Natural Stones with a Self-Cleaning Surface via Self-Assembled Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterizations
3. Results
3.1. Water Wettability of PDA
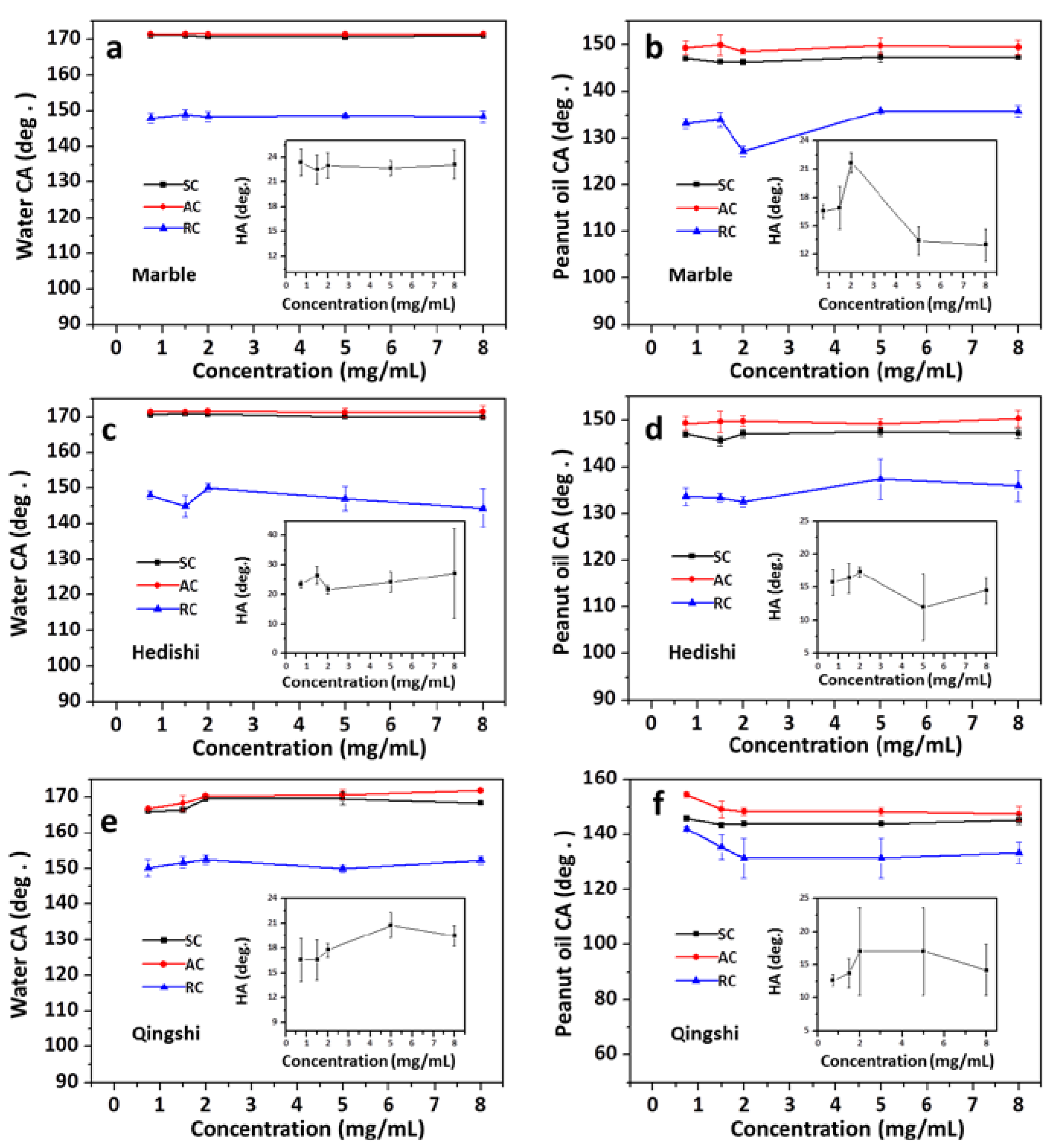
3.2. Impact of PDA Layers on Water/Oil Wettability of the Stone Surfaces
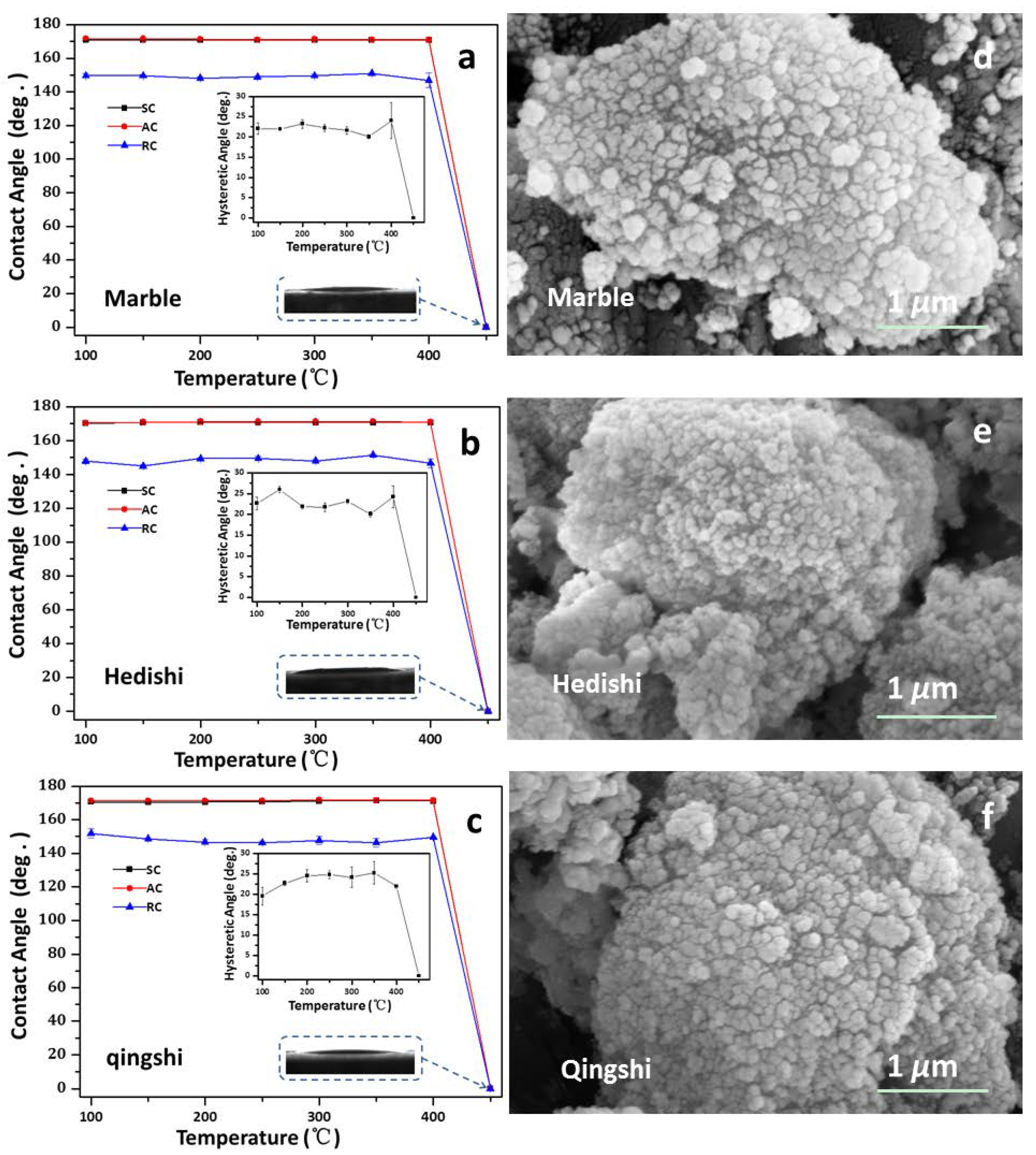
3.3. Thermal Stability and Durability
4. Discussion
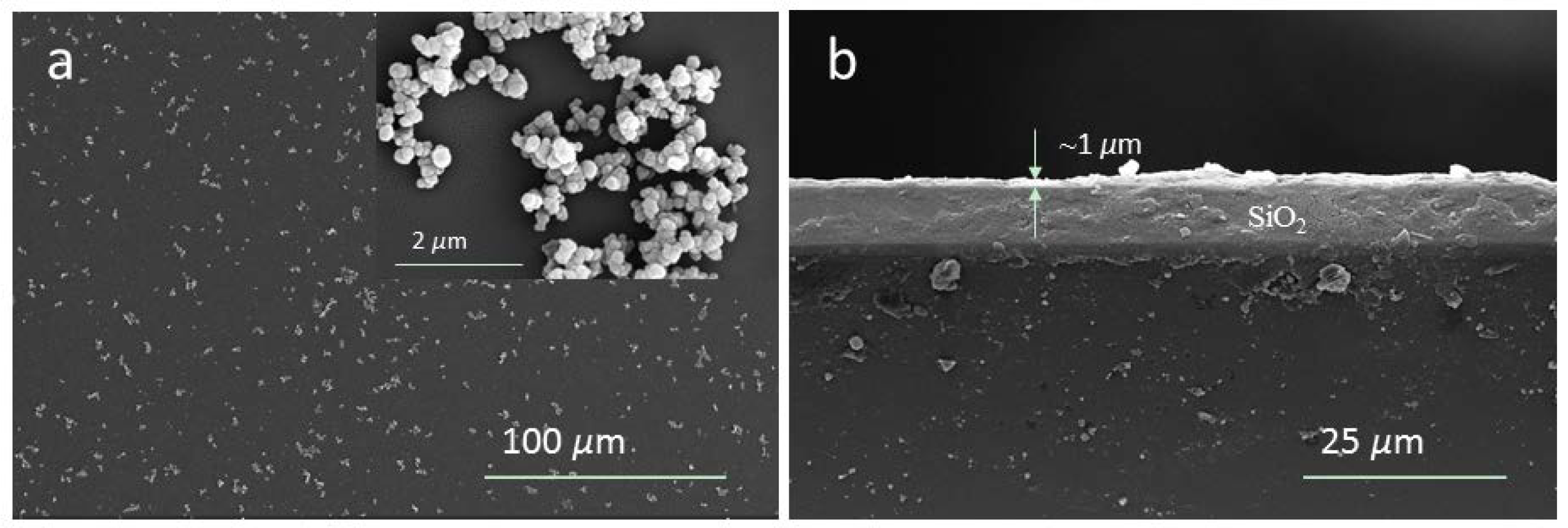
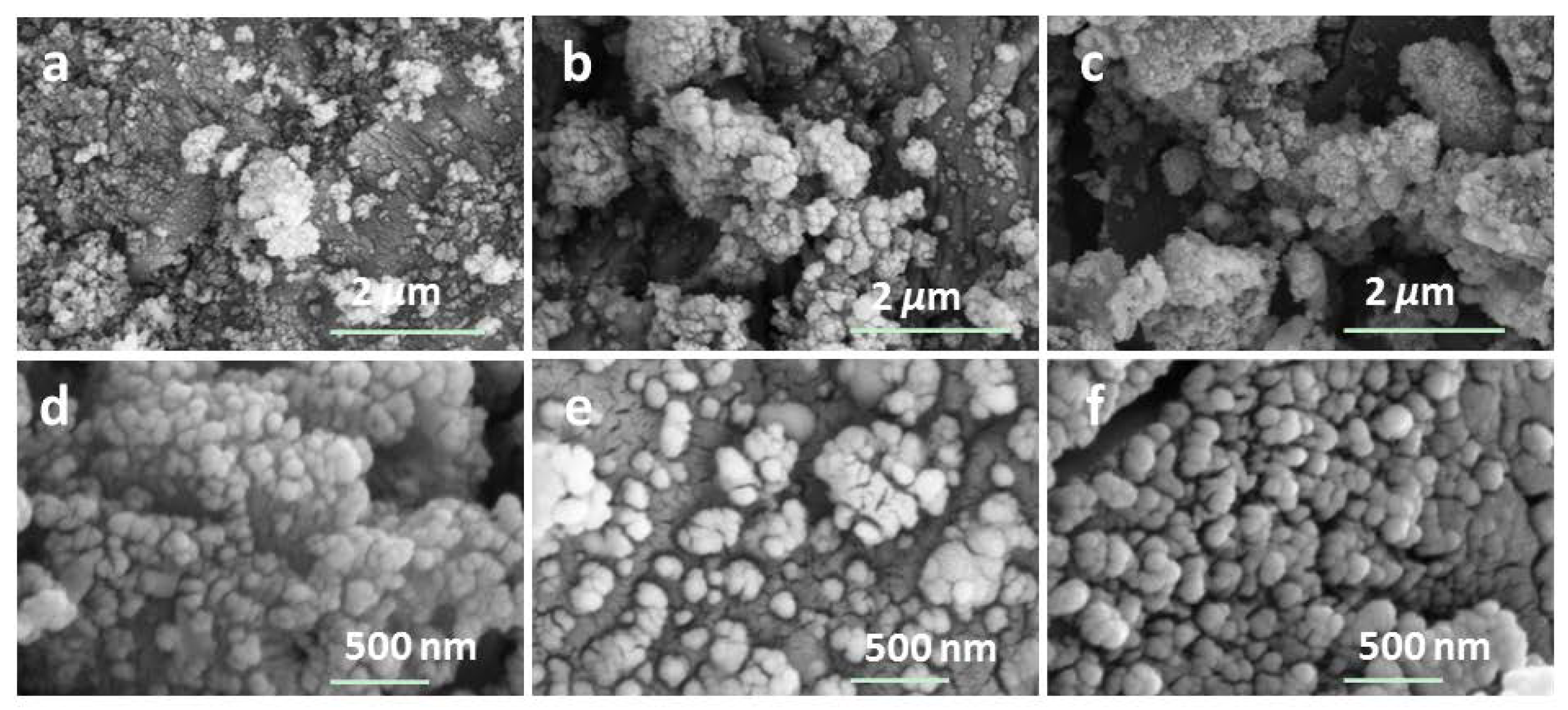
4.1. Growth Mechanism of PDA Layer
4.2. Mechanism of the Liquid Wettability
4.3. Thermal Stability and Durability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The Preservation Damage of Hydrophobic Polymer Coating Materials in Conservation of Stone Relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Doherty, B.; Pamplona, M.; Selvaggi, R.; Miliani, C.; Matteini, M.; Sgamellotti, A.; Brunetti, B. Efficiency and Resistance of the Artificial Oxalate Protection Treatment on Marble Against Chemical Weathering. Appl. Surf. Sci. 2007, 253, 4477–4484. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Porcinai, S. Evaluation of the Application Conditions of Artificial Protection Treatments on Salt-Laden Limestones and Marble. Constr. Built. Mater. 2011, 25, 2723–2732. [Google Scholar] [CrossRef]
- Patankar, N.A. Mimicking the Lotus Effect: Influence of Double Roughness Structures and Slender Pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stabil. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Tsakalof, A.; Manoudis, P.; Karapanagiotis, I.; Chryssoulakis, I.; Panayiotou, C. Assessment of synthetic polymeric coatings for the protection and preservation of stone monuments. J. Cult. Herit. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stabil. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part II: Photo-oxidative and salt-induced weathering of acrylic–silicone mixtures. Polym. Degrad. Stabil. 2007, 92, 335–351. [Google Scholar] [CrossRef]
- Castelvetro, V.; Aglietto, M.; Ciardelli, F.; Chiantore, O.; Lazzari, M.; Toniolo, L. Structure control, coating properties and durability of fluorinated acrylicbased polymer. J. Coat. Technol. 2002, 74, 57–66. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- van Hees, R.P.J.; Brocken, H.J.P. Damage development to treated brick masonry in a long-term salt crystallisation test. Constr. Build. Mater. 2004, 18, 331–338. [Google Scholar] [CrossRef]
- Manoudis, P.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Javaheriannaghash, H.; Ghazavi, N. Preparation and characterization of water-based polyurethane–acrylic hybrid nanocomposite emulsion based on a new silane-containing acrylic macromonomer. J. Coat. Technol. Res. 2012, 9, 323–336. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus or escape from contamination in biological surfaces. Planta 1997, 1, 1–8. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 6, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Lazzarini, L.; Salvadori, O. A reassessment of the formation of the patina called scialbatura. Stud. Conserv. 1989, 34, 20–26. [Google Scholar] [CrossRef]
- Tang, X.; Yu, F.; Guo, W.; Wang, T.; Zhang, Q.; Zhu, Q.; Zhang, X.; Pei, M. A facile procedure to fabricate nano calcium carbonate–polymer-based superhydrophobic surfaces. N. J. Chem. 2014, 38, 2245–2249. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, X.; Gao, Y.; Shi, F.; Zhang, P.; Chen, J. A facile method to prepare superhydrophobic coatings by calcium carbonate. Ind. Eng. Chem. Res. 2011, 50, 3089–3094. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, Z.; Zhao, Z.; Li, R.; Zhou, B.; Yang, D.; Hu, Y. Nano-materials enhanced protectants for natural stone surfaces. Herit. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Deng, R.; Hu, Y.; Wang, L.; Li, Z.; Shen, T.; Zhu, Y.; Xiang, J. An easy and environmentally-friendly approach to superamphiphobicity of aluminum surfaces. Appl. Surf. Sci. 2017, 402, 301–307. [Google Scholar] [CrossRef]
- Zhao, Z.E.; Sun, S.H.; Hu, Y.M.; Zhu, Y. Robust superamphiphobic aluminum surfaces: Fabrication and investigation. J. Coat. Technol. Res. 2019, 16, 1707–1714. [Google Scholar] [CrossRef]
- Guvendiren, M.; Messersmith, P.; Shull, K. Self-Assembly and Adhesion of DOPA-Modified Methacrylic Triblock Hydrogels. Biomacromolecules 2008, 9, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.; Toniazzo, V.; Ruch, D. Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Chen, H.; Zhang, S.; Qiao, L. Application and development of polydopamine functional materials with its usage on environmental protection. J. Yanshan Univ. 2018, 42, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Xi, Z.; Xu, Y.; Zhu, L.; Wang, Y.; Zhu, B. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Kim, H.; McCloskey, B.; Choi, T.; Lee, C.; Kim, M.; Freeman, B.; Park, H. Oxygen Concentration Control of Dopamine-Induced High Uniformity Surface Coating Chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.; Miller, W.; Messersmith, P. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Wu, H.; Tao, S.; Gao, M.; Ou, J. Mechanism and influencing factors for the formation of polydopamine. Jiangxi Chem. Ind. 2017, 4, 4–10. [Google Scholar] [CrossRef]
- Bulusu, A.; Paniagua, S.; MacLeod, B.; Sigdel, A.; Berry, J.; Olson, D.; Marder, S.; Graham, S. Efficient Modification of Metal Oxide Surfaces with Phosphonic Acids by Spray Coating. Langmuir 2013, 29, 3935–3942. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, R.; Zhang, X.; Zhu, Y.; Nie, H. Aluminium films roughened by hot water treatment and derivatized by fluoroalkyl phosphonic acid: Wettability studies. Surf. Eng. 2020, 36, 589–600. [Google Scholar] [CrossRef]
- Wenzel, R. Resistance of solid surface to wetting by water. J. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R. Surface roughness and contact angle. Phys. Coll. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surface. Transm. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.; Mazzella, S.; Rutledge, G.; McKinley, G.; Cohen, R. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]


| Dopamine Concentration (mg/mL) | Static Water CA (°) | |||
|---|---|---|---|---|
| Glass | Marble | Hedishi | Qingshi | |
| 0 | 57.8 ± 2.3 | 52.8 ± 1.9 | 45.3 ± 0.8 | 48.1 ± 1.5 |
| 0.5 | 5.9 ± 0.9 | 10.9 ± 1.6 | 11.5 ± 0.9 | 13.2 ± 0.7 |
| 1.0 | 4.1 ± 0.8 | 10.0 ± 0.6 | 12.0 ± 0.9 | 13.2 ± 1.3 |
| 1.5 | 3.1 ± 0.1 | 11.1 ± 1.3 | 12.0 ± 1.1 | 12.6 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Xie, Z.; Zhou, B.; Yang, X.; Nie, H.-Y.; Hu, Y. Natural Stones with a Self-Cleaning Surface via Self-Assembled Monolayers. Appl. Sci. 2022, 12, 4771. https://doi.org/10.3390/app12094771
Duan Z, Xie Z, Zhou B, Yang X, Nie H-Y, Hu Y. Natural Stones with a Self-Cleaning Surface via Self-Assembled Monolayers. Applied Sciences. 2022; 12(9):4771. https://doi.org/10.3390/app12094771
Chicago/Turabian StyleDuan, Zhuoqi, Zaixin Xie, Bao Zhou, Xiaobo Yang, Heng-Yong Nie, and Yongmao Hu. 2022. "Natural Stones with a Self-Cleaning Surface via Self-Assembled Monolayers" Applied Sciences 12, no. 9: 4771. https://doi.org/10.3390/app12094771
APA StyleDuan, Z., Xie, Z., Zhou, B., Yang, X., Nie, H.-Y., & Hu, Y. (2022). Natural Stones with a Self-Cleaning Surface via Self-Assembled Monolayers. Applied Sciences, 12(9), 4771. https://doi.org/10.3390/app12094771






