Abstract
Significant racial/ethnic disparities in healthcare and diabetes technology use have been observed in Type 2 diabetes mellitus (T2DM), which are associated with nonengagement in diabetes self-management and out-of-range glycemia. This study aimed to assess whether there were differences in the blood glucose levels achieved by several racial/ethnic groups using the same digital tool. Study objectives were to determine whether engagement with the digital tool and blood glucose levels differ among ethnic groups, and to determine whether any differences in the in-target-glycemia are related to engagement levels. The retrospective real-world analysis followed a group of 1000 people with Type 2 diabetes who used the DarioTM digital therapeutic platform over 12 months. Participants included in the study had a blood glucose average > 180 mg/dL (hyperglycemia, high-risk) in their first month. The differences between/within the groups’ average blood glucose level (Avg.bg) and glycemic variability were evaluated. Furthermore, three general linear models were constructed to predict the Avg.bg by the number of blood glucose measurements (Bgm) in Model 1 (with the moderator White persons (WP)/people from racial and ethnic minority groups (REM)) and by the frequency of measurements by months (F.m) within REM and WP in Model 2 and Model 3, respectively. The Avg.bg was significantly reduced in each group over a year with no differences between REM/WP users. Blood glucose measurements in Model 1 and frequency of measurements by months in Model 2 and Model 3 predicted the Avg.bg (β1 = −0.20, p = 0.045; β2 = −4.38, p = 0.009; β3= −3.77, p < 0.001, respectively). Findings indicate a positive association between digital engagement and glycemia, with no differences between REM and WP participants.
1. Introduction
Among people living with diabetes, out-of-range glycemia is associated with long-term diabetes complications that lead to hospitalizations and a high burden of health costs, loss of productivity, disability, and mortality [1,2,3,4]. According to the Centers for Disease Control and Prevention (CDC), 37.3 million people have diabetes (11.3% of the US population) [5], and 8.25 million hospital discharges were reported with diabetes, including 226,000 cases of hyperglycemic crises [3]. Furthermore, diabetes was the seventh leading cause of death in the United States. The diabetes economic burden is also substantial, with an estimated cost of USD 327 billion in the United States’ total population, including racial and ethnic populations [6].
Despite these extreme consequences, most patients fail to achieve optimal glycemia [7,8]. Some demographic and health factors, e.g., age (mainly 60–69), level of education, weight, smoking status, and marital status, are associated with poor adherence to treatment, nonengagement in diabetes self-management, and out-of-range glycemia [9,10,11]. Furthermore, the estimated prevalence of diagnosed and undiagnosed T2DM among USA adults above 20 years of age is higher in ethnic minority groups in comparison with White persons: White 11.1%; Black 16.7%; Mexican American 17.7%; Asian 16% [12].
Being overweight is currently recognized as one of the most important variables in prediabetes and T2DM management [13,14,15], markedly with increasing BMI over 30 kg/m2 [15,16,17]. Previous studies have examined the association between objectively measured BMI and T2DM, showing that increased BMI is positively associated with T2DM [18,19].
Target glycemia is generally assessed by periodic glycated hemoglobin (A1C) and routine repeated measurements of blood glucose; target A1C is considered as 7.0% [20]. According to the American Diabetes Association (ADA) guidelines, adults with diabetes (nonpregnant) and postprandial capillary plasma glucose above 180 mg/dL are considered nontarget glycemic outcomes. Multiple risk factors are involved in the pathogenesis of T2DM with complex underlying gene–environment interactions, including age, ethnicity, family history, low socioeconomic status, obesity, metabolic syndrome, and certain unhealthy lifestyle behaviors [21,22,23,24,25,26,27,28].
Healthcare disparities associated with significant racial/ethnic groups have been observed in T2DM and obesity, including impairments in diagnosis, out-of-range glycemia, and development of diabetes-related complications due to low socioeconomic status, living in rural communities, reduced access to care, and a lack of health education [12,24,29,30,31]. According to the National Diabetes Report 2020 in the United States, the incidence of diagnosed diabetes for 2017–2018 among people aged 18 years or older was higher among people from racial and ethnic minority (REM) populations than White people (WP) populations [32]. In addition, REM (e.g., Black, Latino, and Asian) populations significantly present with cardiovascular and cerebrovascular diseases [33], nephropathy, and lower extremity amputations compared to WP populations [34,35]. Lower rates of health insurance, delivery of medical care, and social policies with limited access to healthy foods and places for physical activity are some of the possible reasons that REM populations do not achieve glycemic goals [36,37,38].
Some social and cultural healthcare disparities might be mitigated through digital therapeutics approaches [36]. Digital health implementations have grown markedly and are now frequently used in health education promotion, disease management, and surveillance of many chronic diseases [39,40]. The rapid adoption of mobile devices and Internet services has also reached rural communities and people at increased/higher risk for a chronic condition [41,42,43,44,45]. Digital tools can also help to facilitate automation and health service delivery [46]. Furthermore, wireless and monitoring devices are increasingly used in healthcare and public health practice for communication, data collection, patient monitoring, and education to enhance adherence to chronic disease management. Sensors and context-awareness features allow real-time information collection [44]. Digital platforms using motivating messages, prompts/push notifications, coaching, and/or skills training for people have demonstrated increased engagement in treatment and self-management. Interventions delivered with these technologies can also accommodate easy and simple access to information and encourage users’ participation and literacy levels [47,48].
According to previous reviews, digitally delivered interventions may enhance the self-management and clinical outcomes of chronic diseases [36,45,49,50,51,52]. Currently, digital approaches are used by subgroups with historically impaired outcomes, but are limited by access to certain types of devices or the Internet [36,49,52]. Most digital health solutions are focused on a specific disease, such as diabetes or obesity, and fewer provide comprehensive multiple conditions services and tools [36,52]. Furthermore, limited data support the health benefits of digital tools among varied racial/ethnic groups, and even fewer data support the value for high-risk T2DM patients in such marginalized populations [53,54].
This study leverages a retrospective analysis of a home-use diabetes digital platform with full data capture in a supporting mobile app among T2DM patients with out-of-target blood glucose levels across ethnic/racial groups. The current study aimed to assess whether there were differences in blood glucose levels among high-risk T2DM users using the same digital tool from varied racial/ethnic groups (WP, Latino, Black, or Asian) over a year. Moreover, the study’s objectives were to compare clinical outcomes between racial/ethnic groups and to investigate the association between the engagement level (blood glucose measurements) with platform and clinical outcomes in both groups. The retrospective study was conducted based on real-world data. We hypothesized that the use of digital platforms over a year would be associated with reduced blood glucose levels through increased self-management among racial/ethnic groups. By analyzing average blood glucose differences over a year among the varied races/ethnicities and between BMI groups, we expected to show that different racial/ethnic groups equal in their baseline using the digital health platform will experience comparable improvement. Moreover, we speculated that user engagement with the platform would be linked with improvement in monthly average blood glucose levels across ethnic/racial groups.
2. Materials and Methods
2.1. Platform
The DarioTM digital therapeutic platform used in this study contains solutions for chronic conditions: diabetes, hypertension, and weight management. The digital platform combines a glucometer with a smartphone app available for both Android and iPhone. The glucometer contains a small pocket-sized holder for strips, a lancing mechanism, and a meter. The meter is removed from the holder and plugged directly into a cell phone, effectively converting the cell phone into the display screen for the meter (the meter itself has no screen). Connecting the meter directly to the phone enables data capture during glucose readings saved in the Dario database. The system facilitates 100% data capture and synchronization to the Dario cloud. The analysis is performed on existing data. In addition, it allows the user to open the mobile app with each glucose measurement and encourages the easy acquisition of data in the context of real-time measurement. Measuring blood glucose and using the Dario App features does not require Internet access. During registration, users reported race/ethnicity in the app as follows: White, Latino, Black, and Asian. In addition, users reported height and weight; from this, BMI was calculated and categorized into groups: <25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2 [55]. The data were extracted from a digital health therapeutics solution for chronic diseases that supports self-management of diabetes with the use of a glucometer and mobile app. All users’ data were transferred and stored in compliance with Health Insurance Portability and Accountability Act (HIPAA) requirements, then were anonymized before extraction for this study, and used for the analysis.
2.2. Study Population and Design
A retrospective data study was performed on the DarioTM database on individuals who used the Dario platform between 2019–2021. The users purchased the device via a direct-to-consumer channel. The SRP (suggested retail price) cost is USD 39, and the app itself is free. The inclusion criteria were as follows: users reported T2DM with at least three blood glucose measurements in their first month (T1) and in the 12th month after registration (T12) inclusive, with first-month blood glucose average >180 mg/dL (hyperglycemia, high-risk) [21,22,23,25,56]. The users reported race/ethnicity in the app: White, Latino, Black, or Asian. Ethical & Independent Review Services issued the IRB exemption for this study (18032-01#, March 2018) [57].
2.3. Measures
The average blood glucose levels (Avg.bg) were defined as the mean of the user’s blood glucose measurements that were taken over a 30-day period at the first month (T1) and at the 12th month (T12). A change from T1 to T12 was a core outcome metric.
We were interested to examine if engagement levels (number of measurement and the frequency of measurements) are predictors of reduction of blood glucose average. We designated these two measurements as independent variables. The first was the number of measurements (Bgm) at T1 and at T12. The second was the frequency of measurements by months (F.m), which is the number of months in which the user took at least one measurement.
Dichotomic ethnicity was defined as follows: WP for the user that reported “White” in the app; REM for the users that reported in the app “Latino”, “Black”, or “Asian”.
2.4. Statistical Analyses
Chi-square rank (α = 1%) tests were performed to establish the similarity of demographic data between the WP and REM users. Differences were considered significant at the p < 0.01 level after Bonferroni corrections. Bonferroni correction was used here as a method to counteract the multiple comparisons problem. Incidences are presented as numbers and percentages of patients. Kruskal–Wallis statistical tests were used to evaluate the differences between the Avg.bg between the racial/ethnic groups at T1 and at T12 as follows: between the WP/REM users and between the racial/ethnic groups: WP, Latino, Black, and Asian; as well as between WP/REM users among the BMI groups. Furthermore, paired Wilcoxon statistical rank tests were used to evaluate the changes in the Avg.bg within each group over the year (from T1 to T12). In addition, Kruskal–Wallis statistical tests were used to evaluate the differences between glucose variabilities by the average standard deviation (STD) within the racial/ethnic groups WP/REM at T1 and at T12, representing how much glucose levels fluctuate over time from a given average. Paired T-test was performed in order to evaluate the significance of reduction in average standard deviation from T1 to T12 for both groups.
Three models of the general linear model (GLM) were conducted to evaluate the effect of the independent variables Bgm and the F.m on the dependent variable Avg.bg. The first model (Model 1) was executed on all users, with Bgm as the predictor, with dichotomic ethnicity as a possible moderator. The second and the third models (Model 2 and Model 3, respectively) were performed on the WP and the REM groups separately with F.m as the predictor to test if consistency of measurement for a year predicts blood glucose levels reduction in each group of WP and REM. The typical estimation method was performed with the least squares approximation method. The statistical analysis was conducted with R studio version 4.1.2.
The Independent variables that were included in the GLM models are as follows:
Model 1:
Model 2 and Model 3:
3. Results
The analysis included 1000 users out of a larger group of users with T2DM who used the Dario platform between 2019 and 2021 and measured their blood glucose with at least three blood glucose measurements in the first and 12th months. The group analyzed started from baseline average blood glucose >180 mg/dL and reported their race/ethnicity; ninety-seven percent of the users were located in the United States. Demographic characteristics did not differ between the WP and REM users for sex, major age group 45–65 (WP 59% vs. REM 62%), BMI groups, and insulin treatment intake (Table 1).

Table 1.
Demographic characteristics of the users. WP, White persons; REM, people from racial and ethnic minority groups; BMI, body mass index; n, number of users; (%) percentages are rounded off and are without decimal points.
3.1. Testing Differences in the Monthly Average Blood Glucose Levels throughout the Initial 12 Months for Different Races/Ethnicities
No difference between WP and REM users in the average blood glucose at baseline was observed (Kruskal–Wallis test, p = 0.689). The blood glucose average was significantly reduced in all users and per racial/ethnic group over a year (Figure 1). All users reduced blood glucose by 14% (230 ± 58 vs. 197 ± 47) (p < 0.001); WP by 14% (229 ± 58 vs. 197 ± 47) (p < 0.001); REM by 15% (233.35 ± 60 vs. 198.94 ± 47) (p < 0.001) with the following reduction within each REM racial/ethnic group: Latino by 15% (237 ± 59 vs. 202 ± 48) (p < 0.001); Black by 15% (230 ± 63 vs. 196 ± 48) (p < 0.001), and Asian by 15% (229 ± 55 vs. 195 ± 43) (p < 0.005). The findings show that there were no differences in the glycemic outcomes achieved by several racial/ethnic groups using the same digital tool. Moreover, 27.3% (273 out of 1000) of all users reached an average blood glucose of less than and equal to 154 mg/dL equivalent to A1C 7.0% (Avg.bg mg/dL = 28.7 × A1C − 46.7 by Nathan et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2742903/ (accessed on 13 December 2022). No differences in blood glucose averages between WP and REM were found at T12 (Kruskal–Wallis test, p = 0.668) and no differences in monthly blood glucose averages among WP, Latino, Black, and Asian groups were found at T1 and T12 (Kruskal–Wallis test, p = 0.165/0.751, respectively). In addition, significant reductions in monthly average blood glucose were found in WP users and REM users in three categories of BMI levels (Figure 2). Furthermore, no differences between the dichotomic ethnicity were found at T1 and T12 within each BMI category (Kruskal–Wallis test, p = 0.994/0.988, respectively).
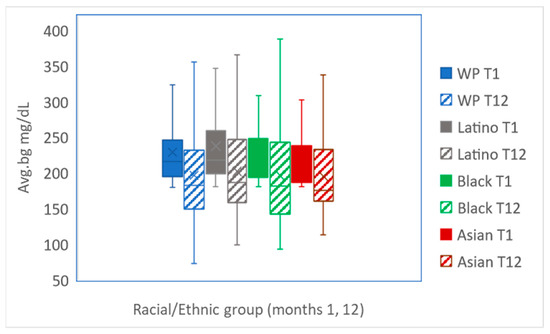
Figure 1.
Average blood glucose levels (mg/dL) presentation by ethnic/racial groups. Avg.bg, the average blood glucose levels in T1 and T12 are presented per group: WP (p < 0.001), Latino (p < 0.001), Black (p < 0.001), and Asian (p < 0.005).
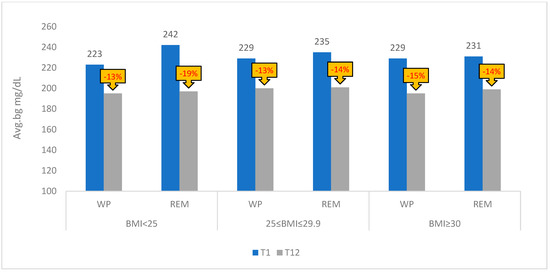
Figure 2.
Average blood glucose levels (mg/dL) presentation by the WP and REM groups and BMI levels. WP, White persons; REM, people from racial and ethnic minority groups; Avg.bg, the average blood glucose levels; races/ethnicities as WP and REM (Latino, Black, and Asian); BMI groups <25 kg/m2; 25–29.9 kg/m2; ≥30 kg/m2; The change in average blood glucose from T1 to T12 is presented as a percentage.
There were no significant differences between REM (10.42 ± 2.46) vs. WP users (10.6 ± 2.31) in F.m averages over the year (Kruskal–Wallis test, p = 0.14). In addition, the distribution of the F.m among REM and WP is similar (Table 2).

Table 2.
Frequency of measurements by month (F.m) presented for White persons (WP) and people from racial and ethnic minority groups (REM); a, chi-square test; b, Kruskal–Wallis test rank. n is the number of users; Avg is the weighted average of the months weighted by the number of users.
3.2. Association between Engagement and Average Blood Glucose Levels
In addition, Kruskal–Wallis statistical tests findings showed no statistically significant difference between glucose variabilities examined by the distribution of the avg STD change (T1 compared to T12) between WP and REM groups (p = 0.40). A paired test for WP and REM users for the average STD in T1 vs. average STD in T12 demonstrated significant reduction over a year (p < 0.001).
Three models of GLM were used to predict the Avg.bg (Table 3) with the coefficients Bgm and F.m. In Model 1, the results presented that Bgm was a significant negative predictor (β1 = −0.20, p = 0.045) or when Bgm was higher (the users performed more blood glucose measurements), the Avg.bg was reduced at T1 and T12 with no interactions between the predictors with dichotomic ethnicity in Model 1. The coefficient F.m, or frequency of glucose measurements, was also a significant negative predictor in Model 2 and Model 3 (β2 = −4.38, p = 0.009; β3 = −3.77, p < 0.001), indicating a positive correlation between engagement and average blood glucose, or, a higher number of active months on the platform is negatively associated with lower average blood glucose level among REM and WP users.

Table 3.
General linear model results. Avg.bg presents the predictable variable—the average blood glucose levels; Bgm presents the number of measurements; WP, White persons; REM presents people from racial and ethnic minority groups; dichotomic ethnicity presents WP/REM. F.m presents frequency of measurements by month.
Model 1:
Model 2:
Model 3:
4. Discussion
4.1. Principle Results
The study results demonstrated that among high-risk T2DM patients who use Dario technology, blood glucose levels (average blood glucose and glucose variability) were improved in different racial/ethnic groups: WP and REM (Latinos, Blacks, and Asians) (Figure 1) and in different BMI groups based on the statistical tests applied (Kruskal–Wallis) (Figure 2). In addition, there were no differences in blood glucose levels between the groups over a year of app usage. The GLM models showed that the frequency of measurements by month (number of months per year (F.m)) and the number of measurements (Bgm) at months 1 and 12 are predictors of blood glucose levels. Our findings strengthen the observation that a higher level of user engagement, reflected by a monthly number of measurements and active months, is associated with a reduction in blood glucose levels in the tested timeframe. Moreover, the improvement in average blood glucose, as examined in Model 1, was not different for separate racial/ethnic groups (WP/REM), consistent with the statistical tests applied (Kruskal–Wallis and paired Wilcoxon). The significant effect of the engagement variables (Bgm and F.m) among WP and REM populations indicated that using the digital therapeutic platform has the potential to enhance diabetes management, contributing to their improved glycemia over a full year.
Multiple factors may contribute to healthcare disparities, such as immigrant status, living in rural regions, socioeconomic status, numeracy, educational level, health literacy, and access to health insurance [58,59,60]. Furthermore, environmental changes, food, and physical insecurity may influence the risk of obesity and diabetes as they are associated with genetics and epigenetic differences between varied races/ethnicities [61,62]. Healthcare providers’ bias may also impair health outcomes amongst racial/ethnic minority populations; such biases may take the form of disparities in treatment recommendations and expectations of treatment adherence [63].
In contrast to traditionally observed racial/ethnic disparities in diabetes treatment participation and outcomes, our study demonstrated the potential of digital therapeutic interventions to support and assist in diabetes management.
Previous studies supporting the current results examined the impact of telemedicine tools, such as Internet-based intervention, on diabetes outcomes. The population participating in these studies were high-risk, low-income, and patients from ethnic minority groups, and showed significant reductions in A1C [36,64,65,66]. Adjustments in usability and availability through a digital platform can engage at-risk populations and may lead to improved glycemic levels. Measuring outcomes among racial/ethnic groups is important to monitor in order to respond to long-standing disparities.
Earlier systematic reviews identified numerous studies evaluating the impact of diabetes self-management interventions delivered via a mobile device and/or Internet on in-target glycemia for adult populations at high risk with T2DM. Most studies reported that A1C improved within the intervention group versus usual care, emphasizing digital health preference above usual care [36]. The current results provide evidence that digital health among varied race/ethnicity groups assists in engaging users and improving their self-management associated with in-target glycemia for a year’s duration.
There is a lack of studies examining the impact of digital health interventions on chronic conditions and disease among populations at high risk, specifically among different racial/ethnic groups of people, with T2DM. Most studies examined the impact of digital health on chronic conditions among vulnerable heterogeneous populations with general and broad definitions, e.g., low-income patients, low level of education, and mixed vulnerable classification. This study utilized real-world data logged into the app by users to stratify the high-risk population purely by race/ethnicity. It revealed no significant differences in their glycemic level over a meaningful period of 12 months. A WP population served here as a type of “control” for the different races/ethnicities assessed, while many previous studies are lacking in their comparison to a control group [36,46,53].
Digital therapeutic platform technology enables the recognition and adjustment of specific required needs (e.g., cultural, linguistic, educational, and more). Our findings provided evidence that a digital tool is equally useful among the varied racial/ethnic users studied. The usability and availability of personalized interventions enable the achievement of healthcare engagement over time. Previous real-world studies presented an association between digital engagement and clinical outcomes improvement among T2DM users [67]. The current study reveals the association between app engagement and clinical outcomes in types of populations known as “challenging”. The study findings showed a positive correlation between engagement and average blood glucose among REM and WP users. Findings also shed light on the mechanism of self-monitoring with a digital therapeutic platform and may assist in developing a better therapeutic approach targeting REM populations with T2DM. The nondifference between improved in-target glycemia may be explained by the ability of digital therapeutics platforms to personalize the solutions according to the user’s needs. The digital platform collects users’ information via the screening process, tracks users’ behaviors and glycemic levels, can personalize language communication, offer relevant educational content, and can provide online consultation with a coach. In addition, the usability and availability of the home glucometer with online feedback on glucose measurements encourage the user to keep tracking these measurements, which may increase healthcare engagement. The availability of important tracking tools with specific adjustments for varied populations’ needs is a gold key in promoting in-target glycemia.
4.2. Limitations
Several limitations are noted in the study. Racial and ethnic categories were limited to the choices available in the application. There was no designed control group in this study comparing digital to traditional self-management strategies. However, we used a comparable group from our real-world data (REM vs. WP). Both groups were characterized by no substantial difference in demographics. As in all studies involving retrospective real-world data, groups were not randomly assigned and treatment protocols were not prescribed. Both factors create challenges for indicating causal effects. It is certainly possible that users who chose to purchase the device and to be engaged were the most motivated to change. However, the users in all groups analyzed in the study were persons with T2DM with hyperglycemia levels (all the users started with an average blood glucose of above 180 mg/dL in the first month of activity since registration). That may indicate that these users were not highly engaged in their treatment prior to joining DarioTM despite their complex condition. An implication of racial and ethnic groups that were coded in the application may not capture results from groups that are not included in the app, and it is possible that people who chose to report their race/ethnicity were those who were the most motivated to change. Our study was designed to ensure that both groups analyzed showed evidence of being motivated about their diabetes care. Additionally, self-reported data are vulnerable to response bias. Specifically, users’ racial/ethnic identities were collected via self-report, which is subject to response bias.
In contrast to a substantial portion of health studies that require follow-up assessment from the experimenter party, these assessments were performed exclusively remotely. This reduced the study bias and enhanced the advantages of real-world data.
Another limitation regarding the study array is assessing the average blood glucose levels only at two time points (1st month and 12th month). Following frequent fluctuations in blood glucose levels is important in diabetes management. We used a longitudinal collection of data of blood glucose measurements and statistical analysis (GLM) to allow robust estimation of the digital health effect over the evaluation period. Additionally, we applied in our models the frequency of measurements by month (the coefficient; F.m) represented in the month’s duration engagement of the users. Blood glucose measurements and frequency of measurements across ethnicities were examined as predicting variables for reduction in blood glucose levels over time, with the limitation that there may be other predicting variables contributing to reduction in blood glucose, such as users’ diet and eating habits or other unknown or unreported medical or mental conditions. However, these variables were found based on the model as significant predictors. Finally, we observed BMI outcomes with the limitation of absent information regarding users’ eating behaviors or exercise regimes. However, the purpose of observing BMI outcomes is to add a related biochemical marker to blood glucose levels and to demonstrate a broader range of metabolic variables. In addition, the country of residence for the participants of this study was skewed towards the USA; therefore, it is not clear if the results are equally applicable to less economically developed countries. Hopefully, our results will encourage further research among less economically developed and more racially/ethnically diverse populations outside of the USA.
5. Conclusions
Our findings demonstrate improvement in blood glucose levels in high-risk racial/ethnic minority populations with T2DM, showing that in this group of users who are motivated to use a digital device there appears to be no difference in the outcomes between racial/ethnic groups. In particular, the study emphasizes the capabilities of digital approaches to deliver digital health services to populations at risk and to personalize the services according to their unique needs. The study highlights the necessity for future research to focus on disparities in various aspects of living with diabetes and to investigate actionable digital strategies that promote in-range glycemia, reduce hyperglycemia, and encourage diabetes self-management engagement by comparing results for diabetes care, technology use, and outcomes among different populations along expanded periods of time. Moreover, the study also sheds light on the association between engagement factors and glycemic outcomes in underserved populations, highlighting the importance of future research exploring how habits change over time and the unique needs of varied diverse populations.
Author Contributions
Methodology, M.D.R., D.L.H. and Y.F.-H.; Formal analysis, T.G.; Investigation, T.G. and Y.F.-H.; Writing—original draft, T.G.; Writing—review & editing, M.D.R., D.L.H., O.M., T.D.-P. and Y.F.-H.; Supervision, Y.F.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Institutional Review Board (or Ethics Committee) of Ethical and Independent Review Services (18032-01#, March 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available.
Conflicts of Interest
T.G., Y.F.H., and O.M. are employees of DarioHealth. M.R. and D.H. serve as DarioHealth scientific advisory board members, and T.P. is an external consultant who has no conflict of interest.
References
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Coexisting Conditions and Complications|Diabetes|CDC. (January 19 2022). Available online: https://www.cdc.gov/diabetes/data/statistics-report/coexisting-conditions-complications.html (accessed on 7 July 2022).
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Statistics Report|Diabetes|CDC. (2022, January 20). Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 27 July 2022).
- The Cost of Diabetes|ADA. (n.d.). Available online: https://diabetes.org/about-us/statistics/cost-diabetest (accessed on 22 August 2022).
- Alzaheb, R.A.; Altemani, A.H. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhu, D.; Cheng, J.; Zhu, Y.; Xu, N.; Cui, S.; Li, W.; Cheng, X.; Wang, F.; Hu, Y.; et al. The status of glycemic control: A cross-sectional study of outpatients with type 2 diabetes mellitus across primary, secondary, and tertiary hospitals in the jiangsu province of China. Clin. Ther. 2010, 32, 973–983. [Google Scholar] [CrossRef]
- Davies, M. The reality of glycaemic control in insulin treated diabetes: Defining the clinical challenges. Int. J. Obes. 2004, 28, S14–S22. [Google Scholar] [CrossRef]
- Kassahun, T.; Eshetie, T.; Gesesew, H. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: A cross-sectional survey in Ethiopia. BMC Res. Notes 2016, 9, 78. [Google Scholar] [CrossRef]
- Moreira, E.D.; Neves, R.C.S.; Nunes, Z.O.; de Almeida, M.C.C.; Mendes, A.B.V.; Fittipaldi, J.A.S.; Ablan, F. Glycemic control and its correlates in patients with diabetes in Venezuela: Results from a nationwide survey. Diabetes Res. Clin. Pract. 2010, 87, 407–414. [Google Scholar] [CrossRef]
- Thornton, P.L.; Kumanyika, S.K.; Gregg, E.W.; Araneta, M.R.; Baskin, M.L.; Chin, M.H.; Crespo, C.J.; de Groot, M.; Garcia, D.O.; Haire-Joshu, D.; et al. New research directions on disparities in obesity and type 2 diabetes. Ann. N. Y. Acad. Sci. 2019, 1461, 5–24. [Google Scholar] [CrossRef]
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef]
- Astrup, A.; Finer, N. Redefining Type 2 diabetes: ‘Diabesity’ or ‘Obesity Dependent Diabetes Mellitus’? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S113–S124. [Google Scholar] [CrossRef]
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, Fat Distribution, and Weight Gain as Risk Factors for Clinical Diabetes in Men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef]
- Gray, N.; Picone, G.; Sloan, F.; Yashkin, A. Relation between BMI and Diabetes Mellitus and Its Complications among US Older Adults. South. Med. J. 2015, 108, 29–36. [Google Scholar] [CrossRef]
- Nyamdorj, R.; Pitkäniemi, J.; Tuomilehto, J.; Hammar, N.; Stehouwer, C.D.A.; Lam, T.H.; Ramachandran, A.; Janus, E.D.; Mohan, V.; For the DECODA and DECODE Study Groups; et al. Ethnic comparison of the association of undiagnosed diabetes with obesity. Int. J. Obes. 2009, 34, 332–339. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Walker, M. Overweight and obesity and weight change in middle aged men: Impact on cardiovascular disease and diabetes. J. Epidemiol. Community Health 2005, 59, 134–139. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S46–S59. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S83–S96. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 13. Older Adults: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S195–S207. [Google Scholar] [CrossRef]
- Becker, C.D.; Sabang, R.L.; Cordeiro, M.F.N.; Hassan, I.F.; Goldberg, M.D.; Scurlock, C.S. Hyperglycemia in Medically Critically Ill Patients: Risk Factors and Clinical Outcomes. Am. J. Med. 2020, 133, e568–e574. [Google Scholar] [CrossRef]
- Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Fradkin, J.E.; Gregg, E.W.; Knowled, W.C.; Barrett-Connor, E.; et al. (Eds.) Diabetes in America, 3rd ed.; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. Available online: http://www.ncbi.nlm.nih.gov/books/NBK567985/ (accessed on 25 July 2022).
- Dhatariya, K.; Corsino, L.; Umpierrez, G.E. Management of Diabetes and Hyperglycemia in Hospitalized Patients. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Wilson, D.P., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK279093/ (accessed on 15 December 2022).
- Kyrou, I.; Tsigos, C.; Mavrogianni, C.; Cardon, G.; Van Stappen, V.; Latomme, J.; Kivelä, J.; Wikström, K.; Tsochev, K.; Nanasi, A.; et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: A narrative review with emphasis on data from Europe. BMC Endocr. Disord. 2020, 20 (Suppl. S1), 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Kanaya, A.M.; Araneta, M.R.G.; Saydah, S.H.; Kahn, H.S.; Gregg, E.W.; Fujimoto, W.Y.; Imperatore, G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019, 322, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Haw, J.S.; Shah, M.; Turbow, S.; Egeolu, M.; Umpierrez, G. Diabetes Complications in Racial and Ethnic Minority Populations in the USA. Curr. Diabetes Rep. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Walker, R.J.; Strom Williams, J.; Egede, L.E. Influence of Race, Ethnicity and Social Determinants of Health on Diabetes Outcomes. Am. J. Med. Sci. 2016, 351, 366–373. [Google Scholar] [CrossRef]
- CDC. National Diabetes Statistics Report 2020. Estimates of Diabetes and Its Burden in the United States; U.S. Department of Health and Human Services: Washington, DC, USA, 2020; 32p. [Google Scholar]
- Karter, A.J.; Laiteerapong, N.; Chin, M.H.; Moffet, H.H.; Parker, M.M.; Sudore, R.; Adams, A.S.; Schillinger, D.; Adler, N.S.; Whitmer, R.A.; et al. Ethnic Differences in Geriatric Conditions and Diabetes Complications Among Older, Insured Adults with Diabetes. J. Aging Health 2015, 27, 894–918. [Google Scholar] [CrossRef]
- Karter, A.J.; Ferrara, A.; Liu, J.Y.; Moffet, H.H.; Ackerson, L.M.; Selby, J.V. Ethnic Disparities in Diabetic Complications in an Insured Population. JAMA 2002, 287, 2519–2527. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Fradkin, J.E.; Saydah, S.H.; Rust, K.F.; Cowie, C.C. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People with Diabetes, 1988–2010. Diabetes Care 2013, 36, 2271–2279. [Google Scholar] [CrossRef]
- Mayberry, L.S.; Lyles, C.R.; Oldenburg, B.; Osborn, C.Y.; Parks, M.; Peek, M.E. mHealth Interventions for Disadvantaged and Vulnerable People with Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Mortensen, K.; Chen, J. The Great Recession and Racial and Ethnic Disparities in Health Services Use. JAMA Intern. Med. 2013, 173, 315–317. [Google Scholar] [CrossRef][Green Version]
- Nwasuruba, C.; Khan, M.; Egede, L.E. Racial/Ethnic Differences in Multiple Self-Care Behaviors in Adults with Diabetes. J. Gen. Intern. Med. 2007, 22, 115–120. [Google Scholar] [CrossRef]
- Insideout. (n.d.). Telehealth Basics. ATA. Available online: https://www.americantelemed.org/resource/why-telemedicine/ (accessed on 5 July 2022).
- Auerbach, A.D. Evaluating Digital Health Tools—Prospective, Experimental, and Real World. JAMA Intern. Med. 2019, 179, 840–841. [Google Scholar] [CrossRef]
- Smartphone Penetration Worldwide. (n.d.). Statista. Available online: https://www.statista.com/statistics/203734/global-smartphone-penetration-per-capita-since-2005/ (accessed on 4 July 2022).
- Mobile Phone Access Reaches Three Quarters of Planet’s Population. (n.d.). World Bank. Text/HTML. Available online: https://www.worldbank.org/en/news/press-release/2012/07/17/mobile-phone-access-reaches-three-quarters-planets-population (accessed on 4 July 2022).
- Tomlinson, M.; Rotheram-Borus, M.J.; Swartz, L.; Tsai, A.C. Scaling Up mHealth: Where Is the Evidence? PLoS Med. 2013, 10, e1001382. [Google Scholar] [CrossRef]
- Free, C.; Phillips, G.; Watson, L.; Galli, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The Effectiveness of Mobile-Health Technologies to Improve Health Care Service Delivery Processes: A Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001363. [Google Scholar] [CrossRef]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth Chronic Disease Management on Treatment Adherence and Patient Outcomes: A Systematic Review. J. Med. Internet Res. 2015, 17, e52. [Google Scholar] [CrossRef]
- Peiris, D.; Praveen, D.; Johnson, C.; Mogulluru, K. Use of mHealth Systems and Tools for Non-Communicable Diseases in Low- and Middle-Income Countries: A Systematic Review. J. Cardiovasc. Transl. Res. 2014, 7, 677–691. [Google Scholar] [CrossRef]
- Lyles, C.; Schillinger, D.; Sarkar, U. Connecting the Dots: Health Information Technology Expansion and Health Disparities. PLoS Med. 2015, 12, e1001852. [Google Scholar] [CrossRef]
- PatientEngagementHIT. (2021, March 10). Is the Digital Divide the Newest Social Determinant of Health? PatientEngagementHIT. Available online: https://patientengagementhit.com/news/is-the-digital-divide-the-newest-social-determinant-of-health (accessed on 23 August 2022).
- Riazi, H.; Larijani, B.; Langarizadeh, M.; Shahmoradi, L. Managing diabetes mellitus using information technology: A systematic review. J. Diabetes Metab. Disord. 2015, 14, 49. [Google Scholar] [CrossRef]
- Cole-Lewis, H.; Kershaw, T. Text Messaging as a Tool for Behavior Change in Disease Prevention and Management. Epidemiologic Rev. 2010, 32, 56–69. [Google Scholar] [CrossRef]
- Saffari, M.; Ghanizadeh, G.; Koenig, H.G. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2014, 8, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, J.; Tummers, L.; Bosma, N. Adherence to Electronic Health Tools Among Vulnerable Groups: Systematic Literature Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e11613. [Google Scholar] [CrossRef] [PubMed]
- Reiners, F.; Sturm, J.; Bouw, L.J.; Wouters, E.J. Sociodemographic Factors Influencing the Use of eHealth in People with Chronic Diseases. Int. J. Environ. Res. Public Health 2019, 16, 645. [Google Scholar] [CrossRef] [PubMed]
- Enyioha, C.; Hall, M.; Voisin, C.; Jonas, D. Effectiveness of Mobile Phone and Web-Based Interventions for Diabetes and Obesity Among African American and Hispanic Adults in the United States: Systematic Review. JMIR Public Health Surveill. 2022, 8, e25890. [Google Scholar] [CrossRef] [PubMed]
- CDC. (2022, June 3). All about Adult BMI. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html (accessed on 23 June 2022).
- Yatabe, T.; Inoue, S.; Sakaguchi, M.; Egi, M. The optimal target for acute glycemic control in critically ill patients: A network meta-analysis. Intensiv. Care Med. 2017, 43, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ethical and Independent Review Services. (n.d.). Available online: https://www.eandireview.com/ (accessed on 26 June 2022).
- Nelson, L.A.; Ackerman, M.T.; Greevy, R.A.; Wallston, K.A.; Mayberry, L.S. Beyond Race Disparities: Accounting for Socioeconomic Status in Diabetes Self-Care. Am. J. Prev. Med. 2019, 57, 111–116. [Google Scholar] [CrossRef]
- Lee, W.; Lloyd, J.T.; Giuriceo, K.; Day, T.; Shrank, W.; Rajkumar, R. Systematic review and meta-analysis of patient race/ethnicity, socioeconomics, and quality for adult type 2 diabetes. Health Serv. Res. 2020, 55, 741–772. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Kao, W.L.; Clark, J.M.; Brancati, F.L.; Cheng, C.-Y.; Pankow, J.S.; Selvin, E. Does Genetic Ancestry Explain Higher Values of Glycated Hemoglobin in African Americans? Diabetes 2011, 60, 2434–2438. [Google Scholar] [CrossRef]
- Agardh, E.; Allebeck, P.; Hallqvist, J.; Moradi, T.; Sidorchuk, A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. Leuk. Res. 2011, 40, 804–818. [Google Scholar] [CrossRef]
- Selvin, E. Are There Clinical Implications of Racial Differences in HbA1c? A Difference, to Be a Difference, Must Make a Difference. Diabetes Care 2016, 39, 1462–1467. [Google Scholar] [CrossRef]
- Golden, S.H.; Joseph, J.J.; Hill-Briggs, F. Casting a Health Equity Lens on Endocrinology and Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e1909–e1916. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.G.; Schwartz, R.; Jennings, T.; Fedders, M.; Vittoria, I. Feasibility of an Internet-Based Intervention for Improving Diabetes Outcomes Among Low-Income Patients with a High Risk for Poor Diabetes Outcomes Followed in a Community Clinic. Diabetes Educ. 2013, 39, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Weinstock, R.S.; Teresi, J.A.; Palmas, W.; Starren, J.; Cimino, J.; Lai, A.; Field, L.; Morin, P.C.; Goland, R.; et al. A Randomized Trial Comparing Telemedicine Case Management with Usual Care in Older, Ethnically Diverse, Medically Underserved Patients with Diabetes Mellitus: 5 Year Results of the IDEATel Study. J. Am. Med. Inform. Assoc. 2009, 16, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Kothari, D.; Teresi, J.A.; Kong, J.; Eimicke, J.P.; Lantigua, R.A.; Palmas, W.; Weinstock, R.S. Social Impact Analysis of the Effects of a Telemedicine Intervention to Improve Diabetes Outcomes in an Ethnically Diverse, Medically Underserved Population: Findings from the IDEATel Study. Am. J. Public Health 2013, 103, 1888–1894. [Google Scholar] [CrossRef]
- Fundoiano-Hershcovitz, Y.; Hirsch, A.; Dar, S.; Feniger, E.; Goldstein, P. Role of Digital Engagement in Diabetes Care Beyond Measurement: Retrospective Cohort Study. JMIR Diabetes 2021, 6, e24030. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).