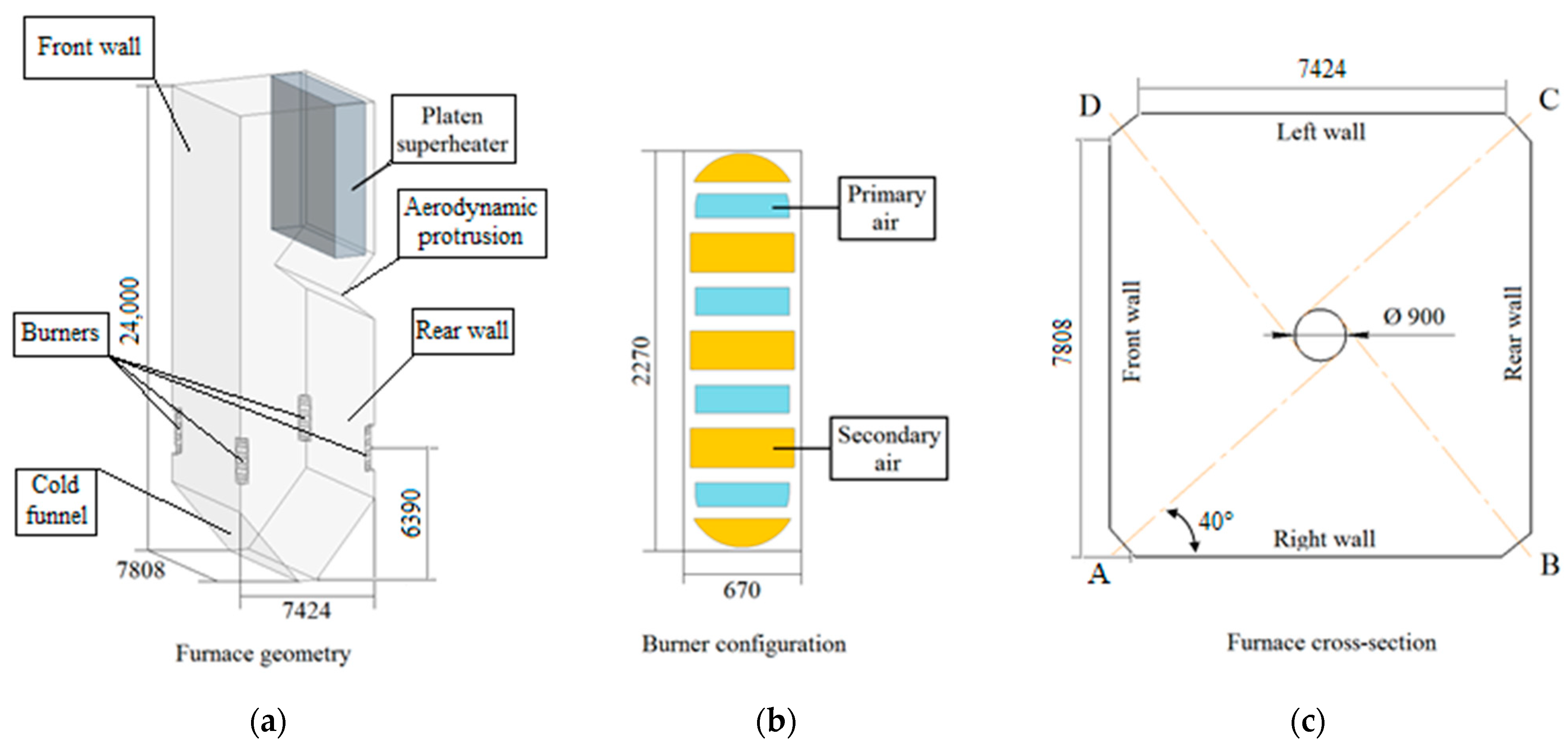
1. Introduction
Composite fuels based on carbon-containing industrial waste and wastewaters, including those containing oil products, are promising for practical use in conditions of shortage and rising prices of traditional energy resources [
1,
2]. The following various solid and liquid components can be used as fuel components: coal sludge, coal refining waste (coal slurry), waste process oils, oil extraction and oil refining waste, chemical waste, various types of biomass [
3,
4,
5].
Involvement of coal and oil refining wastes in the form of coal water slurries (CWS) and coal water slurries with petrochemicals (CWSP) in industrial thermal power engineering will reduce the environmental burden not only by reducing the area of landfills for waste storage [
3,
6], but also due to the reduction in greenhouse gas emissions [
7,
8]. This effect is achieved by increasing the completeness of the slurry fuel components burnout due to the secondary atomization of inhomogeneous droplets during intense heating [
9,
10,
11]. Components (solid and liquid) of the composite fuel droplets are heated at different rates. As a result, local vaporization centers can appear at the boundary of their contact (including mutually insoluble liquids) [
9,
10]. An increase in stress near the interface between the components (especially solid and liquid ones) can cause a microexplosion, as a result of which a droplet is dispersed into tens and hundreds of finely dispersed fragments [
11,
12,
13]. Droplet fragmentation increases the surface area of heat exchange between the fuel and the heated air, thereby intensifying the evaporation and thermal decomposition of the fuel components, and, consequently, ignition and combustion. In addition, the presence of water in the fuel is a favorable factor for intense oxidative reactions and neutralization of harmful substances occurring at relatively high temperatures in boiler furnaces. At a relatively high temperature, water vapor thermally dissociates, resulting in the release of oxygen and hydrogen molecules. These molecules intensify the oxidation process, thereby improving the completeness of fuel burnout [
14,
15]. Aditionally, favorable factors for replacing coal with CWS and CWSP include a reduction in fire hazards (due to the presence of water in the fuel composition) at the stages of preparation, transportation and storage, as well as lower fuel costs [
16].
Combustion of composite fuels with solid components in their composition (in the form of slurries), as well as the use of solid fuels in their initial form, is accompanied by the formation of a solid residue in the form of ash and slag. Formation intensity and ash residue proportion depend on the elemental composition of the solid component. The higher the content of the mineral part in the fuel composition, the higher this characteristic. Slagging is aggravated if the coal contains pyrite (iron disulfide) [
17].
Ash and slag deposits are formed not only in the lower part of the boiler furnace, but also directly on the heat exchange surfaces in the radiative and convective parts of the furnace. Build-up of softened or molten fly ash on heat exchanger tubes occurs when the temperature regime of the boiler furnace deviates (increases) from the design one, as well as when the main fuel is replaced with an alternative one without adjusting the temperature regime. Ash and slag deposits in boiler furnaces on the surface of heat exchanger tubes are a serious problem. They cause high-temperature corrosion of the metal, which threatens to cause accidents. Slag is characterized by relatively low thermal conductivity, so the intensity of heat transfer from the source (hot gases) to the coolant through the slagged surfaces is significantly reduced [
18]. This leads to an increase in the temperature of the flue gases above the design values, a decrease in the overall efficiency of the boiler and excessive fuel consumption. Violations of the aerodynamic regime on the side of flue gases are possible, due to a decrease in the gap between the heat exchanger tubes.
Steam blowing of waterwall tubes is used as the main tool to reduce slagging [
19]. Usually, the process of furnace cleaning from slag deposits occurs at a given frequency, which is not always effective. Excessive use of steam blowing leads to intensive wear of the waterwall tubes, and too lengthy breaks between blowings negatively affect the boiler efficiency. A promising solution to the problem of removing slag deposits from the surfaces is the individual selection of the cleaning mode, considering the features of deposit formation for a specific fuel composition and specified design parameters of the boiler. Creation of such regime charts is possible using mathematical modeling tools that allow prediction of the characteristics of ash and slag deposits’ formation [
20,
21].
The results of numerical studies using different methods are known. For example, within the framework of computational fluid dynamics (CFD), there are two main approaches: steady [
22] and transient [
23,
24]. Steady models save computation time, as they track only a specific number of particles in the combustion products but do not consider the dynamics of changes in the thickness of the sediment layer. The characteristics of this process can be controlled within the framework of transient models, but this approach requires a large number of calculations at a small time step, which leads to large computational resources.
The authors of [
25,
26] present a complex approach to the development of a mathematical model for the formation of slag deposits on the heat exchange surface during the combustion of solid fossil fuel. As a condition for ash particles’ build-up, critical values of viscosity were used, which, in turn, were obtained from the results of experimental studies. The developed model [
25] is represented by several sub-models for calculating the characteristics of the various following stages of slagging: the formation and transport of fly ash particles, particle build-up to the surface and growth of the sediment layer and particle collision. Chernetskiy and Butakov [
25] obtained a good qualitative agreement between the theoretical and experimental results, but due to the multifactorial nature of the process (the formation of ash primary layer, the variability of the slag particles properties, the spontaneous deposits destruction, etc.), the quantitative results differ.
The review [
27] presents general recommendations for improving mathematical models of heat exchange surfaces slagging. The following areas for improvement have been highlighted [
27]: to increase the reliability, it is necessary to complicate the models, including by refining the spatial grid and the integration step; for an adequate description of real physical processes, it is necessary to carefully choose the criteria of build-up particles, to consider the non-uniformity and variability of their viscosity; it is necessary to consider the formed deposits’ structure, the processes of erosion and spontaneous shedding [
27].
Given the wide variety of processes occurring during boiler slagging, as well as the recommendations formulated above, it can be concluded that there is a need for complex mathematical models of slagging. They should describe the processes occurring during the combustion of fuel in the boiler at the macro level (on the scale of the entire furnace) and on the micro level (on the scale of one particle or one element of the heat exchanger). It should be noted that recently, an urgent task in practice is the transfer of coal-fired steam boilers to fuel mixtures, including in the form of slurries. Therefore, the mechanisms for a reliable assessment of the dynamics of heating surfaces’ slagging are the basis for the development of scientifically based practical measures aimed at changing the thermal regime of the boiler.
When boilers are transferred to non-design composite fuels containing low-grade combustible components and water, the flame temperature, as a rule, decreases. At the same time, the problem of slagging remains relevant, as many components of multicomponent fuels (for example, some industrial waste and biomass) are characterized by a relatively high content of alkali and alkaline earth metals [
28,
29]. The structure of ash and slag deposits forming during combustion of CWS and CWSP can be multi-layered and heterogeneous in chemical composition, which complicates the fight against this negative phenomenon by standard means.
The objectives of this work are as follows: (1) the development of two interrelated mathematical models of slagging in the furnace of a steam boiler with flame combustion of a fuel; (2) study of the effect of combustion parameters for three fuels (coal dust, droplets of CWS and CWSP) on the slagging characteristics of heating surfaces. The developed predictive complex is one of the components of a comprehensive algorithm for assessing the prospects of the practical use of composite fuels instead of solid fossil fuels.
3. Results and Discussion
3.1. Boiler Furnace Slagging
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8 illustrate typical temperature fields in a boiler furnace and typical fields of slagging rates of furnace walls for the three fuels being burned.
Table 9 presents the average growth rate of slag deposits on the furnace walls and the conversion degree of the fuel coke residue.
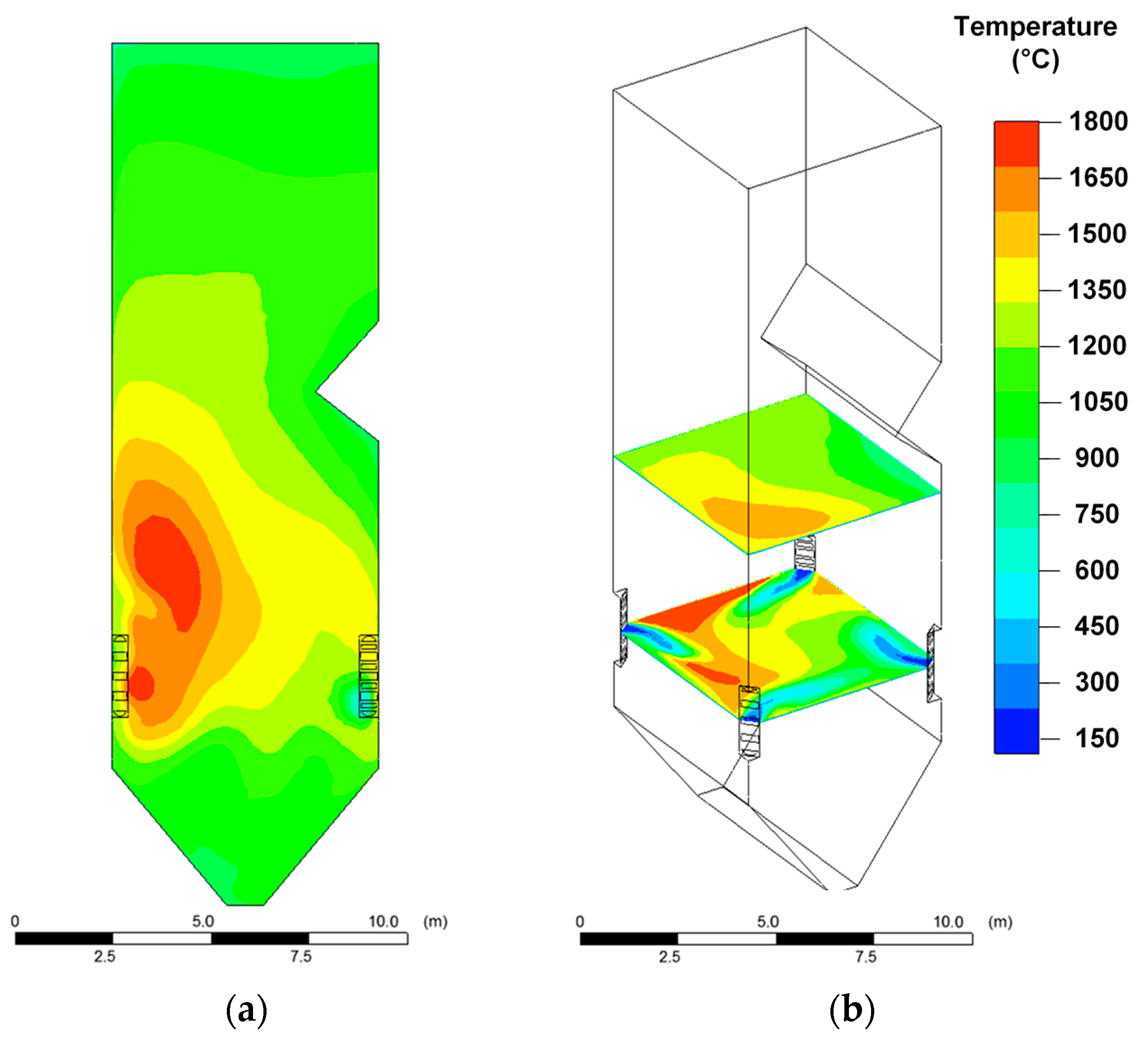
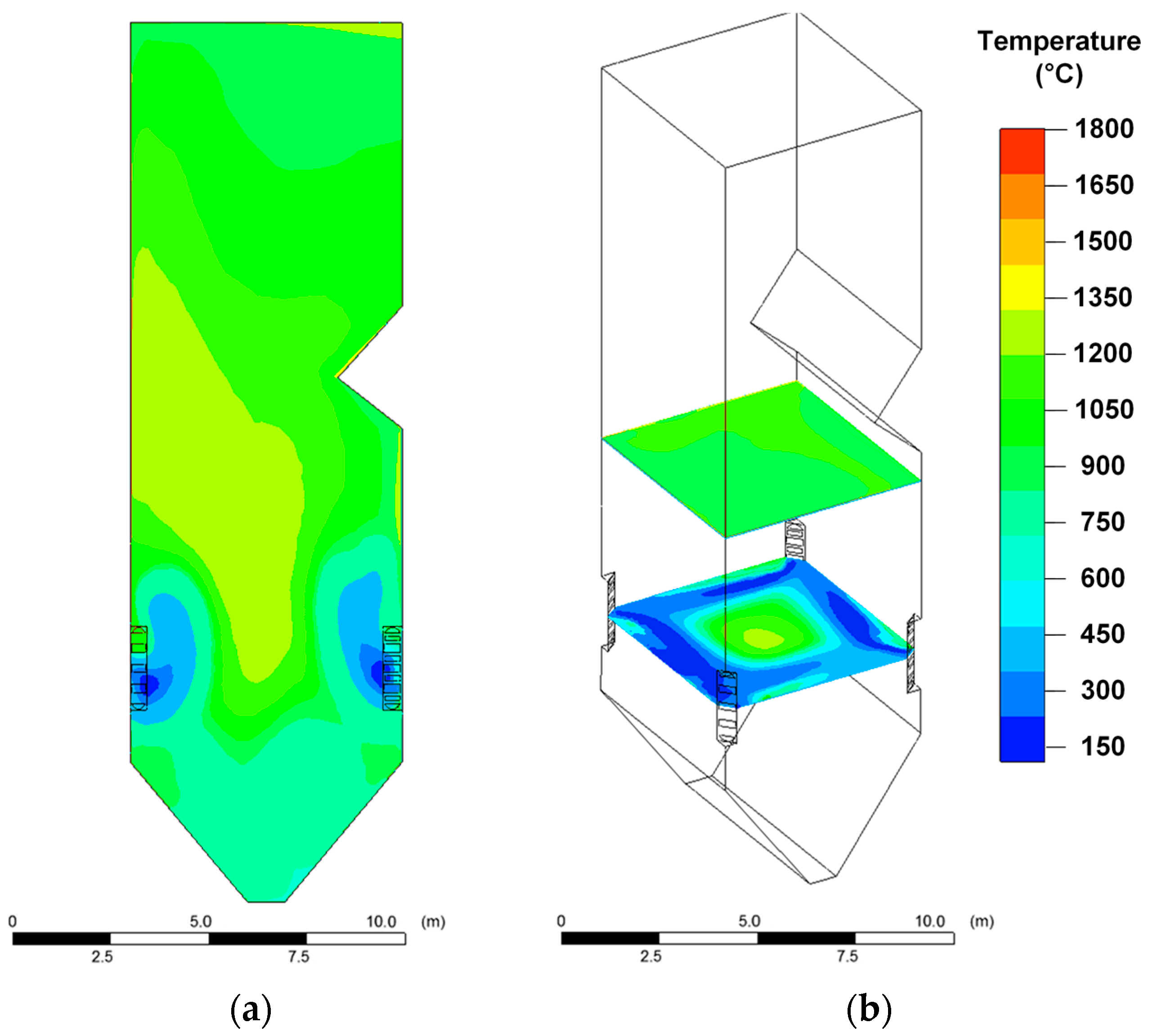
Relatively high temperatures in the boiler furnace are achieved when dry coal dust is burned (
Figure 3). In this case, in different areas of the furnace, the temperature varied in the widest range of 300–1800 °C (
Figure 3) compared with other fuels (CWS and CWSP). When CWS is burned, the temperature varied from 200 °C to 1200 °C (
Figure 4); when CWSP is burned, from 200 °C to 1350 °C (
Figure 5). At the same time, the flue gas temperatures in the upper part of the furnace near the platen superheaters had fairly close values (1000–1050 °C) for all three fuels under consideration (
Figure 3,
Figure 4 and
Figure 5).
When CWS and CWSP are burned, the relatively low temperature zones (200–600 °C) near the fuel burners are much wider than when coal dust is burned. This is especially noticeable when analyzing the temperature distribution in the cross section of the furnace (
Figure 4 and
Figure 5). The result is due to the relatively high moisture content in the composition fuel slurries. When injected into the furnace, a droplet of a slurry, in contrast to a particle of dry coal, undergoes a relatively long evaporation stage, accompanied by thermal energy consumption and, as a consequence, a decrease in the gas temperature. This suggests that particles of softened ash falling into these zones will harden, i.e., the intensity of formation of the slag layer on the furnace heating surfaces and on the burners themselves may be less than when burning coal dust.
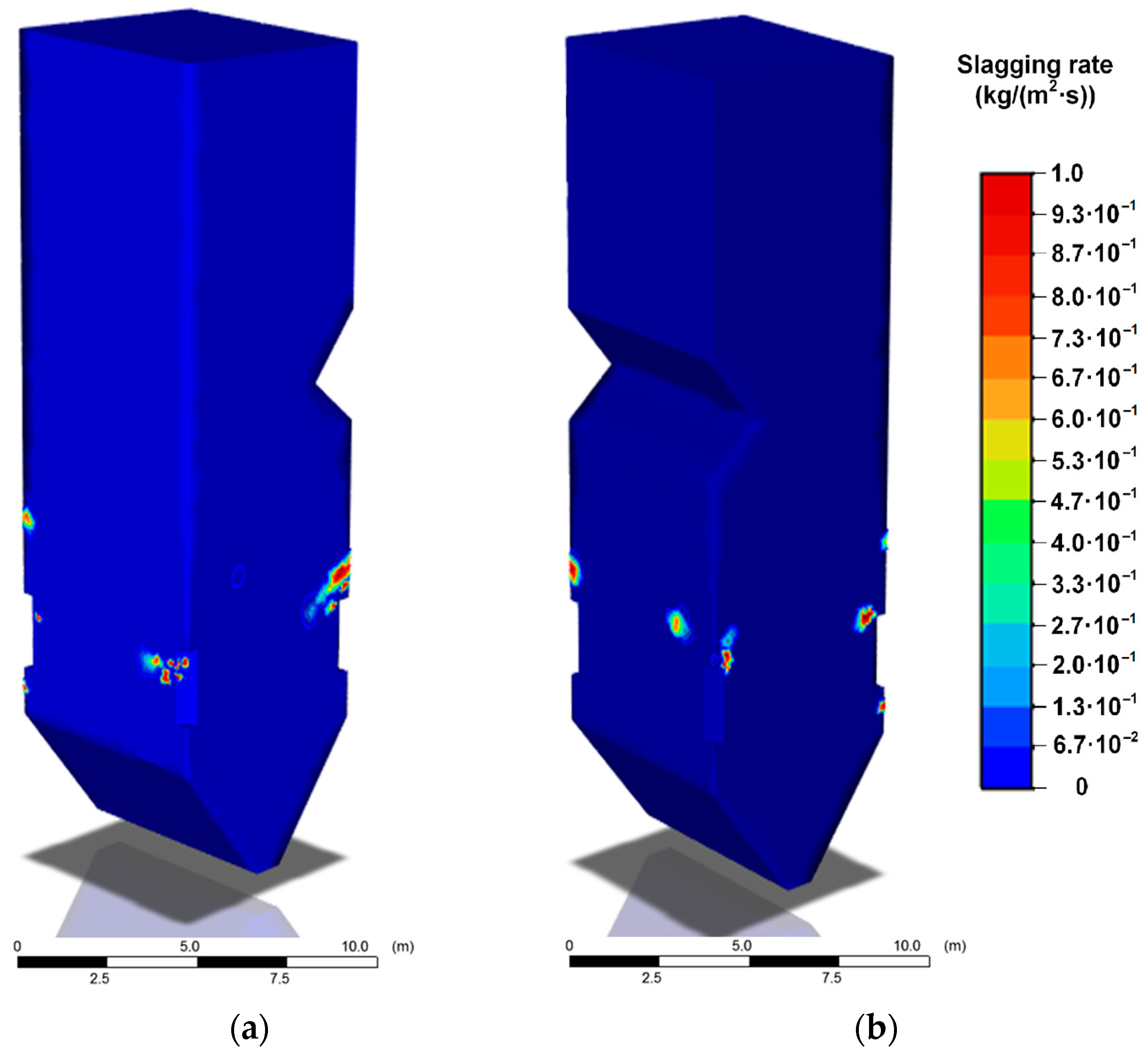
The temperature distribution inside the boiler furnace is one of the factors that determine the slagging intensity of heating surfaces. As can be seen from
Figure 6,
Figure 7 and
Figure 8, the areas of high slagging rates are the most extensive in the case of coal dust combustion (
Figure 6). These areas are concentrated in the lower and middle parts of the furnace in the radiation heat exchange zone. It is during the coal dust combustion that the temperatures of the gases are significantly higher than the softening temperature of the ash (1120 °C). When CWS and CWSP are burned, the well-defined areas of slagging (
Figure 7 and
Figure 8) are characterized by lower rates of this process. In addition, the zones of slag formation are localized on small areas of heating surfaces.
Table 9 provides the average growth rates of slag deposits on the furnace walls for different fuels being burned.
As can be seen from the above results, coal combustion provides the highest peak temperatures in the boiler and the most complete fuel burnout. However, in this case, the slagging rate will be maximum. The use of CWS instead of coal makes it possible to reduce the average rate of slagging of furnace surfaces by almost 12 times. However, combustion of CWS is characterized by rather low temperatures in the furnace and a low degree of burnout compared with pulverized coal, due to a significant proportion of water in the composition. To improve the energy characteristics of CWS, including the combustion temperature and completeness of burnout, additives of liquid combustible organic components are used.
As the simulation results show, in addition to an increase in peak temperatures in the furnace by 13–38%, the average rate of slag deposit growth decreases by 5% when burning CWS compared to CWSP. The latter result is explained by the difference in the component composition of these fuels. CWSP contains fewer components that form ash residue during combustion.
3.2. Heat Transfer through the Tube Wall of the Heat Exchanger When Slagging
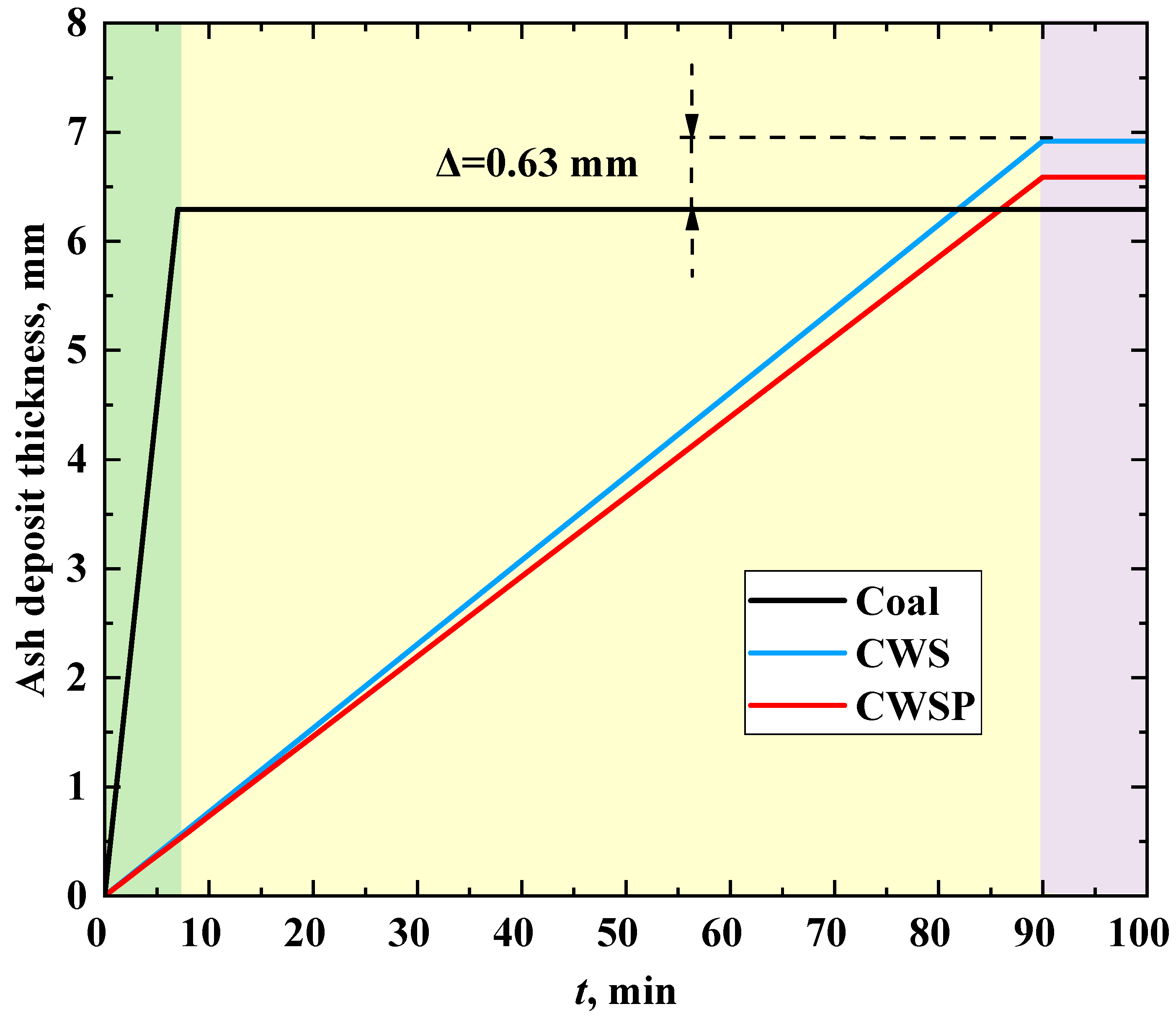
Figure 9 illustrates the change in the thickness of a slag layer on the surface of the heat exchanger tube during combustion of different fuels. When burning coal, the limiting value of the ash deposit thickness of 6.3 mm is reached in 7 min (areas in the boiler furnace subject to the most intense slagging, see
Figure 6). When CWS and CWSP are burned, the formation of an ash layer in similar areas (
Figure 7 and
Figure 8) occurs at a slower rate (a possible maximum is reached in 90 min). When burning coal, the growth rate of the slag layer thickness is 0.90 mm/min. When burning CWS and CWSP, this parameter is 0.077 mm/min and 0.072 mm/min, respectively.
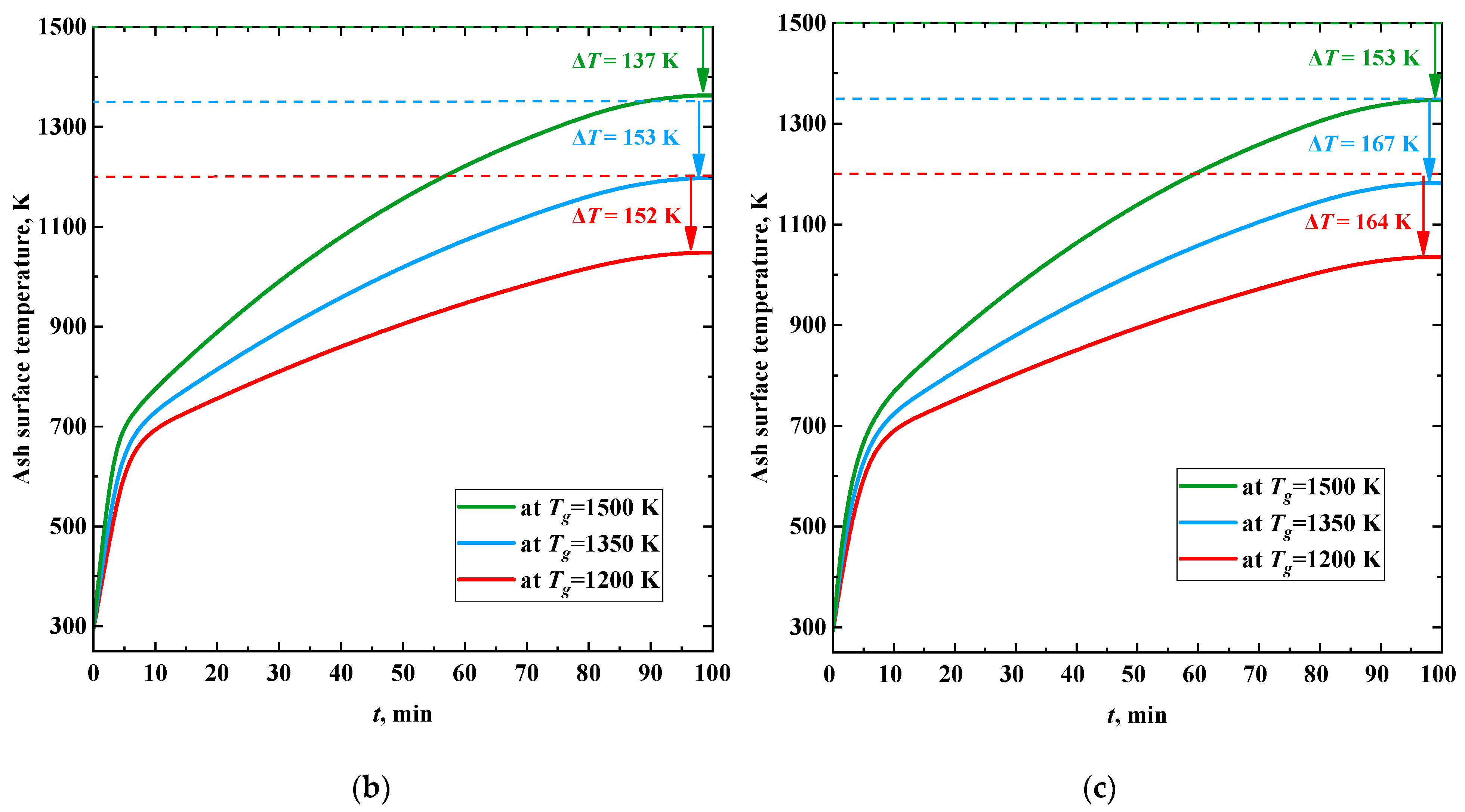
Figure 10 shows the trends in the surface temperature of the slag layer at different temperatures of the combustion products. An intense temperature increase is most typical at the stages of formation and growth of a slag layer (
Figure 10) on the heat exchanger surface. A constant surface temperature is reached after the completion of the slag layer formation. The time delay (about 6 min) between the moments of stabilization of the surface temperature and the thickness of the slag layer is because the thermal conductivity of the latter increases with an increase in the degree of ash sintering under the heating by flue gas [
59,
60].
When burning coal, the calculated rate of increase in the slag surface temperature was about 140 K/min (
Figure 10a). During the combustion of CWS and CWSP (
Figure 10b,c), the increase in the surface temperature of the ash layer was non-monotonic. At the beginning of the process, the average temperature growth rate was about 70 K/min. After 5–7 min and until the values stabilized, the average rate of temperature increase in the ash layer was about 5 K/min. In this case, the higher the temperature of the surrounding gas, the higher the intensity of heating of the ash layer surface.
After stabilization of the thickness of the ash layer, the temperature of its surface no longer reaches the temperature of the surrounding high-temperature gases. Temperature losses during slagging of heating surfaces are 13–17% when coal is burned, 10–15% when CWS is burned, and 11–16% when CWSP is burned. Thus, the maximum difference between the temperature of the gases and the surface of the ash layer can be expected during the combustion of coal dust. For slurry fuels, this difference is expressed to a lesser extent. The results obtained suggest that, in terms of the ash layer thermal diffusivity, the combustion of CWS or CWSP is more efficient than coal combustion.
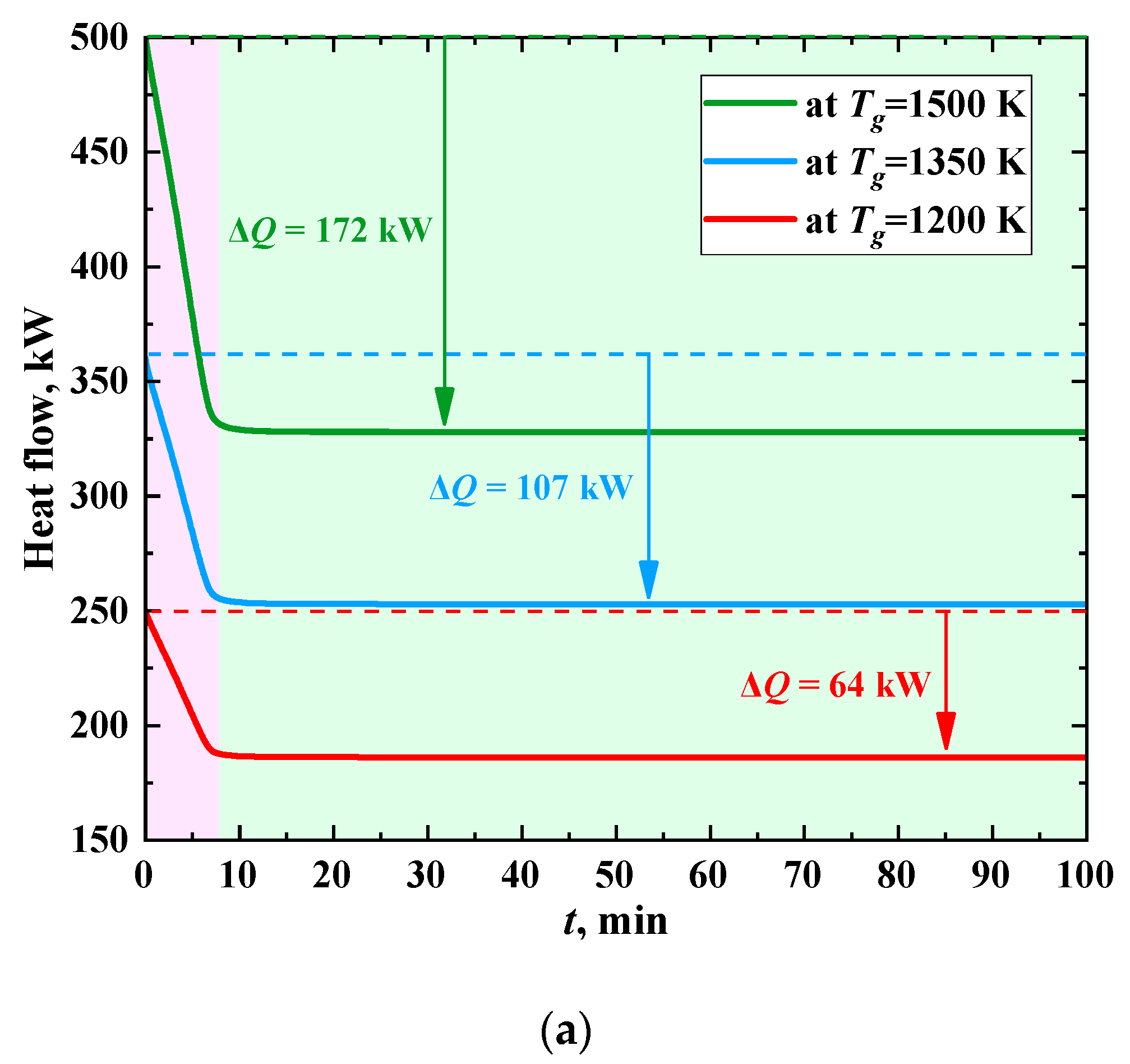
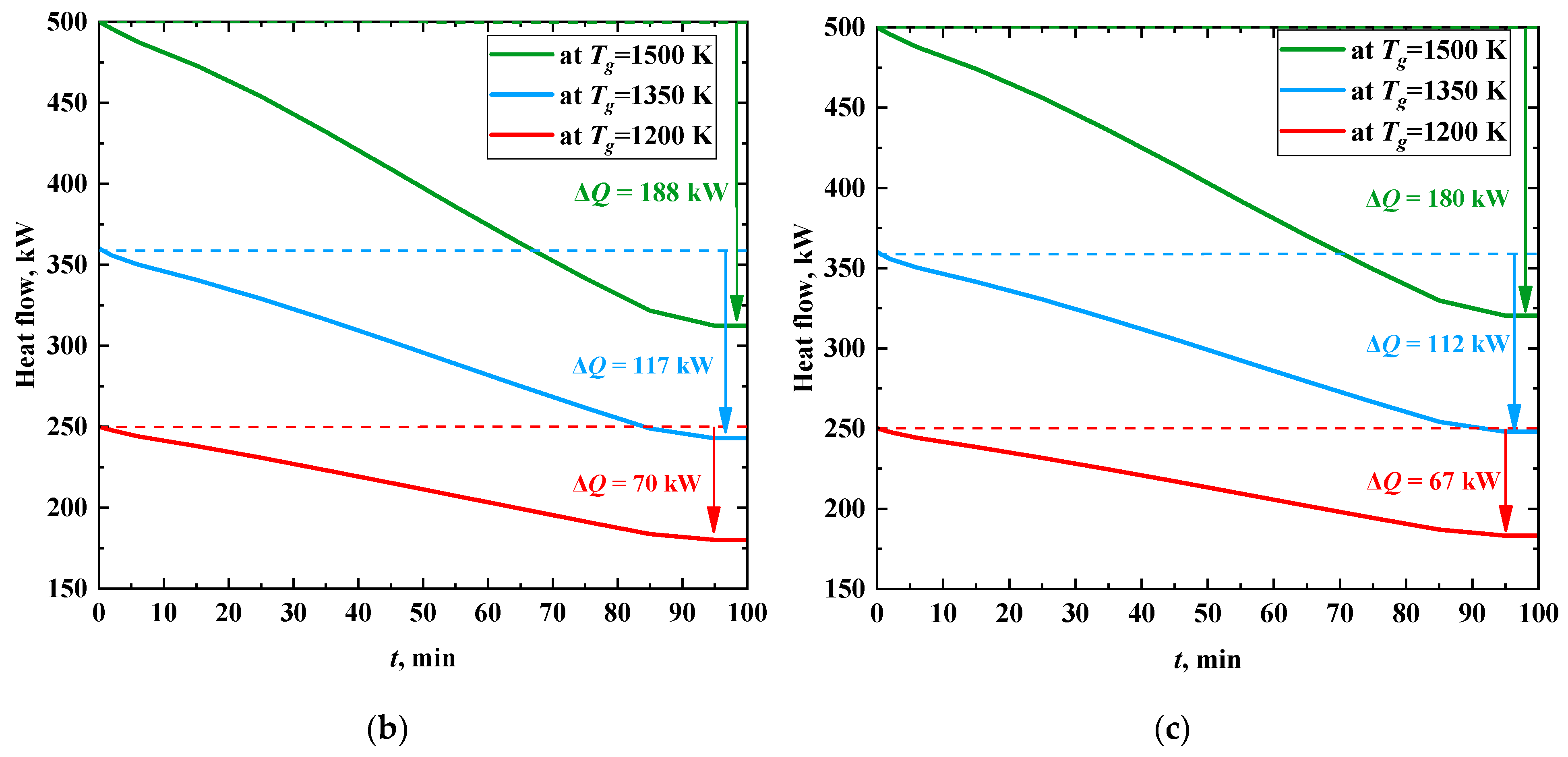
Figure 11 shows the time change of the heat flux to a heat transfer fluid when burning different fuels at several temperatures of the gases. As the thickness of the ash layer increases, the heat flux decreases significantly and stabilizes after the formation of the layer, similarly to the ash surface temperature. The heat flow is reduced due to insulation of heat exchanger tubes during their slagging by 33–50% when coal is burned, and by 39–60% and 35–55%, when CWS and CWSP are burned, respectively. The intensity of heat flux decrease is at its maximum in the first 7 min of the process. When coal dust is burned (
Figure 11a), the values of this parameter vary from 9.14 to 24.57 kW/min. Further, the value of the heat flux takes a steady value and does not change (
Figure 11a). If CWS or CWSP are burned (
Figure 11b,c), the decrease in heat flux is much slower (0.67–1.87 kW/min).
The considered composite fuels contain one common component: lignite with the ash deformation temperature of 1120 °C. However, the presence of a large proportion of moisture in lignite based CWS and CWSP leads to strong differences in the temperature field in the boiler furnace when such fuel slurries are burned, compared with dry coal. Therefore, the tendencies to slagging when replacing coal with CWS and CWSP will differ. Flame temperatures during coal combustion can reach 1800 °C, while during combustion of CWS and CWSP, the maximum temperature in the furnace did not exceed 1200 °C and 1350 °C, respectively. When burning coal dust, the zones of intense slagging are predominantly located along the line of burners, as well as above and below it. When CWS and CWSP are burned, the areas of intense slagging are many times smaller. The average growth rate of the slag layer during coal combustion is almost 12 times higher than when slurries are burned. Modeling the dynamics of the formation of ash layer and its temperature characteristics showed that when coal dust is burned, negative processes proceed at a faster rate.
When burning CWS and CWSP, an ash layer of critical thickness is formed almost 13 times slower than when dry coal dust is burned. Even though the heat flux through the heat exchanger wall is at its maximum during coal combustion, in this case it is possible to predict a greater decrease in heat transfer with an increase in slag layer thickness. The results of numerical simulation showed that the difference between the temperature of the gases and the surface of the ash layer in the case of CWS and CWSP combustion is 3–5% less than in the case of coal combustion. Thus, it can be assumed that when burning slurries, the risks associated with the effect of slagging on the efficiency and functionality of a boiler will be less than when burning coal dust. Since slurries are less harmful with respect to slagging, smaller furnaces can be used for their combustion than for coal combustion (in this case, the issue of the efficiency of burning slurry droplets in small furnaces requires a separate study). The data obtained in this work can be used to develop technologies for controlling and preventing slagging, in particular, for adjusting the temperature regime, the boiler load, the frequency of cleaning and the placement of blowers. The developed algorithm is quite universal and is a concept for a comprehensive study of the characteristics of the boiler furnace slagging at different discretization levels. To ensure the reliability of the forecast results for specific boiler furnaces and fuels, it is necessary to perform new calculations. Each such calculation is moderately time consuming and can be performed in a relatively short time on a modern PC. In the future, the presented algorithm may become the basis of a commercial product that will be applicable for solving the problems of predicting the characteristics of slagging and setting up systems for automated cleaning of heating surfaces.