The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature
Abstract
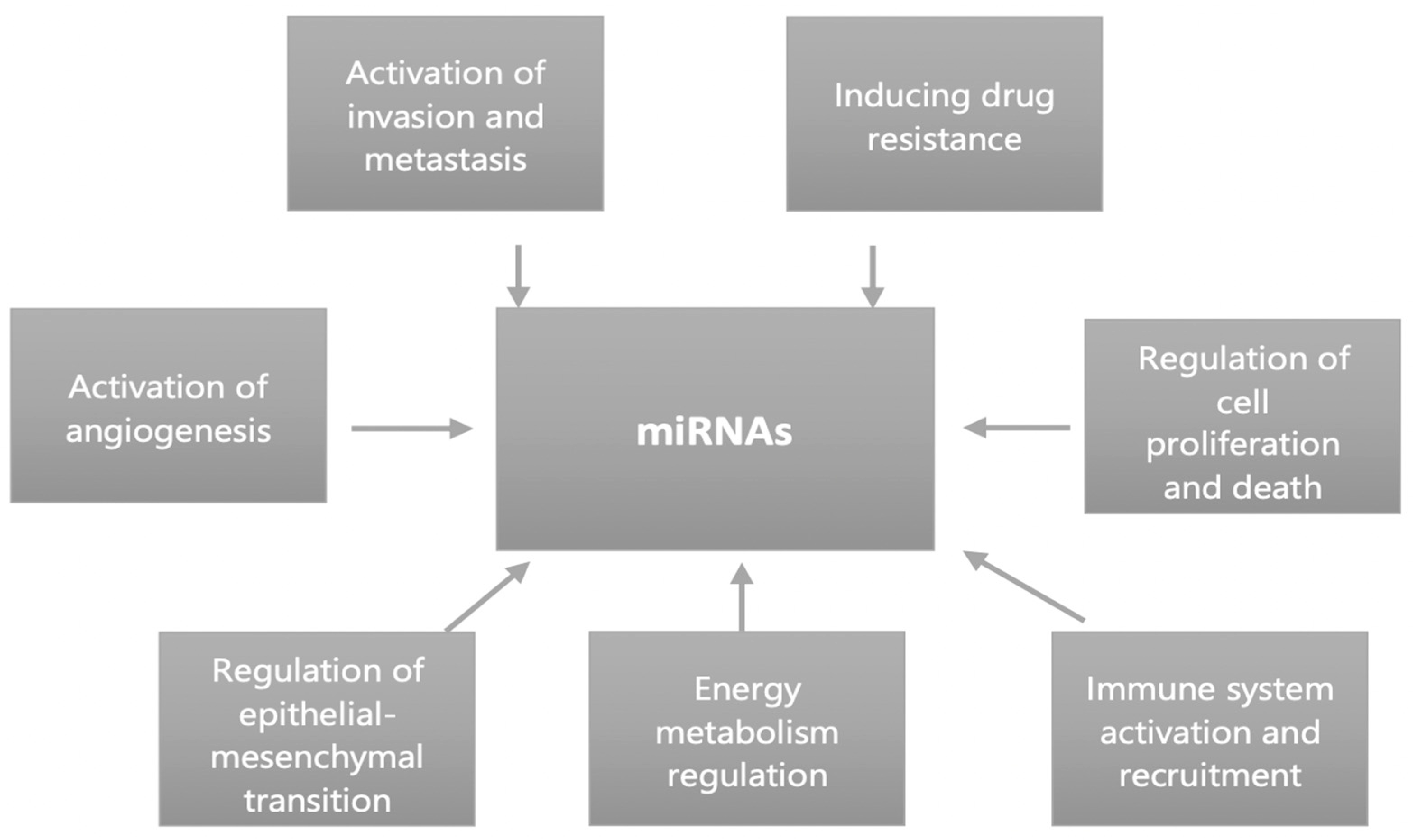
1. Introduction
2. Materials and Methods
3. miRNA Isolation
4. Localized RCC
4.1. Diagnosis
4.2. Prognosis
4.3. Treatment
5. Locally Advanced and Metastatic RCC
5.1. Diagnosis
5.2. Prognosis
5.3. Treatment
| miRNA | Detection Site | Expression | Gene Target | Biological Functions | Role | Clinical Function |
|---|---|---|---|---|---|---|
| miR-25 [11] | plasma | ↑ or ↓ | PTEN, BCL2L11 | Enhance cell migration and increase expression of N-cadherin and Slug | Prognosis | Under-expression after surgery |
| miR-126 [11] | RCC tissue, plasma | ↓ | SLC7A5, SERPINE1, VEGF | Decrease cell migration, Limits mTOR pathway | Diagnosis | High levels in pRCC Reduction after surgery |
| miR-200 [11] | RCC tissue, plasma | ↑ | VEGFA, PTEN, TIMP2 | Promote cell proliferation | Diagnosis | High levels in chRCC and low in oncocytoma Under-expression after surgery |
| miR301 [11] | plasma | ↑ | TIMP2, PTEN BCL2L11 | Promote cell proliferation | Diagnosis and Prognosis | Overexpression associated with metastatic risk and poor prognosis Downregulation after surgery |
| miR-1293 [11] | Plasma | - | - | - | Diagnosis and Prognosis | Overexpression after surgery Under-expression in metastatic patients Poor prognosis and metastatic risk |
| miR-376 [17] | RCC tissue | - | - | - | Therapy | Overexpression in RCC of patients with primary resistance versus long-term response to Sunitinib |
| miR-15 [29] | RCC tissue | ↓ | DMTF1 | Tumor suppressor by activating the transcription of ARF | Diagnosis | |
| miR-16 [29] | RCC tissue | ↑ | SRPR_HUMAN | Guarantees the correct targeting of the proteins to the endoplasmic reticulum membrane | Diagnosis | |
| miR-27a [29] | RCC tissue | ↑ | Cadherin-5 | Favors angiogenesis | Diagnosis | Positive association with advanced clinical stage and metastatic risk |
| miR-17 [29] | RCC tissue | ↓ | E2F1 | Tumor suppressor protein with a crucial role in the control of cell cycle | Diagnosis | |
| miR-103 [29] | RCC tissure | ↓ | FBXW11 | Prevent cell cycle progression | Diagnosis | |
| miR-34a [29] | RCC tissure | ↑ | DLL1 | Promotes cell to cell communication | Diagnosis | |
| miR143 [29] | RCC tissue | ↑ | MAPK7 | Promotes mitotic activity | Diagnosis | |
| miR-31 [30] | RCC tissure | ↑ | YAP1 | Promotes the transcription of cyclin D1 | Diagnosis | High levels in pRCC |
| miR-210 [30] | RCC tissue | ↑ | ISCU 1/2 | Promotes the synthesis and maturation of protein for cell cycle progression | Diagnosis | High levels in p- and ccRCC |
| miR-221 [30] | RCC tissue | ↑ | KIT | Promote cell proliferation, migration, invasion and inhibit apoptosis | Diagnosis | High concentration in oncocytoma and chRCC |
| miR-30 [31] | RCC tissue, urine, and serum | ↓ (c) and ↑ (a) | GRP78, Beclin-1, ITGA4, NRP2 | Promotes cell growth | Prognosis | (c) Downregulation- poor prognosis (a) Upregulation-metastatic disease |
| miR-32 [31] | RCC tissue | - | - | - | Prognosis | Positive association with poor outcome |
| miR-186 [31] | RCC tissue | ↓ | SENP1 | Inhibit cell proliferation, invasion and induce apoptosis, decrease level of p-IkBa and p-p65 | Diagnosis and Prognosis | High levels in oncocytoma and low in chRCC |
| miR-197 [31] | RCC tissue | - | - | - | Diagnosis and Prognosis | High concentration in chRCC and low in oncocytoma |
| miR-203 [31] | RCC tissue | ↓ | p63 | Decrease tumor growth, metastasis and increase the expression of E-cadherin, PTEN, p21 and p27 | Diagnosis and Prognosis | Overexpression in ch- and ccRCC and under-expression in papillary RCC and oncocytoma |
| miR-424 [31] | RCC tissue | ↑ | WEE1 | Limit cell cycle | Prognosis | High levels in clear cell RCC |
| miR-320 [31] | RCC tissue | ↓ | CFL2 | Reduce tumor growth | Diagnosis | Low levels in chRCC and high in oncocytoma |
| miR-135a [32] | RCC tissue | ↓ | RASSF1A | Inhibit proliferation | Diagnosis | Downregulation in ccRCC |
| miR-154 [32] | RCC tissue | - | - | - | Diagnosis | Downregulation in ccRCC |
| miR-377 [32] | RCC tissue | - | - | - | Diagnosis | Downregulation in ccRCC |
| miR-411 [32] | RCC tissue | - | - | - | Diagnosis | Downregulation in ccRCC |
| miR-337 [32] | RCC tissue | - | - | - | Diagnosis | Downregulation in ccRCC |
| miR-130a [33] | RCC tissue | ↑ | PTEN/PI3K/AK | Cell proliferation | Prognosis | Under-expression in poor prognosis |
| miR-9 [33] | RCC tissue | ↑ | VEGF, E-cadherin, PRDM1 | Angiogenesis, cell proliferation | Prognosis and Therapy | High levels predict recurrence rate |
| miR-18a [33] | RCC tissue | Prognosis | High levels predict recurrence rate | |||
| miR-149 [33] | RCC tissue | ↑ | FOXM1 | Inhibit cell migration, invasion and proliferation | Prognosis | Positive association with higher recurrence rate |
| miR-183 [33] | RCC tissue | - | - | - | Prognosis | Positive association with higher recurrence rate |
| miR-21 [33] | RCC tissue | ↑ | PTEN, PDCD4, PIK3R1, TIMP3 | Increase cell proliferation, invasion, migration and reduce apoptosis | Prognosis | Association of these mi-RNAs with worse survival and higher recurrence rates |
| miR-146 [33] | RCC tissue | - | - | - | Prognosis | Positive association with higher recurrence rate |
| miR-335 [33] | RCC tissue | - | - | - | Prognosis | Positive association with higher recurrence rate |
| miR-625 [33] | RCC tissue | - | - | - | Prognosis | Positive association with higher recurrence rate |
| miR-497 [34] | RCC tissues | ↓ | PDL1 | Inhibit cell proliferation, migration and increase apoptosis | Prognosis | Under-expression associated with worse tumor stage and higher histological grade in clear cell RCC |
| miR-223 [35] | RCC tissue | ↑ | HMGCS1 | - | Prognosis | Positive association with higher recurrence rate and worse survival and in ccRCC |
| miR-365 [35] | RCC tissue | ↑ | HMGCS1 | Prognosis | Direct correlation with worse survival and higher recurrence rates | |
| miR-1260b [36] | RCC tissue | ↓ | Wnt | Promote cell proliferation | Therapy | High concentration in RCC tissue Genistein promoted its downregulation |
| miR-26 [43] | RCC tissue | ↓ | Coronin-3 | inhibiting the migration and invasion | Therapy | Overexpression inhibits RCC in cell growth and metastasis via down-regulating coronin-3 |
| miR-139 [45] | RCC tissue | ↑ | TGFβ, Wnt, Rho, | Promotes cell proliferation | Diagnosis and Prognosis | High concentration in oncocytoma and low in chRCC Positive association with metastatic risk |
| miR-144 [45] | RCC tissue | ↑ | MAP3K8 | Suppress cell proliferation, migration and invasion | Diagnosis and Prognosis | Positive association with metastatic risk |
| miR-206 [47] | RCC tissue | - | - | - | Diagnosis | Direct correlation with higher pT-stage and metastasis risk |
| miR-452 [51] | RCC tissue | ↓ | SMAD4 | Suppresses RCC progression targeting various gene | Prognosis | Poor prognosis in mRCC patients Sunitinib reduces its expression |
| miR-192 [54] | Serum | - | - | - | Therapy | High concentration in poor responder patients to TKI therapy |
| miR-193 [54] | Serum | ↑ | ST3GalIV | Promote cell proliferation, invasion, migration and inhibit apoptosis, improve expression of PI3k and p-Akt | Therapy | High concentration in poor responder patients to TKI therapy |
| miR501 [54] | Serum | - | - | - | Therapy | High levels in poor responder patients to TKI therapy |
6. miRNA Role in Clinical Practice and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Esteban, E.; Barrionuevo-Castillo, P.; Domínguez-Rullán, J.; Gómez-Aparicio, M.; Ferri-Molina, M.; Sáez-Bueno, P.; Zalabarría-Zarrabeitia, Z.; Scorsetti, M.; Arcangeli, S.; López-Campos, F.; et al. Stereotactic Body Radiotherapy for Kidney Cancer: Ready for Prime Time? Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Dabestani, S.; Thorstenson, A.; Lindblad, P.; Harmenberg, U.; Ljungberg, B.; Lundstam, S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: A population-based study. World J. Urol. 2016, 34, 1081–1086. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Berindan-Neagoe, I.; Monroig Pdel, C.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar] [CrossRef]
- Roest, H.P.; Ijzermans, J.N.M.; van der Laan, L.J.W. Evaluation of RNA isolation methods for microRNA quantification in a range of clinical biofluids. BMC Biotechnol. 2021, 21, 48. [Google Scholar] [CrossRef]
- Brown, R.A.M.; Epis, M.R.; Horsham, J.L.; Kabir, T.D.; Richardson, K.L.; Leedman, P.J. Total RNA extraction from tissues for microRNA and target gene expression analysis: Not all kits are created equal. BMC Biotechnol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Dias, F.; Teixeira, A.L.; Nogueira, I.; Morais, M.; Maia, J.; Bodo, C.; Ferreira, M.; Silva, A.; Vilhena, M.; Lobo, J.; et al. Extracellular Vesicles Enriched in hsa-miR-301a-3p and hsa-miR-1293 Dynamics in Clear Cell Renal Cell Carcinoma Patients: Potential Biomarkers of Metastatic Disease. Cancers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, J.; Min, L.; He, Y.; Guan, H.; Wang, J.; Peng, X. Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int. J. Cancer 2020, 146, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, E.; Geng, B.; Gao, S.; Yu, H.; He, X.; Li, X.; Dong, G.; You, B. Tumor-associated macrophage-derived exosomes transmitting miR-193a-5p promote the progression of renal cell carcinoma via TIMP2-dependent vasculogenic mimicry. Cell Death Dis. 2022, 13, 382. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Ishihara, M.; Sharrow, A.C.; Flora, K.; He, Y.; Wu, L. The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3005. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Guan, J.; Zheng, Z.; Hao, J.; Sheng, Z.; Wang, M.; Xu, T.; Guo, G.; Yao, L. Exosomal Circsafb2 Reshaping Tumor Environment to Promote Renal Cell Carcinoma Progression by Mediating M2 Macrophage Polarization. Front. Oncol. 2022, 12, 808888. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.; Teixeira, A.L.; Ferreira, M.; Adem, B.; Bastos, N.; Vieira, J.; Fernandes, M.; Sequeira, M.I.; Maurício, J.; Lobo, F.; et al. Plasmatic miR-210, miR-221 and miR-1233 profile: Potential liquid biopsies candidates for renal cell carcinoma. Oncotarget 2017, 8, 103315–103326. [Google Scholar] [CrossRef] [PubMed]
- Kovacova, J.; Juracek, J.; Poprach, A.; Kopecky, J.; Fiala, O.; Svoboda, M.; Fabian, P.; Radova, L.; Brabec, P.; Buchler, T.; et al. MiR-376b-3p Is Associated with Long-term Response to Sunitinib in Metastatic Renal Cell Carcinoma Patients. Cancer Genom. Proteom. 2019, 16, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Chanudet, E.; Wozniak, M.B.; Bouaoun, L.; Byrnes, G.; Mukeriya, A.; Zaridze, D.; Brennan, P.; Muller, D.C.; Scelo, G. Large-scale genome-wide screening of circulating microRNAs in clear cell renal cell carcinoma reveals specific signatures in late-stage disease: Circulating MicroRNAs Signatures in CcRCC. Int. J. Cancer 2017, 141, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Tusong, H.; Maolakuerban, N.; Guan, J.; Rexiati, M.; Wang, W.-G.; Azhati, B.; Nuerrula, Y.; Wang, Y.-J. Functional analysis of serum microRNAs miR-21 and miR-106a in renal cell carcinoma. Cancer Biomark. 2017, 18, 79–85. [Google Scholar] [CrossRef]
- Li, R.; Lu, C.; Li, X.; Chen, X.; Huang, G.; Wen, Z.; Li, H.; Tao, L.; Hu, Y.; Zhao, Z.; et al. A Four-MicroRNA Panel in Serum as a Potential Biomarker for Screening Renal Cell Carcinoma. Front. Genet. 2022, 13, 897827. [Google Scholar] [CrossRef]
- Liu, S.; Deng, X.; Zhang, J. Identification of dysregulated serum miR-508-3p and miR-885-5p as potential diagnostic biomarkers of clear cell renal carcinoma. Mol. Med. Rep. 2019, 20, 5075–5083. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, X.; Chen, Z.; Wang, J.; Zhang, C.; Chen, X.; Peng, X.; Liu, K.; Zhao, L.; Lai, Y.; et al. A Three-microRNA Panel in Serum: Serving as a Potential Diagnostic Biomarker for Renal Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, H.; Wang, J.; Peng, X.; Liu, K.; Zhao, L.; Zhang, C.; Chen, X.; Lai, Y.; Ni, L. Combination of tumor suppressor miR-20b-5p, miR-30a-5p, and miR-196a-5p as a serum diagnostic panel for renal cell carcinoma. Pathol. Res. Pract. 2020, 216, 153152. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu-Maekawa, Y.; Kawakami, K.; Fujita, Y.; Takai, M.; Kato, D.; Nakane, K.; Kato, T.; Tsuchiya, T.; Koie, T.; Miura, Y.; et al. Profiling of Serum Extracellular Vesicles Reveals miRNA-4525 as a Potential Biomarker for Advanced Renal Cell Carcinoma. Cancer Genom. Proteom. 2021, 18, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ni, M.; Su, Y.; Wang, H.; Zhu, S.; Zhao, A.; Li, G. MicroRNAs in Serum Exosomes as Potential Biomarkers in Clear-cell Renal Cell Carcinoma. Eur. Urol. Focus 2018, 4, 412–419. [Google Scholar] [CrossRef]
- Outeiro-Pinho, G.; Barros-Silva, D.; Aznar, E.; Sousa, A.-I.; Vieira-Coimbra, M.; Oliveira, J.; Gonçalves, C.S.; Costa, B.M.; Junker, K.; Henrique, R.; et al. MicroRNA-30a-5pme: A novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J. Exp. Clin. Cancer Res. 2020, 39, 98. [Google Scholar] [CrossRef]
- Fedorko, M.; Juracek, J.; Stanik, M.; Svoboda, M.; Poprach, A.; Buchler, T.; Pacik, D.; Dolezel, J.; Slaby, O. Detection of let-7 miRNAs in urine supernatant as potential diagnostic approach in non-metastatic clear-cell renal cell carcinoma. Biochem. Med. 2017, 27, 411–417. [Google Scholar] [CrossRef]
- Mytsyk, Y.; Dosenko, V.; Borys, Y.; Kucher, A.; Gazdikova, K.; Busselberg, D.; Caprnda, M.; Kruzliak, P.; Farooqi, A.A.; Lubov, M. MicroRNA-15a expression measured in urine samples as a potential biomarker of renal cell carcinoma. Int. Urol. Nephrol. 2018, 50, 851–859. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, Y.; Yang, J.; Chen, T.; Yin, Y.; Tang, M.; Hu, C.; Zhang, L. Microarray analysis of microRNA expression in renal clear cell carcinoma. Eur. J. Surg. Oncol. 2009, 35, 1119–1123. [Google Scholar] [CrossRef]
- Fridman, E.; Dotan, Z.; Barshack, I.; Ben David, M.; Dov, A.; Tabak, S.; Zion, O.; Benjamin, S.; Benjamin, H.; Kuker, H.; et al. Accurate Molecular Classification of Renal Tumors Using MicroRNA Expression. J. Mol. Diagn. 2010, 12, 687–696. [Google Scholar] [CrossRef]
- Petillo, D.; Kort, E.J.; Anema, J.; Furge, K.A.; Yang, X.J.; Teh, B.T. MicroRNA profiling of human kidney cancer subtypes. Int. J. Oncol. 2009, 35, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Juan, D.; Alexe, G.; Antes, T.; Liu, H.; Madabhushi, A.; Delisi, C.; Ganesan, S.; Bhanot, G.; Liou, L.S. Identification of a MicroRNA Panel for Clear-cell Kidney Cancer. Urology 2010, 75, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-Z.; Wu, R.; Xin, H.; Zhu, M.; Lu, T.-Z.; Liu, H.; Xu, Z.; Yu, P.; Zhao, Y.-C.; Li, M.-H.; et al. A tumor-specific microRNA signature predicts survival in clear cell renal cell carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, Z.; Xu, W.; Hou, J.; Du, X. Down-regulation of miR-497 is associated with poor prognosis in renal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 758–764. [Google Scholar]
- Huang, M.; Zhang, T.; Yao, Z.-Y.; Xing, C.; Wu, Q.; Liu, Y.-W.; Xing, X.-L. MicroRNA related prognosis biomarkers from high throughput sequencing data of kidney renal clear cell carcinoma. BMC Med. Genom. 2021, 14, 72. [Google Scholar] [CrossRef]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef]
- Sekino, Y.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. miR-130b Promotes Sunitinib Resistance through Regulation of PTEN in Renal Cell Carcinoma. Oncology 2019, 97, 164–172. [Google Scholar] [CrossRef]
- Kang, H. MicroRNA-Mediated Health-Promoting Effects of Phytochemicals. Int. J. Mol. Sci. 2019, 20, 2535. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suárez, M.; Torres, J.; Salvado, J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef]
- Nwaeburu, C.C.; Bauer, N.; Zhao, Z.; Abukiwan, A.; Gladkich, J.; Benner, A.; Herr, I. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget 2016, 7, 58367–58380. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, B.; Ibragimova, I.; Zhou, Y.; Slifker, M.J.; Devarajan, K.; Al-Saleem, T.; Uzzo, R.G.; Cairns, P. MicroRNA expression signatures of stage, grade, and progression in clear cell RCC. Cancer Biol. Ther. 2014, 15, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Yan, Z.J.; Luo, G.C.; Chen, Y.Y.; Bai, P.M. miR-26 suppresses renal cell cancer via down-regulating coronin-3. Mol. Cell. Biochem. 2020, 463, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.G.; Tolkach, Y.; Deng, M.; Schmidt, D.; Perner, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Serum miR-122-5p and miR-206 expression: Non-invasive prognostic biomarkers for renal cell carcinoma. Clin. Epigenet. 2018, 10, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Han, P.; Lu, Y.; Zhong, X.; Yang, Y.; Li, D.; Liu, D.; Li, Q.; Pan, N.; et al. Inverse correlation of miR-27a-3p and CDH5 expression serves as a diagnostic biomarker of proliferation and metastasis of clear cell renal carcinoma. Pathol. Res. Pract. 2021, 220, 153393. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur. Urol. 2021, 80, 417–424. [Google Scholar] [CrossRef]
- Heinzelmann, J.; Arndt, M.; Pleyers, R.; Fehlmann, T.; Hoelters, S.; Zeuschner, P.; Vogt, A.; Pryalukhin, A.; Schaeffeler, E.; Bohle, R.M.; et al. 4-miRNA Score Predicts the Individual Metastatic Risk of Renal Cell Carcinoma Patients. Ann. Surg. Oncol. 2019, 26, 3765–3773. [Google Scholar] [CrossRef]
- Zhai, W.; Li, S.; Zhang, J.; Chen, Y.; Ma, J.; Kong, W.; Gong, D.; Zheng, J.; Xue, W.; Xu, Y. Sunitinib-suppressed miR-452-5p facilitates renal cancer cell invasion and metastasis through modulating SMAD4/SMAD7 signals. Mol. Cancer 2018, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Lin, X.; Korytowsky, B.; Matczak, E.; Lechuga, M.; Wiltshire, R.; Motzer, R. Sunitinib objective response in metastatic renal cell carcinoma: Analysis of 1059 patients treated on clinical trials. Eur. J. Cancer 2014, 50, 351–358. [Google Scholar] [CrossRef]
- Ralla, B.; Busch, J.; Flörcken, A.; Westermann, J.; Zhao, Z.; Kilic, E.; Weickmann, S.; Jung, M.; Fendler, A.; Jung, K. miR-9-5p in Nephrectomy Specimens is a Potential Predictor of Primary Resistance to First-Line Treatment with Tyrosine Kinase Inhibitors in Patients with Metastatic Renal Cell Carcinoma. Cancers 2018, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Pozo, A.; Antón-Aparicio, L.M.; Bayona, C.; Borrega, P.; Sancho, M.I.G.; García-Domínguez, R.; de Portugal, T.; Ramos-Vázquez, M.; Pérez-Carrión, R.; Bolós, M.V.; et al. MicroRNA Expression Profiling of Peripheral Blood Samples Predicts Resistance to First-line Sunitinib in Advanced Renal Cell Carcinoma Patients. Neoplasia 2012, 14, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Porta, C.; Olive, D.; De Luca, I.; Brando, C.; Rizzo, M.; Messina, C.; Rediti, M.; et al. Baseline Plasma Levels of Soluble PD-1, PD-L1, and BTN3A1 Predict Response to Nivolumab Treatment in Patients with Metastatic Renal Cell Carcinoma: A Step toward a Biomarker for Therapeutic Decisions. OncoImmunology 2020, 9, 1832348. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Brando, C.; Bono, M.; De Luca, I.; Algeri, L.; Bonasera, A.; Corsini, L.R.; Scurria, S.; et al. A “Lymphocyte MicroRNA Signature” as Predictive Biomarker of Immunotherapy Response and Plasma PD-1/PD-L1 Expression Levels in Patients with Metastatic Renal Cell Carcinoma: Pointing towards Epigenetic Reprogramming. Cancers 2020, 12, 3396. [Google Scholar] [CrossRef]
- Gottardo, F.; Liu, C.G.; Ferracin, M.; Calin, G.A.; Fassan, M.; Bassi, P.; Sevignani, C.; Byrne, D.; Negrini, M.; Pagano, F.; et al. Micro-RNA profiling in kidney and bladder cancers. Urol. Oncol. 2007, 25, 387–392. [Google Scholar] [CrossRef]
- Nakada, C.; Matsuura, K.; Tsukamoto, Y.; Tanigawa, M.; Yoshimoto, T.; Narimatsu, T.; Nguyen, L.T.; Hijiya, N.; Uchida, T.; Sato, F.; et al. Genome-wide microRNA expression profiling in renal cell carcinoma: Significant down-regulation of miR-141 and miR-200c. J. Pathol. 2008, 216, 418–427. [Google Scholar] [CrossRef]
- Mytsyk, Y.; Dosenko, V.; Borys, Y.; Ilchyshyn, O.; Chernova, N.; Mytsyk, Y.; Maksymovych, I.; Illiuk, P. The Possibility of Application of Detected in Urine MicroRNA-15a for Diagnostics of Renal Cell Carcinoma. Exp. Clin. Physiol. Biochem. 2017, 2017, 49–53. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Wang, X. A three-microRNA signature as a diagnostic and prognostic marker in clear cell renal cancer: An In Silico analysis. PLoS ONE 2017, 12, e0180660. [Google Scholar] [CrossRef]
- Wulfken, L.M.; Moritz, R.; Ohlmann, C.; Holdenrieder, S.; Jung, V.; Becker, F.; Herrmann, E.; Walgenbach-Brünagel, G.; Von Ruecker, A.; Müller, S.C.; et al. MicroRNAs in Renal Cell Carcinoma: Diagnostic Implications of Serum miR-1233 Levels. PLoS ONE 2011, 6, e25787. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zeng, H.; Fan, J.; Huang, W.; Yu, X.; Li, S.; Wang, F.; Long, X. A novel nine-microRNA-based model to improve prognosis prediction of renal cell carcinoma. BMC Cancer 2022, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lei, T.; Xu, C.; Li, H.; Ma, W.; Yang, Y.; Fan, S.; Liu, Y. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7-H3. Biochem. Biophys. Res. Commun. 2013, 438, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Vergho, D.; Kneitz, S.; Rosenwald, A.; Scherer, C.; Spahn, M.; Burger, M.; Riedmiller, H.; Kneitz, B. Combination of expression levels of miR-21 and miR-126 is associated with cancer-specific survival in clear-cell renal cell carcinoma. BMC Cancer 2014, 14, 25. [Google Scholar] [CrossRef]
- Fritz, H.K.; Lindgren, D.; Ljungberg, B.; Axelson, H.; Dahlbäck, B. The miR21/10b ratio as a prognostic marker in clear cell renal cell carcinoma. Eur. J. Cancer 2014, 50, 1758–1765. [Google Scholar] [CrossRef]
- Vergho, D.C.; Kneitz, S.; Kalogirou, C.; Burger, M.; Krebs, M.; Rosenwald, A.; Spahn, M.; Löser, A.; Kocot, A.; Riedmiller, H.; et al. Impact of miR-21, miR-126 and miR-221 as Prognostic Factors of Clear Cell Renal Cell Carcinoma with Tumor Thrombus of the Inferior Vena Cava. PLoS ONE 2014, 9, e109877. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, Z.; Pan, D.; Zhang, W.; Xu, L.; Zhu, Y.; Liu, H.; Xu, J. Tumor mi R-125b predicts recurrence and survival of patients with clear-cell renal cell carcinoma after surgical resection. Cancer Sci. 2014, 105, 1427–1434. [Google Scholar] [CrossRef]
- Cai, Y.; Li, H.; Zhang, Y. Downregulation of microRNA-206 suppresses clear cell renal carcinoma proliferation and invasion by targeting vascular endothelial growth factor A. Oncol. Rep. 2016, 35, 1778–1786. [Google Scholar] [CrossRef]
- Lou, N.; Ruan, A.-M.; Qiu, B.; Bao, L.; Xu, Y.-C.; Zhao, Y.; Sun, R.-L.; Zhang, S.-T.; Xu, G.-H.; Ruan, H.-L.; et al. miR-144-3p as a novel plasma diagnostic biomarker for clear cell renal cell carcinoma. Urol. Oncol. 2017, 35, 36.e7–36.e14. [Google Scholar] [CrossRef]
- Trevisani, F.; Ghidini, M.; Larcher, A.; Lampis, A.; Lote, H.; Manunta, P.; Alibrandi, M.T.S.; Zagato, L.; Citterio, L.; Dell’Antonio, G.; et al. MicroRNA 193b-3p as a predictive biomarker of chronic kidney disease in patients undergoing radical nephrectomy for renal cell carcinoma. Br. J. Cancer 2016, 115, 1343–1350. [Google Scholar] [CrossRef]
- Nofech-Mozes, R.; Khella, H.W.Z.; Scorilas, A.; Youssef, L.; Krylov, S.N.; Lianidou, E.; Sidiropoulos, K.G.; Gabril, M.; Evans, A.; Yousef, G.M. Micro RNA-194 is a Marker for Good Prognosis in Clear Cell Renal Cell Carcinoma. Cancer Med. 2016, 5, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Van der Aa, M.N.; Zwarthoff, E.C.; Steyerberg, E.W.; Boogaard, M.W.; Nijsen, Y.; van der Keur, K.A.; van Exsel, A.J.; Kirkels, W.J.; Bangma, C.; van der Kwast, T.H. Microsatellite Analysis of Voided-Urine Samples for Surveillance of Low-Grade Non-Muscle-Invasive Urothelial Carcinoma: Feasibility and Clinical Utility in a Prospective Multicenter Study (Cost-Effectiveness of Follow-Up of Urinary Bladder Cancer Trial [CEFUB]). Eur. Urol. 2009, 55, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Margulis, V. Tumour and patient factors in renal cell carcinoma—Towards personalized therapy. Nat. Rev. Urol. 2015, 12, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Eisen, T.; Sternberg, C.N.; Robert, C.; Mulders, P.; Pyle, L.; Zbinden, S.; Izzedine, H.; Escudier, B. Targeted Therapies for Renal Cell Carcinoma: Review of Adverse Event Management Strategies. J. Natl. Cancer Inst. 2012, 104, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zeng, J.; Tang, K.; Xiao, H.; Hu, J.; Huang, C.; Yao, W.; Yu, G.; Xiao, W.; Guan, W.; et al. miR-490-5p suppresses tumour growth in renal cell carcinoma through targeting PIK3CA. Biol. Cell 2016, 108, 41–50. [Google Scholar] [CrossRef]
- Xiao, H.; Xiao, W.; Cao, J.; Li, H.; Guan, W.; Guo, X.; Chen, K.; Zheng, T.; Ye, Z.; Wang, J.; et al. miR-206 functions as a novel cell cycle regulator and tumor suppressor in clear-cell renal cell carcinoma. Cancer Lett. 2016, 374, 107–116. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, L.; Orecchia, L.; Giulioni, C.; Carbonara, U.; Tavella, G.; Lizzio, L.; Fimognari, D.; De Palma, A.; Gheza, A.; Grosso, A.A.; et al. The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature. Appl. Sci. 2023, 13, 275. https://doi.org/10.3390/app13010275
Napolitano L, Orecchia L, Giulioni C, Carbonara U, Tavella G, Lizzio L, Fimognari D, De Palma A, Gheza A, Grosso AA, et al. The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature. Applied Sciences. 2023; 13(1):275. https://doi.org/10.3390/app13010275
Chicago/Turabian StyleNapolitano, Luigi, Luca Orecchia, Carlo Giulioni, Umberto Carbonara, Giovanni Tavella, Leonardo Lizzio, Deborah Fimognari, Antonio De Palma, Alberto Gheza, Antonio Andrea Grosso, and et al. 2023. "The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature" Applied Sciences 13, no. 1: 275. https://doi.org/10.3390/app13010275
APA StyleNapolitano, L., Orecchia, L., Giulioni, C., Carbonara, U., Tavella, G., Lizzio, L., Fimognari, D., De Palma, A., Gheza, A., Grosso, A. A., Falagario, U., Parodi, S., Fasulo, V., Romantini, F., Rosiello, G., Viganò, S., Rabito, S., Ceccato, T., Pinelli, M., ... Flammia, R. S. (2023). The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature. Applied Sciences, 13(1), 275. https://doi.org/10.3390/app13010275







