Featured Application
Environmentally friendly HS-GC-MS methods for the identification of volatile organic compounds in industrial water effluents were optimized. The removal of the pollutants from the samples via a new hybrid electrothermochemical wastewater treatment technology was carried out. The removal efficiency factors REFi were defined for the inspection of contaminants’ degradation.
Abstract
This study presents a compact system developed to treat paint-industrial water contaminated by the residues of volatile organic compounds (VOCs) using hybrid electrothermochemical wastewater treatment technology. Different treatment parameters (sample dilution, working current) were studied, and the power of the removal was expressed by the removal efficiency factor. It was shown that for all of the VOCs, significant removal was obtained with dilution 1:3 (industrial water: deionized water, V:V) and electric current set at 30 A. For advanced inspection of the treatment process, a simple and solventless method has been developed and validated, using headspace sampling combined with gas chromatography-mass spectrometry. Parameters affecting the headspace sampling efficiency were thoroughly studied, including the temperature, time, and mixing rate. The proposed method was partially validated utilizing the selected sampling parameters. The limits of detection ranged between 0.19 µg/L and 4.02 μg/L. The validated analytical method was an efficient tool for the inspection of residual VOCs in paint-industrial water and treated water samples. The new electrochemical water treatment was shown to be helpful in the paint industry’s effluent reuse.
1. Introduction
Water is a very important staple for industries, where it has different uses. Water is usually used as a solvent, but it should also be applied for manufacturing goods, heating, or cooling. The main part of water is then converted into industrial wastewater, and only a little portion of the supplied water is present within the product or is eliminated by evaporation. Industrial effluents represent an enormous environmental problem. The largest problem of industrial wastewater policy is the indiscriminate discharge of these wastewater streams into the environment, which can cause soil contamination, pollute the receiving bodies of water, and can be the source of air pollution by generating obnoxious gases [1]. These industrial effluents can contain high amounts of contaminants, such as volatile organic compounds (VOCs), including monoaromatic hydrocarbons, such as benzene, toluene, ethylbenzene, and xylene isomers (BTEX) which are used as industrial solvents and as the raw materials to produce many pharmaceuticals, agrochemicals, polymers, explosives, paints, cosmetics, etc. [2]. Accidental spills and leakage from storage tanks can cause frequent contaminations of groundwaters, soils, and sediments with VOCs and other pollutants, and also, environmental pollution can be involved in and associated with piping, improper waste disposal practices, and leaching landfills [3,4]. Some of these pollutants are on the list of priority toxic substances by the US Environmental Protection Agency (US EPA) [5]. VOCs pose a serious threat to the ecosystem and human health due to their high toxicity and migration to groundwater [6]; they are chemically inert, with a relatively high ability to be dissolved in water, and are toxic. Therefore, VOCs are common targets of environmental monitoring [7]. The struggle with the local environment and diffuse pollution of the VOCs spills is an issue of wide interest. Developing efficient and useful wastewater treatment technologies is needed to meet the standards of rigorous environmental regulations [8].
For the elimination of volatile pollution, several treatment processes have been mentioned in the published materials, and many of them are currently used. These differ in many aspects and include processes from the most used and cost-effective to the most advanced and costliest ones [9]. The mostly applied process for the degradation of VOCs in water samples is microbe-mediated biodegradation, where microbes with specific functions act as degraders, and they transform VOCs into nontoxic compounds [10]. Many bacteria which should effectively degrade VOCs have been isolated in recent years, and biodegradation pathways have been explored [5]. However, there are still leaks in the VOC-degrading, strains and the main disadvantage of these techniques is that the biodegradation processes of VOCs take a long time, more than 48 h [8]. Therefore, biofiltration is considered the most important technology among biological treatment systems [11,12]. Furthermore, this technology has been shown to be a low-cost technology to control pollution by odors and VOCs [13]. There are some applications of advanced oxidation processes (AOPs) for the elimination of VOCs in aqueous solutions [8,14]. These methods are sufficient and effective treatment methods without producing high amounts of sludge as waste, because of the generation of hydroxyl radicals having a high oxidation power which is useful for the mineralization of the pollutants [8]. In the comparison of the use of membrane processes with the adsorption process or any other conventional methods for industrial water treatment, membrane processes have a significant advantage because the separation of mixtures can be achieved effectively at low energy consumption. Therefore, membrane technology could be an encouraging technique for the elimination of VOCs from contaminated water sources. On the other hand, the availability of an effective and low-cost membrane unit is required to achieve VOCs’ amount reduction [15]. In recent years, electrochemical (EC) methods have started to be used for wastewater treatment. EC processes possess some advantages over the above-described technologies, such as the use of fewer amounts of added chemicals, simple installation, lower secondary toxicants occurrence, odor and/or pigment removal, and shorter residence time [16]. Electrocoagulation and electroflotation are the most widespread electrochemical wastewater treatment processes, which capitalize on the simultaneous generation of metal hydroxide and hydrogen microbubbles at the anode and cathode, respectively [17,18]. Dissolved metal ions from the anode form metal hydroxide, which grows into large flocs that act as highly effective coagulating agents for capturing contaminants, while the hydrogen bubbles carry the precipitated solids to the surface of treated water, thus enabling simple separation of contaminants. Dissolved organic compounds undergo electrochemical oxidation/reduction in the vicinity of electrodes and are captured by hydroxide flocs [17].
To ensure that the treatment process is successful, safe disposal limits must be respected; in addition to traditional parameters such as toxicity, total organic carbon content (TOC), chemical oxygen demand (COD), biological oxygen demand (BOD), turbidity, etc., the individual identification of the industrial wastewater contaminants can be realized via advanced analytical methods allowing the specification of individual contaminants, particularly through separation and appropriate spectrometric detection. Within the analytical method, the sample preparation step is very time-consuming and for par trace-level VOCs present in a complex matrix, it is highly challenging due to the difficulty [19]. This means that sample preparation is a crucial and unavoidable part of the analytical method for the detection of low-level analytes in multicomponent matrices [20]. Sample pretreatment techniques such as liquid–liquid extraction (LLE) [21], solid-phase extraction (SPE) [22], liquid-phase microextraction (LPME) [23], and solid-phase microextraction (SPME) [24] have been exploited for the isolation of VOCs from environmental samples. However, there are several disadvantages to these approaches [25]. In the case of industrial wastewater, the purge and trap method is the first choice for the pre-concentration and analysis of VOCs [26,27]. Apart from sensitivity, purge and trap has the advantages of precision and the possibility of automation. The drawbacks are elaborated instrumentation, water vapor which is produced during the extraction and could cause interferences, use of large amounts of cryogen, trap contaminations, especially in highly polluted samples such as industrial wastewater, and a long time of analysis. For the determination of VOCs, headspace techniques are more suited. In static headspace sampling (SHS), the sample is sealed in a gas-tight vial with a septum, and after the equilibrium, headspace vapor is injected to the GC by a gastight syringe [28]. The advantage of HS techniques is in the direct analysis of the VOCs without any interference from the sample matrix; therefore, the matrix effects are reduced. As the entire analysis runs in a closed system, there is the potential to reach high reproducibility.
In this study, a new electrochemical treatment technology was introduced for the industrial wastewater treatment procedure. The proposed wastewater processing technology uses a proprietary approach to the electrochemical introduction of certain metal consumables into the wastewater and to capture (electrochemically transformed) organic pollutants of the wastewater within a metal hydroxide matrix.
The objective of this work was to study the various electrochemical treatment technology parameters for the elimination of the VOCs from paint-industrial water effluents and the development of an easy, cheap, solventless, and rapid static headspace method combined with GC-MS for the identification of volatile contaminants in raw and treated industrial wastewater. The removal efficiency factors were introduced by applying different water treatment technology parameters. The next task of the paper was the method validation showing the linearity, the limit of detection (LOD), and the limit of quantification (LOQ).
2. Materials and Methods
2.1. Chemicals and Reagents
At first, standard stock solution of individual VOCs (benzene, toluene, ethylbenzene, xylene, and chlorobenzene) (KGaA, Darmstadt, Germany) was prepared at a concentration of 1 mg/mL in methanol (Sigma Aldrich, Steinheim, Germany), and afterwards, mix stock standard solution at a concentration of 0.20 ng/mL in water (Sigma Aldrich, Steinheim, Germany) was prepared. These stock solutions were used for the fortification of distilled water, used for the validation experiments. Stock solutions and t diluted working solutions were kept in a fridge. Sodium chloride (NaCl) (Lachema a.s., Brno, Czech Republic) was heated at 600 °C (6 h) in a furnace.
2.2. Samples
The real samples of the industrial effluents were obtained from the paint and coating company. In all, 40 L of the sample was directly sampled from the water tanks and delivered to the laboratory. The samples were collected into 1 L glass bottles and stored at temperature-controlled conditions at 4 °C. Before the extraction procedures, the samples needed to be filtrated using a 0.45 mm cellulose membrane filter (GF/F, Whatman, UK).
2.3. Industrial Water Treatment
Wastewater treatment was performed in a flow-through electrochemical setup working on electrocoagulation and electroflotation principles and running in a continuous loop mode. The setup operated in an open system in terms of pressurizing, i.e., the treated effluent from the electrochemical reactor was discharged into an open glass chamber, where it was mixed and then injected back into the electrochemical reactor for further processing during the next loop round. The electrochemical reactor was equipped with 4 rectangular carbon steel electrodes having a total electrode gap volume of 150 mL. The electrodes were interconnected in a so-called monopolar regime, i.e., only the two boundary electrodes were connected to the positive and negative polarity of the power supply. The total water flow rate was 5 Lpm due to avoiding sludge adhesion and further clogging inside the electrode gaps. The total voltage drop on the electrode system did not exceed 15 V thanks to the naturally high electrical conductivity of the sampled wastewater. Fundamental electrochemistry is based on a real-time metal hydroxide formation and further cyclical multi-loop utilization of organic adsorption processes which, however, cannot be described in deeper detail since the technology is under patent evaluation processes. Microbubble generator stones were mounted at the bottom of the glass chamber for separation of the formed foam/sludge via microbubble floatation. The final filter-pressed metalized sludge consisted of metallic oxides and hydroxides bound with organics, which were aimed to be further utilized as an admixture in concrete in the building industry (the scope of an ongoing industrial project).
Before starting the electrochemical process, the entire system was watered with the help of a pump, so that the water would circulate in the entire device before starting the process. Electrolysis took place at different current values (20 A, 30 A) for 10 min under regulated flotation (0–2.5 min without flotation, then flotation was switched on at an interval of 30 s and 1 min off during the measurement period). For the treatment processes, a portion of the samples was utilized. Different testing modes were used for the industrial water effluent in the verification test. The experiment aimed to select and demonstrate the effect of primary and secondary boundary conditions.
- −
- The industrial wastewater was diluted with deionized water 1:1 (treated water 1: deionized water V:V), and a working electric current of 20 A was used for electrochemical treatment;
- −
- The industrial wastewater was diluted with deionized water 1:1 (treated water 2: deionized water V:V), and a working electric current of 30 A was used for electrochemical treatment;
- −
- The industrial wastewater was diluted with deionized water 1:3 (treated water 3: deionized water V:V), and a working electric current of 20 A was used for electrochemical treatment;
- −
- The industrial wastewater was diluted with deionized water dilution 1:3 (treated water 4: deionized water, V:V), and a working electric current of 30 A was used for electrochemical treatment.
The test duration was 10 min, and the floatation was turned on during the whole process.
2.4. Headspace Sampling
For the extraction of volatile pollution from the industrial wastewater, static headspace sampling was applied, which is one of the simplest and most efficient ways for the separation of volatilized sample components. The procedure is based on the formation of gas in the space over the sample on the top of the sample vials. A volume of 30 mL of filtered water sample was placed in a 70 mL headspace glass vial sealed with a headspace cap. The sample was kept at a temperature of 70 °C to reach the equilibria for the time of 20 min. For separation and detection of volatile components, 40 µL of the headspace gas was introduced to GC-MS.
2.5. GC-MS Analysis
The GC-MS analyses of the headspace phases were evaluated using an Agilent 6890 (Agilent, Little Falls, DE, USA) gas chromatograph coupled with an Agilent 5975 (Agilent, Little Falls, DE, USA) mass spectrometric detector. The headspace phase was introduced into the system using a programmable temperature vaporization (PTV) injector maintained at a constant temperature of 220 °C. In all, 40 µL of the gas phase (headspace) was taken using a gas-tight microsyringe and injected in GC using splitless mode and setting a splitless time at 1 min. A narrow browed capillary column (15 m length × 0.15 mm I.D. × 0.15 μm film thickness) with 5% diphenyl 95% dimethylsiloxane stationary phase (CP-Sil 8 CB; Agilent Technologies, Middelburg, Netherlands) was used for the separation of the analytes. Non-polar deactivated precolumn 1 m long with an internal diameter of 0.32 mm was used. The oven temperature-program conditions were: 60 °C held for 1.75 min, then raised with a gradient of 60 °C/min to 150 °C, then increased to 23.8 °C/min to 300 °C, and the final isothermal part was 1.90 min. The flow of Helium as the carrier gas was set at 1.2 mL/min in constant flow mode.
The mass spectrometer worked in electron ionization mode (70 eV) with the temperature of the ion source at 250 °C. The mass range of 50–550 m/z was set up for a full scan. For quantification purposes, the selected ion monitoring (SIM) mode was employed.
3. Results and Discussion
It is known that a high number of organic compounds are contained in widely used primers, top-finish paints, barrier paints, fire-retardant paints, decoration adhesives, and diluents [29]. There are four major components of paints. The most abundant components are the different types of solvents; important components are pigments and binders, and also different types of chemical additives are used in paints. This study is focused on solvents, which should also be the main component of paint-industrial effluents. Solvents are volatile and are used in the paint industry to dissolve or distribute the binder or make the production of the paints easier and have a positive effect on application conditions [30]. Painting and drying painted surfaces, solvents should be evaporated into the air mainly as mixtures of organic hydrocarbons, alcohols, esters, and ketones. On the other hand, high volumes of these organic solvents should be found in wastewater effluents from paint industries because water is used for the cleaning of barrels, tubes, and machines, which are used for the production and packaging of the products.
3.1. Evaluation of HS Sampling and Identification of Pollutants in HS Gas
Organic solvents, which are used during paint production and are the typical representation of VOCs, are a group of relevant environmental pollutants [31]. One of the ideal methods for sampling VOCs is the headspace technique. The major advantages of this simple method are the cost (it is a cheap technique), the instrumentation (without using complicated instruments), and its solventless character. Headspace sampling requires only the thermodynamic equilibrium between the aquatic and gaseous phases in a closed-tempered vial. After reaching equilibrium, the volatile compounds are represented in gas phase which is injected into the GC and analyzed without the influence of the matrix or extraction solvent. On the other hand, the disadvantage of headspace sampling is the restriction of the sensitivity of the method by the concentration of the VOCs in the headspace phase. The concentration of VOCs in the headspace phase depends on several parameters, such as the initial concentration in the water, the phase ratio between the liquid phase and gas phase, and the water/air distribution constant [31].
The paint-industrial water effluent underwent analysis by GC-MS in full scan mode, and some of the VOCs were identified in the HS, namely toluene, ethylbenzene, chlorobenzene, and the isomers of xylene.
The headspace sampling parameters were studied by comparing the chromatographic peak areas of the identified compound with those recorded at different sampling parameters. Different parameters affect the effective transfer of analytes to a headspace gas. The effects of sampling temperature, sampling time, stirring rate, and the volume of the injected gas phase on the efficiency of the VOC transfer to the gas phase were investigated and optimized independently.
At first, the optimal volume of the injected HS phase into the GC-MS system was selected. A 30 mL sample of industrial water was placed in a vial, which was heated in a water bath to a temperature of 60 °C. After the HS sampling time, a volume of 10, 20, 30, 40, and 50 µL was injected into the gas chromatograph, and the chromatographic peaks of the analytes were subsequently compared. A gradual increase in the peak areas of the analytes was observed with an increase in the injected volume of the gas phase. The largest peak areas were obtained in the case of injecting 40 µL of the headspace phase. When the volume was further increased, the chromatographic peak areas of the identified analytes began to decrease, as a result of which, 40 µL was selected as the optimal injecting volume of the HS phase.
As the next parameter, the sampling temperature was examined. The enhanced temperature could increase the distribution of VOCs between a sample and the headspace because higher temperatures cause higher vapor pressure of the analyte, and consequently, its concentration in the headspace increases [28]. According to the literature, the usually applied HS sampling temperature for the BETX sampling from water samples is between 70 and 80 °C [31,32,33]. Therefore, for the selection of the sampling temperature, the sample was exposed for 20 min to temperatures ranging from 60 to 80 °C. HS sampling at the laboratory temperature was also tested, but the extracted analyte concentration was below the limit of detection. It was observed that the quantity of the extracted compounds increases with the temperature (Figure 1). Headspace sampling at 80 °C also caused lower peak resolution than at 70 °C, which complicated the identification of the analytes. At higher temperatures, a higher amount of water may be injected into the GC system, which could cause an increase in the background level [32]. Therefore, to reduce water vapor, 70 °C was preferred as the optimum sampling temperature.
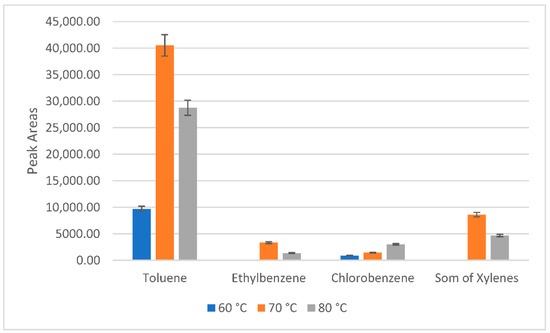
Figure 1.
The effect of the headspace sampling temperature on the efficiency of the sampling of toluene, ethylbenzene, chlorobenzene, and total xylene transfer to the gas phase. The dependence of the peak areas on the sampling temperature.
An important effect on the headspace sampling method efficiency has the sampling time because headspace sampling is an equilibrium-based technique. The influence of the headspace sampling time on the peak area was studied by adjusting the time in the range of 5–30 min. By using a higher sampling time, the transfer of the analytes into the gaseous phase is increased. The equilibrium was observed after 20 min. After this time, no significant changes were noticed in the extraction efficiency for the target analytes (Figure 2). The selected time was enough for the distribution of the analyte between the headspace and water media. However, the small decrease in the peak areas for VOCs after 20 min can be considered not relevant, but it was shown in the literature that there is a degeneration in the method precision for longer sampling times [31,33,34]. Therefore, the sampling time was fixed at 20 min.
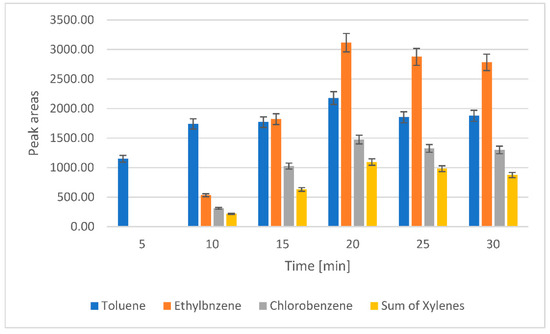
Figure 2.
The effect of sampling time on the efficiency of toluene, ethylbenzene, chlorobenzene, and xylene transfer to the gas phase. The dependence of the peak areas on the sampling time.
The mass transfer of the volatile analytes should be influenced by another important parameter, which is the stirring rate. This parameter has an effect on the time-enrichment profile. It was shown that mixing of the sample solution improves the mass transfers in the aqueous phase and induces convection in the headspace, and consequently, the equilibrium between the aqueous and vapor phases can be more rapidly attained [28]. Stirring rate was evaluated between 0 rpm and 1200 rpm. The increase in the efficiency of volatile compounds’ transfer into the headspace reaching a stirring rate of 1000 rpm was confirmed, which was then sequentially decreased at higher stirring rates. At a higher speed, the stirring bar started to vibrate, and the mixing of the sample was not consistent. This means that higher agitation tends to be uncontrollable, which might cause a change in the equilibrium time and poor measurement precision [28]. Therefore, to reach the highest efficiency of the mass transfer, the stirring rate of 1000 rpm was selected for further experiments.
It was shown that the best HS parameters for the isolation of toluene, ethylbenzene, xylene, and chlorobenzene from the paint-industrial water effluent were the following: 20 min sampling time, 70 °C sampling temperature, 1000 rpm stirring rate, and the volume of the gas phase at 40 µL.
3.2. Validation of the GC-MS Method
The selected validation parameters (such as linearity, correlation coefficient, LODs, and lLOQs) for the identified analytes were studied in spiked water samples and are collected in Table 1. The spiked water samples were analyzed by HS-GC-MS. A seven-point calibration curve was constructed by extracting seven spiked water samples containing all the analytes at different concentration levels in the range from 0.01 to 10 µg/mL. For each calibration level, three replicate headspace samplings were performed. Acceptable linearity was obtained for all the studied VOCs over the whole studied concentration range with correlation coefficients (r2) higher than 0.990. The high correlation coefficient demonstrates a directly proportional relationship between the concentration of the analytes in the HS phase and the initial concentration of the sample. The LODs based on a signal-to-noise ratio (S/N) of 3 ranged from 0.19 to 4.02 µg/L, which is below regulated limits for these compounds in drinking water. The validation parameters are shown in Table 1. The obtained LODs are lower than the established maximum permissible levels of these contaminants in water destined for public supply of 1.0 mg/L (toluene), 0.7 mg/L (ethylbenzene), 10 mg/L (total xylenes), and 0.1 mg/L for chlorobenzene.

Table 1.
The retention times of the identified compounds, the selected fragment ions (m/z), and basic validation parameters.
3.3. Industrial Water Treatment
The most common parameter to be monitored for nontoxic wastewater is COD. The treatment efficiency of the different processes was determined by studying the CODs of wastewater and treated water. The main characteristics of paint-industrial wastewater were a pH value of around 9 and a high value of COD 60 g/L.
Two dilution ratios (1:1 and 1:3) of industrial wastewater and two different working electric currents (20 A and 30 A) were tested. As a result, four different types of treated wastewater were obtained. In all cases, the COD values were rapidly reduced; however, lower values were obtained when the dilution ratio was 1:3 (V:V) for working electric current 20 A and 30 A, respectively. The reduction in CODs is shown in Figure 3. The COD was reduced by between 95% and 99% in both systems. The reduction in the COD values reflects that the system worked with a higher working electric current, 30 A, which was more advantageous because the COD values have been reduced from 60 g/L to 0.63 g/L. The COD values of the untreated diluted effluents were evaluated. The COD has been reduced thanks to the dilution into the values of 21.8 g/L and 33.5 g/L for dilution ratio 1:3 (wastewater:deionized water, V:V) and 1:1 (wastewater:deionized water, V:V), respectively.
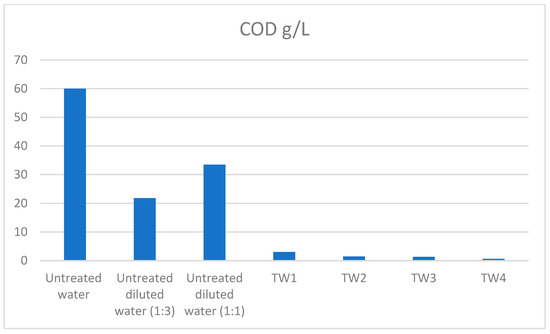
Figure 3.
The reduction in the COD values using the different parameters during the wastewater treatment process. TW1—dilution 1:1 (treated water 1: deionized water V:V), working electric current: 20 A; TW2—dilution 1:1 (treated water 2: deionized water V:V), working electric current: 30 A; TW3—dilution 1:3 (treated water 3: deionized water V:V), working electric current: 20 A; TW4—dilution 1:3 (treated water 4: deionized water, V:V), working electric current: 30 A.
The most commonly used method for paint-industrial wastewater treatment is the physicochemical treatment method; however, COD removal efficiency is lower in comparison with the hybrid electrothermochemical method, and it depends on the sample pH and the type of coagulants and flocculants [35,36,37]. In paint-industrial effluent treatments, electrochemical methods are also very popular and starting to replace traditional processes. However, in the case of COD removal, they are less effective than the combined hybrid electrothermochemical wastewater treatment with COD removal between 66% and 81% [38,39,40]. The new hybrid electrothermochemical wastewater treatment technology provides a similar COD removal, such as Fenton’s oxidation process at 99.5% [41]. The comparison of the different treatment processes in the case of COD removal efficiency is shown in Table 2.

Table 2.
Comparison of different treatment processes used for paint-industrial effluents.
3.4. Study of the Removal Efficiency
The validated HS-GC-MS method in SIM mode was used for the analyses of the treated industrial waters for the inspection of treatment efficiency. The peak areas of the identified compounds in treated water samples were compared with the peak areas obtained for the raw industrial effluent. The evaluations of the data showed significant elimination of the VOCs in all studied treatment systems.
The removal efficiency factors (REFi) [19] were calculated for the characterization of different treatment procedures, for the studied individual compounds:
where A (Mi, TW) is the peak area of individual VOCs determined in the headspace gas taken above the treated water sample, and A (Mi, IW) is the peak area of individual VOCs from the headspace gas taken from the industrial water sample (raw sample).
REFi= (1 − A (Mi, TW)/A (Mi, IW))⋅100%
Significant removal efficiency was obtained when the REF exceeded a percentage of 70%. Removal efficiency was characterized as moderate when the obtained REF was in the range of 30–70%. Below 30%, the removal of selected VOCs was evaluated as weak. Table 3 contains the removal efficiency factors for all studied VOCs for different treatment procedures.

Table 3.
Removal efficiency factors (REFi) for the identified VOCs for studied water treatment technology procedures (TW).
The most abundant compound in industrial water effluent was toluene, and it was shown that the amount was significantly eliminated during the treatment process. In the case of using sample dilution 1:3 before the treatment process (Treatment 3 and 4), significant removal was obtained for all of the studied compounds. On the other hand, when the sample dilution ratio was 1:1 (Treatment 1 and 2), moderate removal was acquired for Toluene and the sum of the xylenes. In all cases, the REFs were significantly higher when the working electric current of 30 A was applied during the treatment procedure for both dilution ratios. For all the VOCs, significant removal was shown in the case of procedure 4. It can be observed that the worst results were obtained when the sample dilution ratio was 1:1 and 20 A of the working electric current was applied. Compared with untreated industrial water, the peak areas of the studied compounds decreased in all cases (Figure 4).
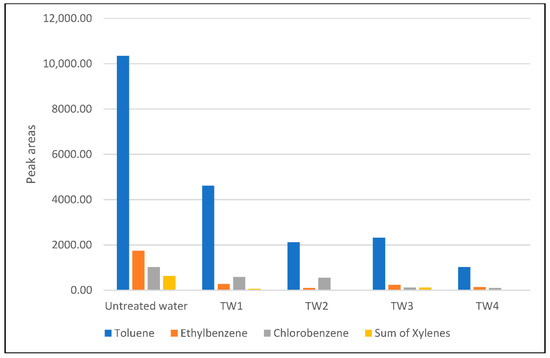
Figure 4.
Dependence of the peak areas of studied VOCs on different treatment processes (TW 1—TW 4—treated water samples under the treatment conditions noted in Table 3).
4. Conclusions
A hybrid electrothermochemical wastewater treatment technology has been proposed for the elimination of selected VOCs from paint-industrial wastewater samples. The elimination of organic contaminants was evaluated by COD; additionally, selected individual components were searched for in detail via HS-GC-MS. The effect of several sampling parameters, such as sampling temperature, sampling time, stirring rate, and the volume of the injected HS phase was evaluated, and the optimal sampling parameters were selected for the separation of the selected VOCs from industrial water effluent. The efficiency of the treatment processes under various working conditions was characterized by the REF of the compounds of interest, and the removal was evaluated. Working conditions eliminated studied VOCs with REF above 90%, and it can be concluded that substantial removal was proposed.
Author Contributions
Conceptualization, A.S. and S.H.; funding acquisition, G.H. and S.H.; investigation, A.S., V.M. and G.H.; methodology, A.S., V.M., G.H. and S.H.; project administration, G.H. and S.H.; supervision, A.S. and S.H.; validation, A.S. and S.H.; writing—original draft, A.S.; writing—review and editing, G.H. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Research and Development Agency under Contract No. APVV-19-0149. The work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic (VEGA project no. 1/0412/20). The work was supported by the project EUREKA EUROSTARS2 114774.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patwardhan, A.D. Industrial Wastewater Treatment, 2nd ed.; Eastern Economy Edition; PHI Learning: Delhi, India, 2017. [Google Scholar]
- Smith, M.R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation 1990, 1, 191–206. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Fedorov, K.; Plata-Gryl, M.; Khan, J.A.; Boczkaj, G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020, 397, 122804. [Google Scholar] [CrossRef]
- Lueders, T. The ecology of anaerobic degraders of BTEX hydrocarbons in aquifers. FEMS Microbiol. Ecol. 2017, 93, fiw220. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef] [PubMed]
- Godin, S.; Kubica, P.; Ranchou-Peyruse, A.; Le Hecho, I.; Patriarche, D.; Caumette, G.; Szpunar, J.; Lobinski, R. An LC-MS/MS Method for a Comprehensive Determination of Metabolites of BTEX Anaerobic Degradation in Bacterial Cultures and Groundwater. Water 2020, 12, 1869. [Google Scholar] [CrossRef]
- Lecompte, B.F.C.; Kakar, D.; Mehrvar, M. Photochemical treatment of benzene, toluene, ethylbenzene, and xylenes (BTEX) in aqueous solutions using advanced oxidation processes: Towards a cleaner production in the petroleum refining and petrochemical industries. J. Clean. Prod. 2018, 186, 609–617. [Google Scholar] [CrossRef]
- Caetano, M.O.; Schneider, I.A.H.; Gomes, L.P.; Kieling, A.G.; Miranda, L.A.S. A compact remediation system for the treatment of groundwater contaminated with BTEX and TPH. Environ. Technol. 2016, 38, 1408–1420. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Elsner, M.; Griebler, C.; Lueders, T.; Stumpp, C.; Aamand, J.; Agathos, S.N.; Albrechtsen, H.J.; Bastiaens, L.; Bjerg, P.L. Biodegradation: Updating the concepts of control for microbial cleanup in contaminated aquifers. Environ. Sci. Technol. 2015, 49, 7073–7081. [Google Scholar] [CrossRef]
- Zamir, S.M.; Halladj, R.; Nasernejad, B. Removal of toluene vapors using a fungal biofilter under intermittent loading. Process Saf. Environ. 2011, 89, 8–14. [Google Scholar] [CrossRef]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, K.I.; Albayati, M.T. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Leili, M.; Farjadfard, S.; Sorial, G.A.; Ramavandi, B. Simultaneous biofiltration of BTEX and Hg from a petrochemical waste stream. J. Environ. Manag. 2017, 204, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef] [PubMed]
- Unuigbe, C.F.; Fayemiwo, O.M.; Daramola, M.O. Performance evaluation of iron nanoparticles infused polyethersulphone (Fe-NPs/PES) membrane during treatment of BTEX-contaminated wastewater. Water Environ. J. 2020, 34, 74–86. [Google Scholar] [CrossRef]
- Sharma, D.; Chaudhari, P.K.; Dubey, S.; Prajapati, A.K. Electrocoagulation treatment of electroplating wastewater: A review. J. Environ. Eng. 2020, 146, 03120009. [Google Scholar] [CrossRef]
- Muddemann, T.; Haupt, D.; Sievers, M.; Kunz, U. Electrochemical reactors for wastewater treatment. ChemBioEng Rev. 2019, 6, 142–156. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Alardhi, S.M.; Ahmed, S.A.; Salman, A.D.; Juzsakova, T.; Cretescu, I.; Le, P.C.; Chung, W.J.; Chang, S.W.; Nguyen, D.D. Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Environ. Res. 2022, 214, 113890. [Google Scholar] [CrossRef]
- Szarka, A.; Viktoryová, N.; Horváth, G.; Szalay, Z.; Šimo, F.; Hrouzková, S. GC–MS methods for the evaluation of the performance of electrochemical water treatment for the degradation of pollutants from paint industry effluents. Monatsh. Chem. 2022, 153, 161–169. [Google Scholar] [CrossRef]
- Andraščíková, M.; Matisová, E.; Hrouzková, S. Liquid Phase Microextraction Techniques as a Sample Preparation Step for Analysis of Pesticide Residues in Food. Sep. Purif. Rev. 2015, 44, 1–18. [Google Scholar] [CrossRef]
- Larriba, M.; Navarro, P.; García, J.; Rodríguez, F. Liquid-liquid extraction of BTEX from reformer gasoline using binary mixtures of [4empy][Tf2N] and [emim] [DCA] ionic liquids. Energy Fuel 2014, 28, 6666–6676. [Google Scholar] [CrossRef]
- Serrano, A.; Gallego, M. Fullerenes as sorbent materials for benzene, toluene, ethylbenzene, and xylene isomers preconcentration. J. Sep. Sci. 2006, 29, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Faraji, H.; Tajbakhsh, M.; Helalizadeh, M. Validation and optimization of a liquid-phase microextraction method for trace analysis of BTEX in water samples. Anal. Methods 2012, 4, 3372–3380. [Google Scholar] [CrossRef]
- Maya, F.; Ghani, M. Ordered macro/micro-porous metal-organic framework of type ZIF-8 in a steel fiber as a sorbent for solid-phase microextraction of BTEX. Microchim. Acta 2019, 186, 425. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Tong, Y.; Li, N.; Ouyang, S.; Yang, H.; Xu, J.; Ouyang, G. Sample bottle coated with sorbent as a novel solid-phase extraction device for rapid on-site detection of BTEX in water. Anal. Chim. Acta 2021, 1152, 338226. [Google Scholar] [CrossRef] [PubMed]
- Barco-Bonilla, N.; Plaza-Bolaños, P.; Fernández-Moreno, J.L.; Romero-González, R.; Frenich, G.A.; Vidal, J.L.M. Determination of 19 volatile organic compounds in wastewater effluents from different treatments by purge and trap followed by gas-chromatography coupled to mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Liu, Y.T.; Wu, B.Z.; Nian, H.C.; Chen, H.J.; Chiu, K.H.; Lo, J.G. Process sampling module coupled with purge and trap-GC-FID for in situ auto-monitoring of volatile organic compounds in wastewater. Talanta 2009, 80, 903–908. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Hasheminasab, K.S.; Baghdadi, M.; Khakpour, A. A simple and rapid method based on direct transfer of headspace vapor into the GC injector: Application for determination of BTEX compounds in water and wastewater samples. Anal. Methods 2012, 4, 1996–2001. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, T.; Xu, X.; Wang, H.; Miao, C. Determination of BTEX compounds in solid–liquid mixing paint using the combination of solid phase extraction, thermal desorption and GC-FID. Chromatographia 2010, 71, 1131–1135. [Google Scholar] [CrossRef]
- Erkmen, J. Solvent Bazlı Inşaat Boyalarında Pigment Boyutunun Etkilerinin Uzun Zaman Aralığında Incelenmesi. Master’s Thesis, Atatürk University, Erzurum, Turkey, 2012. [Google Scholar]
- Yilmazcan, O.; Ozer, E.T.; Izgi, B.; Gucer, S. Optimization of static head-space gas chromatography–mass spectrometry–conditions for the determination of benzene, toluene, ethyl benzene, xylene, and styrene in model solutions. Ekoloji 2013, 22, 76–83. [Google Scholar] [CrossRef]
- Hou, M.; Ren, H.; Cheng, W.; Li, L.; Zhang, S.; Chen, Y.; Yu, C.; Li, F.; Tian, S.; Deng, Z. Development of a headspace-gas chromatography/mass spectrometry method based on matrix-matched calibration for evaluating VOC content, characterization, source, and risk in RO membrane. Polym. Test. 2022, 107, 107474. [Google Scholar] [CrossRef]
- Pascale, R.; Bianco, G.; Calace, S.; Masi, S.; Mancini, I.M.; Mazzone, G.; Caniani, D. Method development and optimization for the determination of benzene, toluene, ethylbenzene and xylenes in water at trace levels by static headspace extraction coupled to gas chromatography-barrier ionization discharge detection. J. Chromatogr. A 2018, 1548, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Eshagi, Z.; Ebrahimi, M.; Hosseini, M.S. Optimization of a novel method for determination of benzene, toluene, ethylbenzene, and xylenes in hair and waste water samples by carbon nanotubes reinforced sol–gel based hollow fiber solid phase microextraction and gas chromatography using factorial experimental design. J. Chromatogr. A 2011, 1218, 3400–3406. [Google Scholar]
- Eremektar, G.; Goksen, S.; Babuna, F.; Dogruel, S. Coagulation-flocculation of wastewaters from a water-based paint and allied products industry and its effect on inert COD. J. Environ. Sci. Health A–Toxic Hazard. Subst. Environ. Eng. 2006, 41, 1843–1852. [Google Scholar] [CrossRef]
- Balik, Ö.Y.; Aydin, S. Coagulation/flocculation optimization and sludge production for pre-treatment of paint industry wastewater. Desalin. Water Treat. 2016, 57, 12692–12699. [Google Scholar] [CrossRef]
- Aldemir, A.; Hakkıtanır, E.; Özgüven, A. Determination of optimum treatment conditions for paint industry wastewater with the coagulation/flocculation method. Desalin. Water Treat. 2021, 211, 165–176. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Aktas¸, N.; Tanyolaç, A. Optimization of electrochemical treatment of industrial paint wastewater with res-ponse surface methodology. J. Hazard Mater. 2007, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, D.; Dagdas, E.; Fil, B.A.; Bashir, M.J.K. Central composite modeling for electrochemical degradation of paint manu-facturing plant wastewater: One-step/two-response optimization. Environ. Technol. Innov. 2021, 21, 101264. [Google Scholar] [CrossRef]
- Can-Güven, E.; Guvenc, S.Y.; Ilhan, F.; Varank, G. Application of combined EO/PMS/Me2+ process in organic matter and true color removal from paint manufacturing industry wastewater. Environ. Res. 2022, 212, 113451. [Google Scholar] [CrossRef]
- De Oliveira, I.S.; Viana, L.; Verona, C.; Fallavena, V.L.V.; Azevedo, C.M.N.; Pires, M. Alkydic resin wastewaters treatment by fenton and photo-Fenton processes. J. Hazard. Mater. 2007, 146, 564–568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).