Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
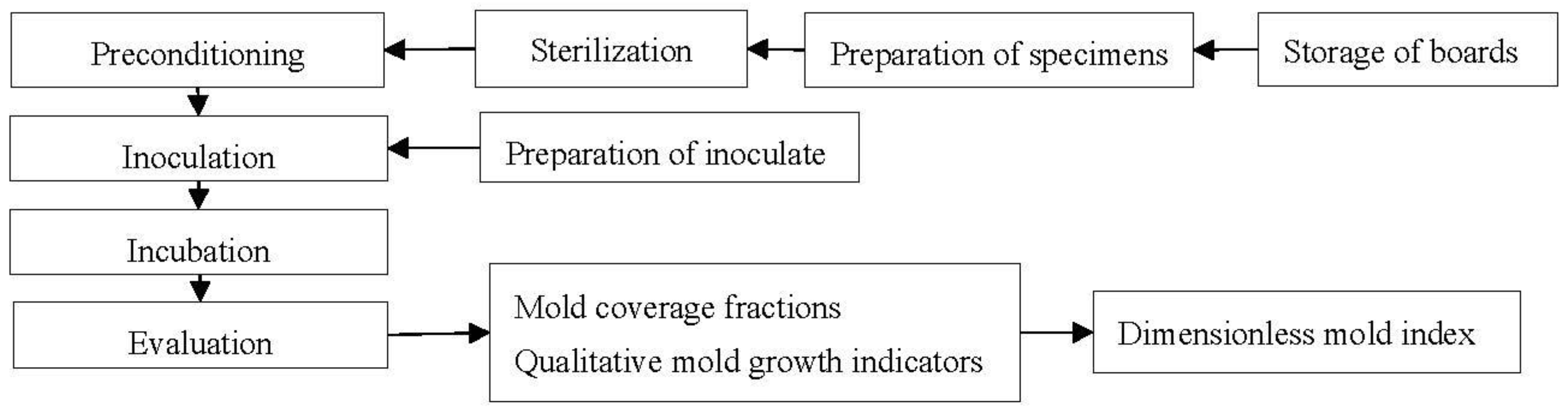
2.1. Experimental Procedure
2.2. Wood Specimens
2.3. Preparation of Inoculate
2.4. Main Experiment
2.4.1. Preconditioning of Specimens
2.4.2. Inoculation
2.4.3. Incubation
2.4.4. Evaluation of Mold Growth
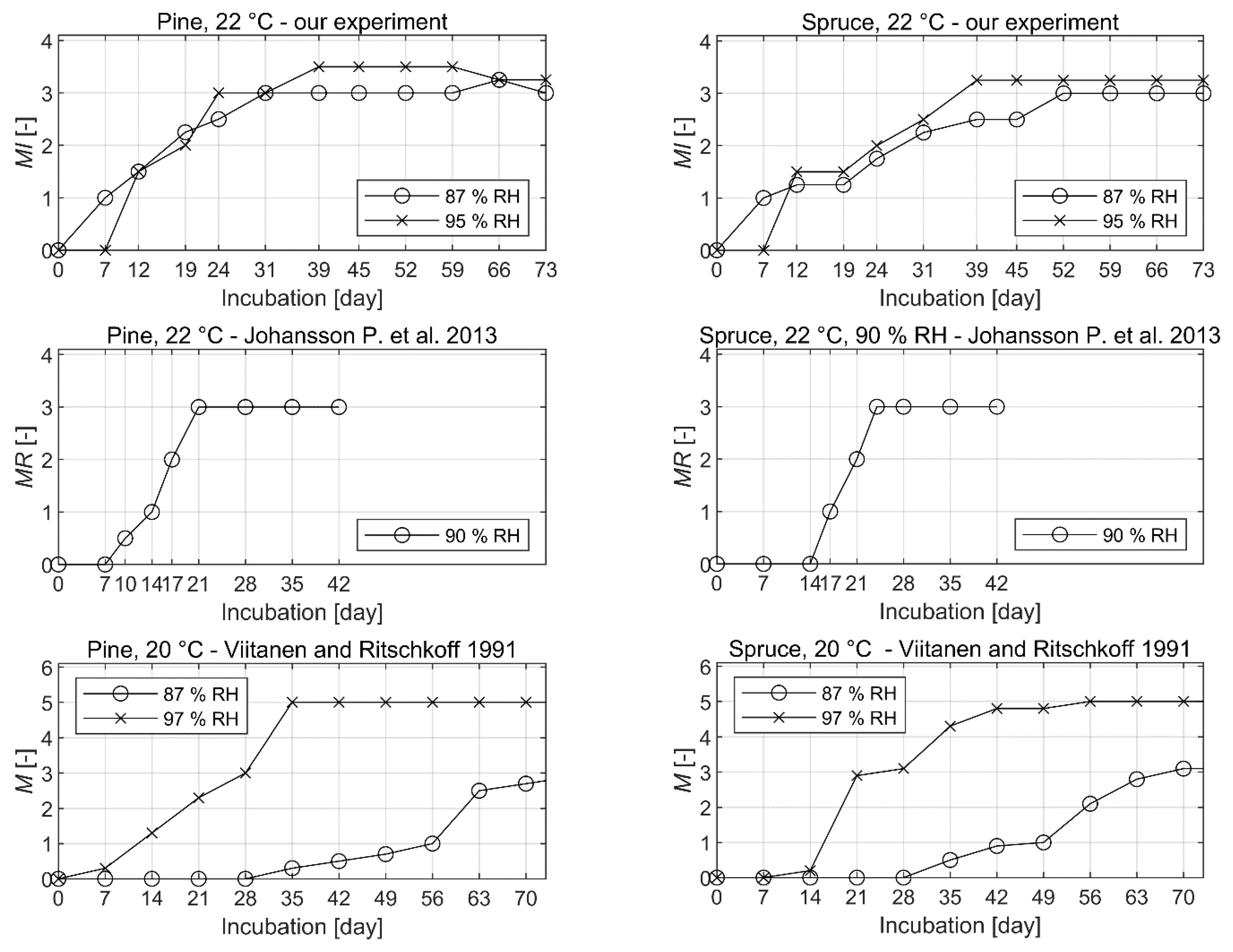
3. Results
4. Discussion
4.1. The Experimental Results
4.2. Comparison to Other Mold Growth Studies
5. Conclusions
- The differences of mold growth between anatomical sections were relatively small and are not considered to be significant.
- The time shift between mold growth curves was observed between the experiment performed and the published mold growth experiments. It is believed that the discrepancy is to some extent related to differences among experimental protocols. Scientific mold growth studies would be improved if a common standardized methodology were to be developed and followed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Selected Microscopic Images


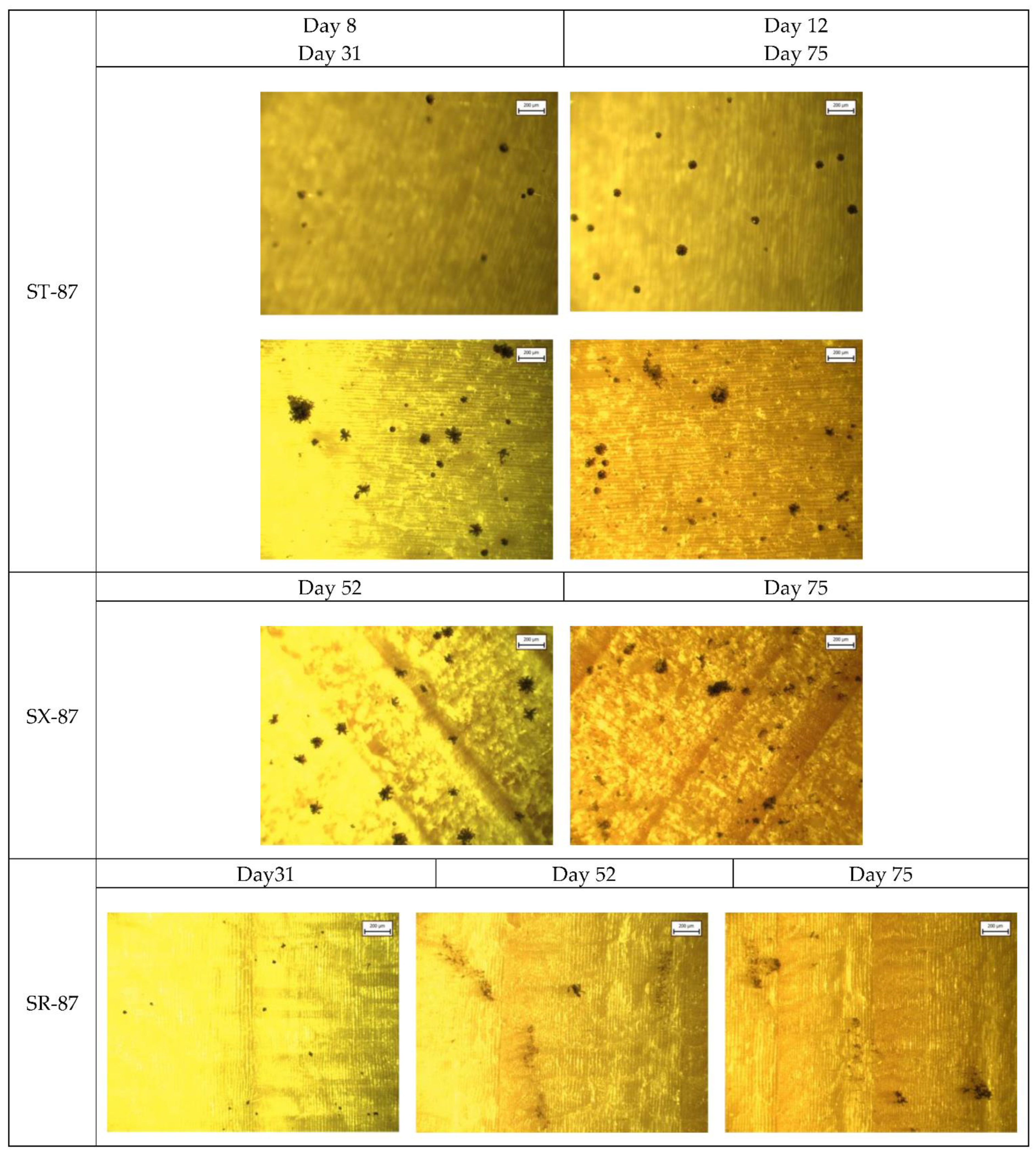
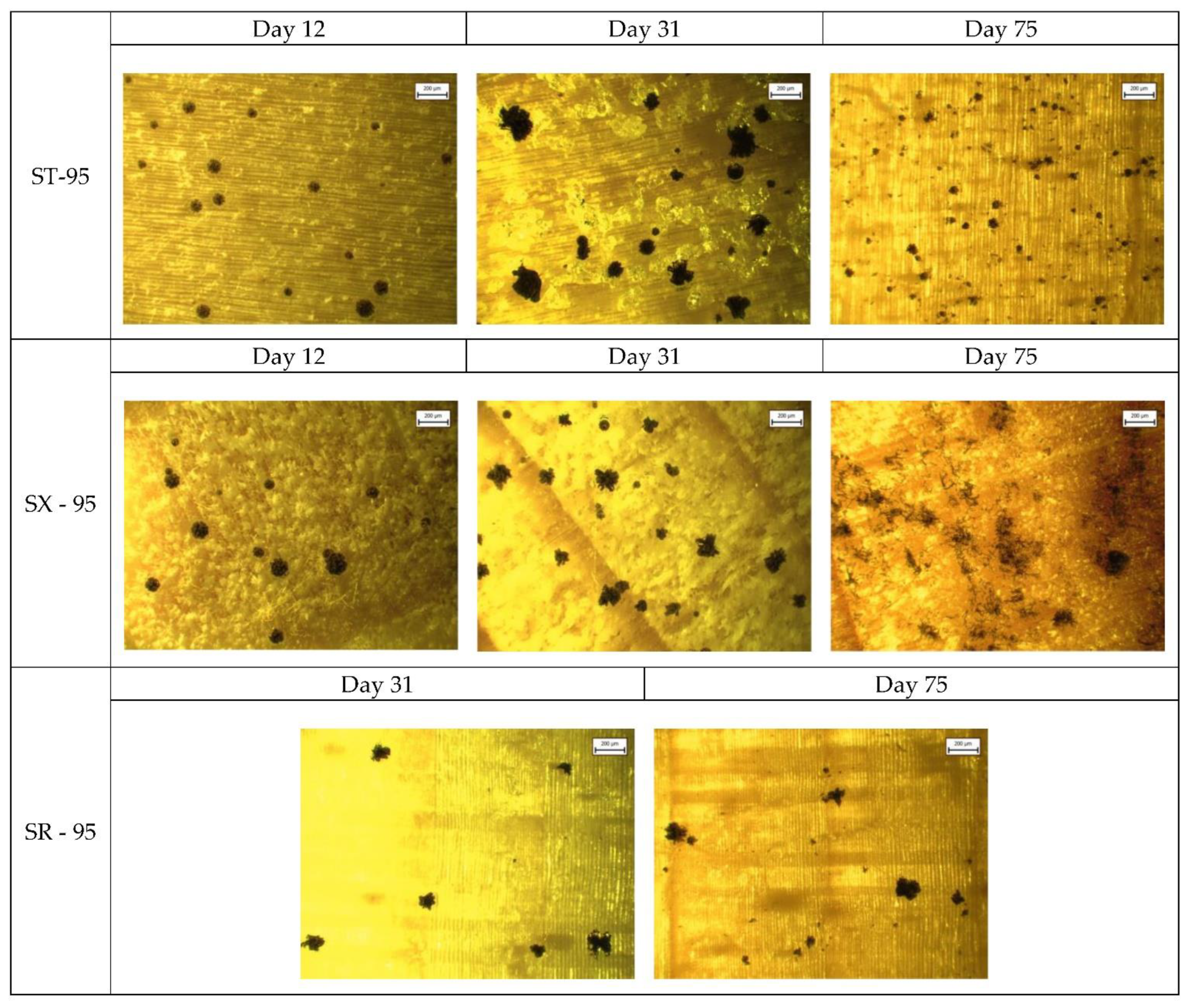
References
- Koziróg, A.; Otlewska, A.; Piotrowska, M.; Rajkowska, K.; Nowicka-Krawczyk, P.; Hachułka, M.; Wolski, G.J.; Gutarowska, B.; Kunicka-Styczyńska, A.; Libudzisz, Z.; et al. Colonising organisms as a biodegradation factor affecting historical wood materials at the former concentration camp of Auschwitz II-Birkenau. Int. Biodeterior. Biodegrad. 2014, 86, 171–178. [Google Scholar] [CrossRef]
- Choidis, P.; Kraniotis, D.; Lehtonen, I.; Hellum, B. A Modelling Approach for the Assessment of Climate Change Impact on the Fungal Colonization of Historic Timber Structures. Forests 2021, 12, 819. [Google Scholar] [CrossRef]
- Ingebretsen, S.B.; Andenæs, E.; Gullbrekken, L.; Kvande, T. Microclimate and Mould Growth Potential of Air Cavities in Ventilated Wooden Façade and Roof Systems—Case Studies from Norway. Buildings 2022, 12, 1739. [Google Scholar] [CrossRef]
- Isaksson, T.; Thelandersson, S.; Ekstrand-Tobin, A.; Johansson, P. Critical conditions for onset of mould growth under varying climate conditions. Build. Environ. 2010, 45, 1712–1721. [Google Scholar] [CrossRef]
- Hukka, A.; Viitanen, H.A. A mathematical model of mould growth on wooden material. Wood Sci. Technol. 1999, 33, 475–485. [Google Scholar] [CrossRef]
- Gradeci, K.; Labonnote, N.; Time, B.; Köhler, J. Mould growth criteria and design avoidance approaches in wood-based materials—A systematic review. Constr. Build. Mater. 2017, 150, 77–88. [Google Scholar] [CrossRef]
- Vereecken, E.; Roels, S. Review of mould prediction models and their influence on mould risk evaluation. Build. Environ. 2012, 51, 296–310. [Google Scholar] [CrossRef]
- Feng, J.; Li, C.; Chen, J.; Chen, M.; Shu, X.; Shi, Q. Evaluation of the association between natural mold resistance and chemical components of nine wood species. BioResources 2018, 13, 6524–6541. [Google Scholar] [CrossRef]
- Sehlstedt-Persson, M.; Karlsson, O.; Wamming, T.; Morén, T. Mold Growth on Sapwood Boards Exposed Outdoors: The Impact of Wood Drying. For. Prod. J. 2011, 61, 170–179. [Google Scholar] [CrossRef]
- Terziev, N.; Boutelje, J. Effect of Felling Time and Kiln-Drying on Color and Susceptibility of Wood to Mold and Fungal Stain During an Above-Ground Field Test. Wood Fiber Sci. 1998, 4, 360–367. [Google Scholar]
- Johansson, P.; Ekstrand-Tobin, A.; Svensson, T.; Bok, G. Laboratory study to determine the critical moisture level for mould growth on building materials. Int. Biodeterior. Biodegrad. 2012, 73, 23–32. [Google Scholar] [CrossRef]
- Johansson, P.; Bok, G.; Ekstrand-Tobin, A. The effect of cyclic moisture and temperature on mould growth on wood compared to steady state conditions. Build. Environ. 2013, 65, 178–184. [Google Scholar] [CrossRef]
- Viitanen, H.; Salonvaara, M. Moisture Conditions and Biodeterioration Risk Of Building Materials and Structure. Indoor Air no. Figure 1. 2015, pp. 1001–1006. Available online: https://www.irbnet.de/daten/iconda/CIB5413.pdf (accessed on 15 April 2018).
- Nielsen, K.F. Mould Growth on Building Materials. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2002. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/2988232/phd-kfn.pdf (accessed on 15 April 2018).
- Nielsen, K.; Holm, G.; Uttrup, L.; Nielsen, P. Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism. Int. Biodeterior. Biodegrad. 2004, 54, 325–336. [Google Scholar] [CrossRef]
- Segers, F.J.J.; van Laarhoven, K.A.; Huinink, H.P.; Adan, O.C.G.; Wösten, H.A.B.; Dijksterhuis, J. The Indoor Fungus Cladosporium halotolerans Survives Humidity Dynamics Markedly Better than Aspergillus niger and Penicillium rubens despite Less Growth at Lowered Steady-State Water Activity. Appl. Environ. Microbiol. 2016, 82, 5089–5098. [Google Scholar] [CrossRef]
- Viitanen, H.A. Modelling the Time Factor in the Development of Mould Fungi—The Effect of Critical Humidity and Temperature Conditions on Pine and Spruce Sapwood. Holzforschung 1997, 51, 6–14. [Google Scholar] [CrossRef]
- Johansson, P.; Mjörnell, K.; Arfvidsson, J. Examples of characteristics of wood that affect mould growth: A meta-analysis. Eur. J. Wood Wood. Prod. 2016, 75, 603–613. [Google Scholar] [CrossRef]
- Viitanen, H.; Ritschkoff, A.-C. Mould Growth on Pine and Spruce Sapwood in Relation to Air Humidity and Temperature; Rapport nr. 221; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1991. [Google Scholar]
- Gobakken, L.R.; Høibø, O.A.; Solheim, H. Mould growth on paints with different surface structures when applied on wooden claddings exposed outdoors. Int. Biodeterior. Biodegrad. 2010, 64, 339–345. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, H.; Jang, Y.; Cho, Y.; Kim, G.-H.; Kim, J.-J. Phylogenetic analysis of major molds inhabiting woods. Part 4. Genus Alternaria. Holzforschung 2014, 68, 247–251. [Google Scholar] [CrossRef]
- Ryparová, P.; Rácová, Z. The Occurrence of Mold in Construction Materials before Inbuilt into New Building and Protection against this Type of Biodegradation. Key Eng. Mater. 2016, 714, 44–50. [Google Scholar] [CrossRef]
- Viitanen, H. Factors affecting the development of biodeterioration in wooden constructions. Mater. Struct. 1994, 27, 483–493. [Google Scholar] [CrossRef]
- Johansson, P.; Lång, L.; Capener, C.-M. How well do mould models predict mould growth in buildings, considering the end-user perspective? J. Build. Eng. 2021, 40, 102301. [Google Scholar] [CrossRef]
- Yang, D.-Q. Isolation of wood-inhabiting fungi from Canadian hardwood logs. Can. J. Microbiol. 2005, 51, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, E.; Vanoirbeek, K.; Roels, S. Towards a more thoughtful use of mould prediction models: A critical view on experimental mould growth research. J. Build. Phys. 2015, 39, 102–123. [Google Scholar] [CrossRef]
- Adan, O. On the Fungal Defacement of Interior Finishes. Ph.D. Thesis, University of Eindhoven, Eindhoven, The Netherlands, 1994. [Google Scholar]
- Johansson, P. Determination of the Critical Moisture Level for Mould Growth on Building Materials. Ph.D. Thesis, Lund University, Lund, Sweden, 2014. [Google Scholar]
- Imken, A.A.; Brischke, C.; Kögel, S.; Krause, K.C.; Mai, C. Resistance of different wood-based materials against mould fungi: A comparison of methods. Eur. J. Wood Wood Prod. 2020, 78, 661–671. [Google Scholar] [CrossRef]


| Tangential Surface (T) | Radial Surface (R) | Transversal Surface (X) | |||
|---|---|---|---|---|---|
| Pine (P) | Spruce (S) | Pine (P) | Spruce (S) | Pine (P) | Spruce (S) |
 |  |  | |||
| PT-75 | ST-75 | PR-75 | SR-75 | PX-75 | SX-75 |
| PT-87 | ST-87 | PR-87 | SR-87 | PX-87 | SX-87 |
| PT-95 | ST-95 | PR-95 | SR-95 | PX-95 | SX-95 |
| Mold Index [-] | Subindex | Observation and Coverage [%] | Description | |
|---|---|---|---|---|
| 0 | Micro | Macro | No mold growth is present. | |
| 1 | 1.0 | <10% | 0% | Sparsely distributed sporangia, hyphae difficult to observe. |
| 1.5 | 10–30% | |||
| 2 | – | >30% | >0% | Sporangia and hyphae observable. |
| 3 | 3.0 | 100% | <30% | Full microscopic coverage, the first macroscopic signs. |
| 3.5 | 30–70% | |||
| 4 | – | >70% | Macroscopic coverage is substantial. | |
| Microscopic | MI = 1 | MI = 1.5 | MI = 2 |
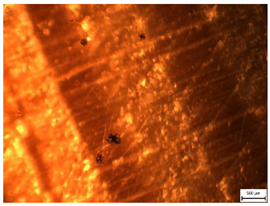 |  | 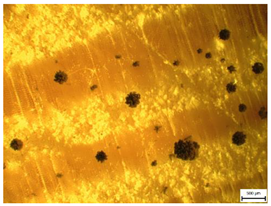 | |
| MI = 3 | MI = 3.5 | MI = 4 | |
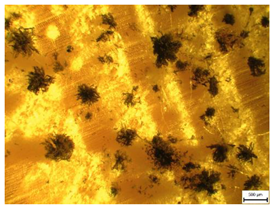 |  |  | |
| Macroscopic | MI = 3 | MI = 3.5 | MI = 4 |
 |  |  |
| Species | Pine | Spruce | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RH [%] | 87 | 95 | 87 | 95 | ||||||||
| Specimens | PT-87 | PX-87 | PR-87 | PT-95 | PX-95 | PR-95 | ST-87 | SX-87 | SR-87 | ST-95 | SX-95 | SR-95 |
| MI = 1 [day] | 7 | 7 | 7 | 12 | 7 | 12 | 7 | 19 | 7 | 12 | 19 | 12 |
| MI = 3 [day] | 24 | 24 | 31 | 19 | 31 | 24 | 31 | 52 | 52 | 24 | 31 | 39 |
| Max MI [day] (MI value)] | 66 (4) | 45 (3) | 31 (3) | 39 (4) | 39 (4) | 24 (3) | 31 (3) | 52 (3) | 52 (3) | 24 (3) | 31 (3) | 39 (3.5) |
| Decay [day] | - | 59 | 66 | - | 66 | 66 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryparová, P.; Kopecký, P.; Staněk, K.; Richter, J.; Tywoniak, J. Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces. Appl. Sci. 2023, 13, 228. https://doi.org/10.3390/app13010228
Ryparová P, Kopecký P, Staněk K, Richter J, Tywoniak J. Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces. Applied Sciences. 2023; 13(1):228. https://doi.org/10.3390/app13010228
Chicago/Turabian StyleRyparová, Pavla, Pavel Kopecký, Kamil Staněk, Jan Richter, and Jan Tywoniak. 2023. "Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces" Applied Sciences 13, no. 1: 228. https://doi.org/10.3390/app13010228
APA StyleRyparová, P., Kopecký, P., Staněk, K., Richter, J., & Tywoniak, J. (2023). Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces. Applied Sciences, 13(1), 228. https://doi.org/10.3390/app13010228








