrossRef] [PubMed]
- Tokarek, T.; Dziewierz, A.; Wiktorowicz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Kleczynski, P.; Dudek, D. Effect of diabetes mellitus on clinical outcomes and quality of life after transcatheter aortic valve implantation for severe aortic valve stenosis. Hell. J. Cardiol. 2018, 59, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Graves, L.E.; Donaghue, K.C. Vascular Complications in Adolescents with Daiabetes Mellitus. Front. Endocrinol. 2020, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, P.; Zhang, Y.; Lin, Y.; Shen, Y.; Shen, X.; Zhao, F.; Yan, S. Relationship between onset age of type 2 diabetes mellitus and vascular complications based on propensity score matching analysis. J. Diabetes Investig. 2022, 13, 1062–1072. [Google Scholar] [CrossRef]
- Sinwar, P.D. The diabetic foot management-recent advance. Int. J. Surg. 2015, 15, 27–30. [Google Scholar] [CrossRef]
- Tang, W.H.w.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbia in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padial, L.; Burgos, J.M.; Barderas, M.G. Coronary flow reserve in degenerative aortic stenosis and diabetes mellitus: An intriguing question. Kardiol. Pol. 2022, 80, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Sattar, N.; McGuire, D.K.; Wallström, O.; Smith, U.; Borén, J.; Bergström, G.; Omerovic, E.; Rosengren, A.; Eliasson, B.; et al. Left-Sided Degenerative Valvular Heart Disease in Type 1 and Type 2 Diabetes. Circulation 2022, 146, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Diabetes Mellitus: Is It Protective against Aneurysm? A Narrative Review. Cardiology 2018, 141, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Orbach, A.; Halon, D.A.; Jaffe, R.; Rubinshtein, R.; Karkabi, B.; Flugelman, M.Y.; Zafrir, B. Impact of diabetes and early revascularisation on the need for late and repeat procedures. Cardiovasc. Diabetolog. 2018, 17, 25. [Google Scholar] [CrossRef]
- Knapp, M.; Tu, X.; Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanism to clinical trials. Metabolism 2020, 111, 154299. [Google Scholar] [CrossRef]
- Williams, S.; Raheim, A.S.; Khan, I.M.; Rubab, U.; Zhao, S.S.; Marshall, A.; Brown, E.; Alam, U. Cardiac Autonomic Neuropathy in Type 1 and 2 Diabetes: Epidemiology, Pathophysiology, and Management. Clin. Ther. 2022, 44, 1394–1416. [Google Scholar] [CrossRef]
- Milek, T.; Forysinski, K.; Myrcha, P.; Ciostek, P. Diabetes association of polyps and colon cancer. Pol. Prz. Chir. 2019, 91, 9–12. [Google Scholar] [CrossRef]
- Peila, R.; Rohan, T.E. Diabetes, Glycated Hemoglobin, and Risk of Cancer in the UK Blobank Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1107–1119. [Google Scholar] [CrossRef]
- Cheuk, N.; Worth, L.J.; Tatoulis, J.; Skillington, P.; Kyi, M.; Fourlanos, S. The relationship between diabetes and surgical site infection following coronary artery bypass graft surgery in current-era models of care. J. Hosp. Infect. 2021, 116, 47–52. [Google Scholar] [CrossRef]
- Listewnik, M.J.; Jedrzejczak, T.; Majer, K.; Szylińska, A.; Mikolajczyk, A.; Mokrzycki, K.; Gorka, E.; Brykczynski, M. Complications in cardiac surgery: An analysis of factors contributing to sternal dehiscence in patients who underwent surgery between 2010 and 2014 and a comparison with the 1990-2009 cohort. Adv. Clin. Exp. Med. 2019, 28, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Giakoumidakis, K.; Nenekidis, I.; Brokalaki, H. The correlation between peri-operative hyperglycemia and mortality in cardiac surgery patients: A systematic review. Eur. J. Cardiovasc. Nurs. 2012, 11, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Carvalho, G.; Sato, T.; Lattermann, R.; Matsukawa, T.; Schricker, T. The Association of Pre-operative Glycemic Control, Intraoperative Insulin Sensitivity, and Outcomes after Cardiac Surgery. J. Clin. Endocrinol. Metab. 2010, 95, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Boreland, L.; Scott-Hudson, M.; Hetherington, K.; Frussinetty, A.; Slyer, J.T. The effectiveness of tight glycemic control on decreasing surgical site infections and readmission rates in adult patients with diabetes undergoing cardiac surgery: A systematic review. Heart Lung 2015, 44, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Leśniowska, J.; Schubert, A.; Wojna, M.; Baran, S.I.; Fedyna, M. Costs of diabetes and its complications in Poland. Eur. J. Health Econ. 2014, 15, 653–660. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Vandenberghe, C.; Castellano, C.A.; Maltais, M.; Fortier, M.; St-Pierre, V.; Dionne, I.J.; Cunnane, S.C. A short-term intervention combining aerobic exercise with medium-chain triglycerides (MCT) is more ketogenic than either MCT or aerobic exercise alone: A comparison of normoglycemic and prediabetic older women. Appl. Physiol. Nutr. Metab. 2019, 44, 66–73. [Google Scholar] [CrossRef]
- Poblete-Aro, C.; Russell-Guzman, J.; Parra, P.; Soto-Munoz, M.; Villegas-Gonzalez, B.; Cofre-bola-Dos, C.; Herrera-Valenzuela, T. Exercise and Oxidative in type 2 diabetes mellitus. Rev. Med. Chile 2018, 146, 362–372. [Google Scholar] [CrossRef]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.M.; Coren, A.T.; Pant, T.; Ciecko, A.E.; Jia, S.; Roethle, M.F.; Simpson, P.M.; Atkinson, S.N.; Salzman, N.H.; Chen, Y.G.; et al. Probiotic normalization of systemic inflammation in siblings of type 1 diabetes patients: An open-label pilot study. Sci. Rep. 2022, 12, 3306. [Google Scholar] [CrossRef]
- Hasain, Z.; Ali, R.A.R.; Ahmad, H.F.; Rauf, U.F.A.; Oon, S.F.; Mokhtar, N.M. The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients 2022, 14, 3878. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Zhao, J.; Han, Y.J.; Zhou, H.X.; Wu, J.; Ji, N.L. Relationship Between Serum Zinc Level and Microvascular Complications in Patients with Type 2 Diabetes. Chin. Med. J. 2015, 128, 3276–3282. [Google Scholar] [CrossRef]
- Yukinori, T. The Role of Zinc Homeostasis in the Prevention of diabetes Mellitus and Cardiovascular Diseases. J. Atheroscler. Thromb. 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycaemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Chang, Q.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell. Infect. Microbiol. 2023, 13, 1069954. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieudorp, M.; Vallance, B.A.; Verchere, C.B.; van Raatle, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet asa preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef] [PubMed]
- Dynka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Fink, J.; Seifert, G.; Bluher, M.; Fichtner-Feigl, S.; Marjanovic, G. Obesity Surgery. Deutches Arztebl. Int. 2022, 119, 70–80. [Google Scholar] [CrossRef]
- Cho, E.Y.; Kemmet, O.; Frenken, M. Biliopancreatic Diversion with Duodenal Switch in Patients with Type 2 Diabetes mellitus: Is the Chance of Complete Remission Dependent on Therapy and Duration of Insulin Treatment. Obes. Facts 2011, 4 (Suppl. S1), 18–23. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clement, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diabetes Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- De Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomized controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejia, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef] [PubMed]
- Kochar, I.S.; Jain, R. Pancreas transplant in type 1 diabetes mellitus: The emerging role of islet cell transplant. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 86–91. [Google Scholar] [CrossRef]
- Shyr, B.S.; Shyr, B.U.; Chen, S.C.; Loong, C.C.; Shyr, Y.M.; Wang, S.E. A comparative study of pancreas transplantation between type 1 and type 2 diabetes mellitus. Hepatobiliary Surg. Nutr. 2021, 10, 443–453. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Kuo, T.; Wang, L.; McKkimpson, W.M.; Son, J.; Watanabe, H.; Kitamoto, T.; Lee, Y.; Creusot, R.J.; et al. Pharmacological conversion of gut epithelial cells into insulin-producing cells lowers glycemia in diabetic animals. J. Clin. Investig. 2022, 132, e162720. [Google Scholar] [CrossRef] [PubMed]
- Contreras, I.; Vehi, J. Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef]
- Nomura, A.; Noguchi, M.; Kometani, M.; Furukawa, K.; Yoneda, T. Art.ificial Intelligence in Current Diabetes Management and Prediction. Curr. Diabetes Rep. 2021, 21, 61. [Google Scholar] [CrossRef]
- Pixley, J.S. Mesenchymal stem cells to treat type 1 diabetes. BBA—Mol. Basis Dis. 2020, 1866, 165315. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota; Implications for neurodegenerative diseases. Neurol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Cani, P.D. Severe obesity and gut microbiota: Does bariatric surgery reset the system? Gut 2019, 68, 5–6. [Google Scholar] [CrossRef]
- Wan, J.; Ma, J. Efficacy of dietary supplements targeting gut microbiota in the prevention and treatment of gestational diabetes mellitus. Front. Microbiol. 2022, 13, 927883. [Google Scholar] [CrossRef]
- Elbere, I.; Kalnina, I.; Silamikelis, I.; Konrade, I.; Zaharenko, L.; Sekace, K.; Radovica-Spalvina, I.; Fridmanis, D.; Gudra, D.; Pirags, V.; et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS ONE 2018, 13, e0204317. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef] [PubMed]
- Garabedian, E.M.; Roberrts, L.S.S.; McNevin, M.S. Examining the Role of Paneth Cells in the Small Intestine by Lineage Ablation in Transgenic Mice. Cell Biol. Metab. 1997, 272, 23729–23740. [Google Scholar] [CrossRef] [PubMed]
- Affinati, A.H.; Esfandiari, N.H. Oral EA, Kraftson AT. Bariatric Surgery in the Treatment of Type 2 Diabetes. Curr. Diabetes Rep. 2020, 19, 156. [Google Scholar] [CrossRef]
- Ciobarca, D.; Catoi, A.F.; Copaescu, C.; Miere, D.; Crisan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrients Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef]
- Debedat, J.; Clement, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11, 1582. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Dollet, L.; Kuefner, M.; Caria, E.; Rizo-Roca, D.; Pendergrast, L.; Abdelmoez, A.M.; Karlsson, H.K.R.; Bjőrnholm, M.; Dalbram, E.; Treebak, J.T.; et al. Glutamine Regulates Skeletal Muscle Immunometabolism in Type 2 Diabetes. Diabetes 2022, 71, 624–636. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Boonthongkaew, C.; Tong-Un, T.; Kanpetta, Y.; Chaungchot, N.; Leelayuwat, C.; Leelayuwat, N. Vitamin C Supplementation Improves Blood Pressure and Oxidative Stress after Acute Exercise in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A randomized, Placebo-Controlled, Cross-over Study. Chin. J. Physiol. 2021, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Du, K.; Zou, C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res. Ther. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]

| Type of Diabetes Mellitus | Prevalence | Predisposing Factor(s) | Influence of Dysbiosis | Underlying Pathology |
|---|---|---|---|---|
| Type 1 DM [52,115,151]. | 5% | Genetic Autoimmunity. | Yes | Absolute insulin deficiency. |
| Type 2 DM [52,152,153,154,155,156]. | 90% | Genetic. Obesity. High fat diet. High calorie diet. Sedentary lifestyle. | Yes | Insulin resistance. Insulin deficiency. |
| Type 3c DM [116,131,155]. | 8–16% | Acute pancreatitis. Chronic pancreatitis. Haemochromatosis. Cystic fibrosis. Pancreatic cancer. | Yes | Exocrine insufficiency. Reduced incretin secretion. Insulin deficiency. Glucagon deficiency. Pancreatic polypeptide deficiency. Hepatic insulin resistance. |
| Neonatal diabetes mellitus [118]. | 1 in 90,000 | Genetic | Unknown. | Abnormal function of β-cells. Anatomical anomaly of pancreas. |
| Post-transplant associated diabetes mellitus [119]. | 10–40% of posttransplant patients | Steroids. Immunosuppressive drugs. | Yes | Insulin resistance. Insulin deficiency. |
| Gestational diabetes mellitus [132]. | Family history. Older age. Obesity. Smoking. | Yes | Insulin resistance. | |
| HIV and ARV-associated DM [136,138,139]. | Antiretroviral drugs. Systemic inflammation. | Yes | Insulin resistance. | |
| Maturity onset diabetes of the young [150]. | 2% | Genetic | Unknown. | High set point of insulin receptors. |
| Drug-induced DM [156]. | Type, dose and duration of treatment. | Unknown. | Insulin resistance. |
| Derangement | Treatment Option |
|---|---|
| Insulin deficiency | Insulin. |
| Islets cell transplant [213]. | |
| Pancreas transplantation [214]. | |
| Mesenchymal stem cell [218]. | |
| Insulin resistance | Exercise [192,219]. |
| Lifestyle modification [219]. | |
| Oral hypoglycaemics. | |
| Insulin. | |
| Artificial pancreas [216]. | |
| Bariatric surgery [200,220]. | |
| Dysbiosis | High-fibre diet [199]. |
| Low-fat diet [221]. | |
| Low-calorie diet [221]. | |
| Ketogenic diet [198]. | |
| Exercise [52,192,219]. | |
| Dietary supplements [221]. | |
| Probiotics [32,194,195]. | |
| Prebiotics [32,200]. | |
| Metformin [200,222]. | |
| Synbiotics [223]. | |
| Faecal transplantation [115,210]. | |
| Synthetic HD-5 [224]. | |
| Bariatric surgery [225,226,227,228]. | |
| Increased gut permeability | Diet [229]. |
| Glutamine [230]. | |
| Herbal medicines [125,211,231]. | |
| Synthetic HD-5 [226]. | |
| Inflammation | Exercise [52]. |
| Cannabinoids receptor agonists [212]. | |
| Faeces transplant [200,208,209,210]. | |
| Oxidative stress | Exercise [219]. |
| Vitamin C [232]. | |
| Metformin [222]. | |
| Herbal medicines [231]. | |
| Ketogenic diet [205]. | |
| Pancreatic β-cell dysfunction | Mesenchymal stem cells [218]. |
| Islet cell transplant [213]. | |
| Pancreas transplant [214].Stem cell therapy [233]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luvhengo, T.; Mabasa, S.; Molepo, E.; Taunyane, I.; Palweni, S.T. Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus. Appl. Sci. 2023, 13, 6605. https://doi.org/10.3390/app13116605
Luvhengo T, Mabasa S, Molepo E, Taunyane I, Palweni ST. Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus. Applied Sciences. 2023; 13(11):6605. https://doi.org/10.3390/app13116605
Chicago/Turabian StyleLuvhengo, Thifhelimbilu, Susan Mabasa, Edith Molepo, Itumeleng Taunyane, and Sechaba Thabo Palweni. 2023. "Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus" Applied Sciences 13, no. 11: 6605. https://doi.org/10.3390/app13116605
APA StyleLuvhengo, T., Mabasa, S., Molepo, E., Taunyane, I., & Palweni, S. T. (2023). Paneth Cell, Gut Microbiota Dysbiosis and Diabetes Mellitus. Applied Sciences, 13(11), 6605. https://doi.org/10.3390/app13116605






