1. Introduction
Dental caries is one of the most common dental public health problems in the world; it is a disease characterized by a progressive and chronic demineralization of tooth tissues when the oral pH becomes more acidic due to salivary factors, modifications in the oral microbiome, and excessive sugar consumption. Monosaccharides induce a rapid lowering of oral pH, and this critical period increases the risk of caries, the development of which is strictly connected with the bacterial fermentation of dietary carbohydrates. For this reason, the energy supply of foods characterized by high sugar contents is potentially cariogenic, and it is very important to educate children about adequate nutrition and to teach them correct oral hygiene habits to prevent the development of carious lesions [
1]. However, insufficient oral health services, difficulties in accessing medical care facilities, illiteracy, inadequate lifestyle habits, and frequent consumption of sweets all represent possible causes of dental caries, the prevalence of which is expected to increase in developing countries [
2]. In many developed and middle-income countries, dental caries has decreased on average, mainly due to the widespread use of fluoride—a compound with F
− ions that is able to reinforce the structure of hydroxyapatite in the teeth. In fact, the impact of fluoride on human health is dosage-dependent—many studies have seen that a fluoride intake lower than 1.5 mg/L helps to significantly reduce the incidence of dental caries. On the other hand, a fluoride intake above 1.5 mg/L is extremely harmful and can cause systemic diseases as well as dental fluorosis. Dental fluorosis is an irreversible defect in dental enamel caused by long-term undesired fluoride intake during tooth formation, and it influences the formation and the mineralization of dental enamel. This represents the effect of long-term intake of excessive amounts of fluoride (over 2 ppm) during the amelogenesis (tooth enamel production) phase in permanent teeth (2–3 years). The ameloblastic activity is damaged by the excessive incorporation of fluorine. This is characterized by an intrinsic, primitive, endogenous dyschromia; therefore, it cannot be eliminated with professional hygiene. Its main feature is the hypomineralization of the enamel; consequently, the interprismatic matrix of the enamel becomes defective.
The clinical manifestations of dental fluorosis may range from white spots to dark brown areas, which can initially represent only an aesthetic problem, but they can also lead to functional problems; in severe cases, pitting and fractures can occur [
3]. The severity of fluorosis in primary dentition was found to be lower than in permanent dentition, probably due to different reasons. First of all, the enamel of primary teeth contains lower concentrations of fluorine than corresponding permanent teeth. Secondly, the enamel layer of primary teeth is thinner and its maturation has been shorter in time. Furthermore, the transfer of fluoride during breastfeeding is shorter, and it is preferentially absorbed by the growing skeleton. Other studies are necessary to better investigate this difference [
4].
Studies have shown that the increase in tooth whiteness caused by mild or very mild fluorosis has a positive effect on the quality of life, increasing tooth and smile attractiveness. In contrast, severe fluorosis lesions have been found to negatively affect health-related quality of life [
5,
6]. Oral health has started to be seen from a strictly clinical perspective with regard to its psychological impacts, with the great importance of appearance and relationships with others. Children affected by fluorosis are perceived to be less attractive, unintelligent, and unhappy as compared to their peers, and this fact influences their social development.
Epidemiological evidence suggests that higher fluoride concentrations in drinking water (1.5 mg/L) increase the risk of dental and skeletal fluorosis [
7,
8,
9]—a progressive and slow condition with many different systemic complications, which can lead to bone fracture, paralysis, respiratory complications, low blood pressure, and other diseases, such as weight loss and anemia. Dental and skeletal fluorosis has been found to affect 200 million people in nearly 25 countries [
7]. According to the literature, high fluoride contents in drinking water are one of the main sources of fluorosis [
8,
9].
The increasing incidence of dental fluorosis in Kenya varies from place to place, and it is closely related to the high levels contained in groundwater, ranging from 0.50 to 72 mg/L [10 bis]. In contrast to surface water, groundwater is rich in fluoride-containing compounds due to contamination from volcanic rocks rich in fluorine, which are characteristic of different areas in East Africa traversed by the Rift Valley. In Nairobi, there are two different sources of water: most people use water from the river, but some residents use water from boreholes, in which the concentration of fluoride is greater [
4]. The prevalence of fluorosis in Kenya is projected to increase in the coming years if the government does not implement adequate countermeasures. Many governments recognize the negative impacts of dental fluorosis on people’s health, and they should invest in methods that can filter or clean water in poor villages without other alternatives or find simple and low-cost defluoridation devices to overcome this problem. Based on the WHO’s recommendations, many boreholes would have to be closed. However, in these areas, economic problems and limited budgets make it difficult to establish defluoridation technologies or to provide water from different places. On the other hand, endemic fluorosis has become a serious public health problem, so it is important to raise affected communities’ awareness of this problem, in order to identify possible solutions to prevent this disease [
4,
10].
According to the WHO, fluoroprophylaxis—understood as the prevention of carious lesions with the use of fluoride—represents a milestone in the prevention of caries, and it is necessary for all individuals. Over the years, different means of administering fluorine have been developed, each with different concentrations, frequencies of use, and posologies, e.g., fluoridated water, milk, salt, tablets, drops, toothpastes, paints, or gels.
The most frequently used means of fluoroprophylaxis are those with topical application. Scientific evidence on systemic fluoroprophylaxis is controversial—many studies in the literature confirm the effectiveness of fluoride supplements in permanent dentition [
11], but this is not clear for primary dentition.
In children with a high risk of carious lesions, or in case of objective difficulty in using toothpaste enriched with fluorine, the WHO recommends the somministration of 0.25 mg/die of fluoride with drops from the age of 6 months to 3 years, and the somministration of 0.50 mg/die of fluoride with drops or tablets in children aged 3–6 years. In some cases, the professional application of fluorinated gels (12,500 ppm), mouthwashes, or chlorhexidine paints can also be used to prevent carious lesions. Even the fluoridation of water is a preventive measure adopted in different countries; in this case, the concentration of fluoride considered optimal for the prevention of caries is 0.7 mg/L, while the maximum permissible concentration of fluoride in drinking water recommended by the WHO is 1.5 mg/L [
10].
The aim of the present epidemiological study was to investigate the oral health status of a group of adolescents residing in the rural area around the city of Nairobi (Kenya), in order to define treatment needs and, therefore, plan prevention and treatment programs.
2. Materials and Methods
Participants: For the current study, a total of 215 adolescents—114 females (53.1%) and 101 males (46.9%), aged 12 (99 subjects, 46.1%) and 15 (116 subjects, 53.9%) years—were included. Four schools were selected to visit the subjects. A questionnaire was designed for acquiring a detailed history of their oral health status. One-hundred subjects from Karen Academy, Banana area; 74 subjects from Ruiru School, Ruiru area; 24 subjects from Kiambaa School, Kiambaa area; and 17 subjects from Nazareth Hospital School, Kimuga area participated in this study and completed the questionnaire. The study was conducted in accordance with the guidelines of the Declaration of Helsinki (2008 version), and informed written consent was obtained from the parent/guardian of each participant.
Data Collection and Analysis: Two examiners with previous experience in epidemiological surveys participated in the study. As detailed elsewhere [
12,
13], the calibration process was performed at the “World Health Organization (WHO) Center of Collaboration for Epidemiology and Community Dentistry” (University of Milano, Milano, Italy). Mean kappa values of 0.95 were obtained for the WHO diagnostic criteria. The DMFT index (Decay, Missing, and Filled Teeth), CPI (Community Periodontal Index), and questionnaire data concerning oral and food hygiene habits, parents’ educational levels, and socioeconomic levels were recorded in accordance with the WHO criteria. The DMFT index represents the sum of the number of decayed teeth, teeth missing due to caries, and filled teeth among the permanent teeth; the mean DMFT index is the sum of the individual DMFT values divided by the sum of the population [
14].
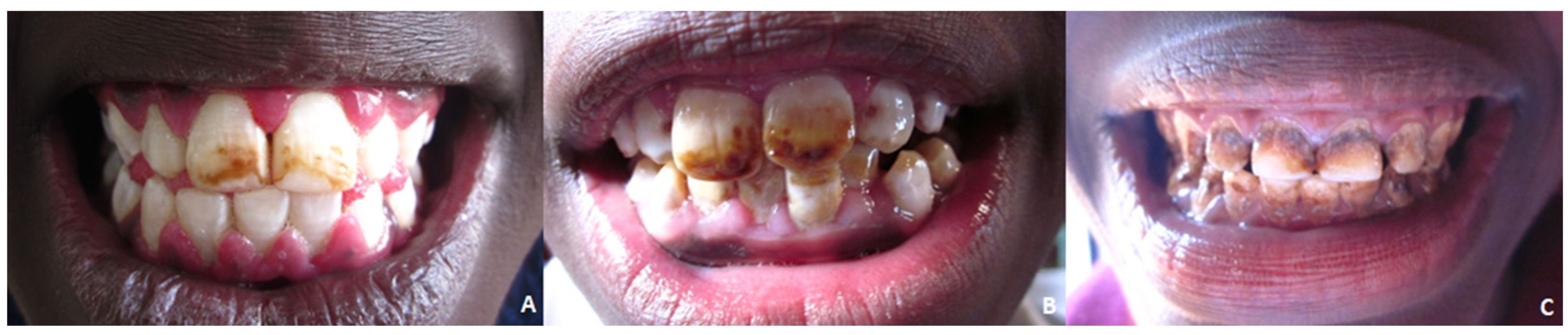
The Thylstrup–Fejerskov (TF) index was also utilized to assess dental fluorosis [
15]. The TF index assesses the dental fluorosis on all surfaces of every tooth, which is classified according to a 0–9 scale; according to this index, all erupted teeth are scored for fluorosis, and the highest score of a patient is described as their overall TF score. In the present study (
Figure 1), when determining the severity of fluorosis, a TF score of 2–3 was considered mild, a TF score of 4–5 was considered moderate, and scores above 5 were considered severe.
Statistical analysis: The odds ratios (ORs) of being diagnosed with dental caries lesions and co-presence of risk factors (i.e., age, gender, parents’ work and educational level, number of subjects in the household, eating times, eating sweets during or between meals, drinking soft drinks during or between meals, and dental care and oral hygiene status) were calculated using multivariable models with logistic regression. The association between outcomes and explanatory variables was evaluated by setting the significance level to a p-value of less than or equal to 0.05.
3. Results
Analyzing all of the data collected, it is clear that there are no differences between the four schools of the study, so the results are presented together.
Characteristics of the sample are shown in
Table 1.
3.1. Carious Lesions
In total, 86 out of 215 subjects presented carious lesions. The most affected sites were the lower and upper molars, and 5% of them were completely decayed or irrecoverable elements.
3.2. Periodontal Status
Regarding periodontal status, 35% did not show gingival inflammation, 45% had mild gingival bleeding on probing, and 20% had roughness on the elements (i.e., presence of calculus).
3.3. Dental Formula
Regarding dental formula, 32% of the 12-year-old subjects had not yet changed their upper and/or lower second deciduous molars into permanent elements. In 18%, other elements such as canines and lateral incisors were also deciduous teeth.
3.4. DMFT Index
The DMFT index was 0.87 in the total sample. The F variable showed a value of 0, the M variable was 0.46, and the D variable was 0.41. In our sample, 21.4% were fluorosis-free and 78.6% were affected by fluorosis; 33% of these had a “score 2–3, mild”, 34.4% “score 4–5, moderate”, and 11.2% “code > 5 severe”.
The odds ratio values for risk factors for developing caries are reported in
Table 2. Each variable indicated in
Table 2 represents a different risk factor for the development of caries, with a different significance; among the variables indicated in the table, only “sweets during meal”, “sweets between meal”, “soft drinks during meal”, “soft drinks between meal”, “toothbrush frequency”, “use of toothpaste”, “bleeding”, and “calculus” are statistically significant (
p < 0.01).
4. Discussion
In Kenya, dental health services are private, and turning to a dental practice is too expensive for much of the population. In most cases, children with caries do not have the opportunity to cure the disease by receiving fillings; therefore, the lesion affects the entire element, which then remains in the oral cavity, infecting the surrounding elements, the mucous membranes, and the entire oral cavity. There may be a relationship with the ascertained lack of vitamins and calcium with which these subjects live, but even more interesting is the potential relationship with fluoride. The caries-preventive benefits of fluoride, due to its incorporation in the structure of dental enamel, reduce the solubility of enamel and inhibit demineralization by promoting remineralization of the teeth. On the other hand, at high concentrations, if the consumption of fluoride is greater than optimal, it can lead to increased enamel porosity, with the consequent manifestation of dental fluorosis.
Fluorosis only affects developing teeth, and certain stages of development are more susceptible than others. As found in the present study, and according to the literature [
16,
17], there is a relationship between delayed dental exchange and excessive intake of fluorine (and in children taking sodium fluoride), with a delay in the eruption of the teeth recorded up to a maximum of 0.7–2 years. Dental fluorosis is also more prevalent in late-erupting permanent teeth, but other studies are necessary to better understand the real relationship between fluoride intake levels and dental fluorosis of late-eruptive teeth.
The high incidence of fluorosis in our sample (almost 80%) is due to the fact that, in Nairobi’s suburbs, the population lives without adequate aqueducts and pipes for drinking water and digs wells that reach as deep as 30 meters to be able to supply themselves with water. These waters are not controlled and are rich in bacteria and elements that are harmful to humans, which in the long run can have serious consequences for the body. One of these elements is fluorine—a substance that, in developed countries, is controlled and filtered by aqueducts in order to allow the supply of drinking water to our homes. The concentration of fluorine in the waters of the rural area around Nairobi ranges from 0.50 to 72 mg/L [
10], and it is often greater than the concentration of 1.5 mg/L recommended by the WHO [
11]. The fact that fluoride accumulates in the body is the reason that U.S. law requires health officials to set a maximum level of contaminants for the amount of fluoride in public water supplies, as set out by the Environmental Protection Agency. The threshold established to avoid only the third disabling stage of this disease is set at 4 ppm or 4 mg per liter. The human body is supposed to retain half of this amount (2 mg); therefore, 4 mg per liter is the safety level. Therefore, a daily dose of 2–8 mg is sufficient to cause the third disabling stage of invalidating skeletal fluorosis [
6].
Regarding dental care, the multivariate model highlighted statistical associations between the presence of caries, the oral hygiene status, and the frequency of consumption of sugary drinks and sweets. This result is shown in
Table 2, where all of the variables investigated and their correlation with the development of new carious lesions are represented. The prevalence of caries was higher in those children who frequently consumed sugary drinks and food between and during meals. This association has been evidenced by many studies [
1,
18,
19] reporting that the daily consumption of sweetened drinks and food increases the risk of developing caries. In developing countries, increasing the tooth-brushing frequency, as well as the quality of oral hygiene, is one of the most important strategies to reduce the incidence of caries. The importance of brushing in preventing dental caries is due to the disruption of dental biofilm, which represents one of the primary factors in the pathogenesis of caries. Individuals who state that they brush their teeth infrequently are at a greater risk for the incidence of or increase in new carious lesions compared to those who brush more frequently, and the incidence is higher in deciduous dentition than in permanent dentition. However, many people are not able to achieve an optimal control of dental biofilm with the use of a toothbrush alone, and in these cases the addition of fluoride to the toothpaste seems to make the difference in preventing carious lesions, although more detailed studies are necessary to analyze how tooth-brushing and fluoridated toothpaste separately influence the incidence of carious lesions [
20,
21].
The use of fluoride toothpaste, at appropriate concentrations, has been reported to prevent caries and seems to be more effective in controlling the risk of dental fluorosis [
22]. Increasing the fluoride content in toothpaste increases the preventive effect against caries [
23], as our participants showed less caries due to the high fluoride content in their drinking water. We observed the same relationship of caries with the frequency of tooth-brushing, as well as with the use of different toothpaste concentrations among our participants.
Research studies [
1,
13] have described a statistically significant association between the prevalence of caries and inadequate oral hygiene (i.e., presence of bleeding and calculus). The subjects with visible plaque on their teeth surfaces had an up to 10-fold increased risk of developing caries compared to the subjects who had adequate oral hygiene.
With regard to the caries problem, the results show that the prevalence was not significantly higher than in Europe. The main characteristic found was the extension of single carious cavities in our population. A low prevalence of caries was detected (60% of the subjects were caries-free), probably due to the high concentration of fluoride in the drinking water. Similarly, the fluoride in the well waters was likely the cause of the high levels of fluorosis observed. The absence of treated dental elements (component F of the index) highlights the lack of services in the territory (i.e., suitable dentists). On the other hand, the score of 0.3 for component M shows that the only treatments carried out are extractive and urgent.
This study has some limitations: The study group was a sample of convenience based on the recruitment of all of the children enrolled in the four included schools, and it was also limited in number and contained only two ranges of age. Data on fluoride dosages were not measured for this study but derived from databases of other studies. Despite this, the results of this first sample indicate the need for more extensive investigations in order to prevent these health problems and implement adequate preventive measures.
5. Conclusions
During the study, the problem of fluorosis emerged with great clinical relevance, both as a prevalence of pathology and as a socio-health problem; the main cause was the consumption of water with a high concentration of fluorine. Similarly, caries was also found as an oral health issue in the population of Kenya, but its prevalence was relatively low compared to Europe. A possible intervention that could avoid the fluorosis pathology is the sanitation of the waters or the installation of hydraulic pipes at homes for the provision of controlled drinking water, in addition to geochemical surveys that help to select borehole sites in suitable hydrogeological areas. On the other hand, carious lesions can be controlled by managing the various risk factors implicated in the occurrence of caries, such as lifestyle and eating habits, including the frequency of tooth-brushing and the use of adequate fluorinated toothpastes. After the new definition of human health by the WHO, including not only physical but also mental and social wellbeing, we cannot ignore the negative effects that dental and skeletal fluorosis can cause on the human psyche and quality of life.
In conclusion, an important element in this perspective is the construction of strategies to guarantee fair access to healthcare services and the promotion of oral prevention programs carried out in the context of general health, in order to significantly reduce the impact of oral diseases in the population studied.