Abstract
Drought is a major environmental constraint, affecting agricultural productivity worldwide. Allelopathic hormesis, the low-dose stimulatory effect of allelochemicals, offers a pragmatic solution in alleviating the adverse effects of drought in plants. This study, therefore, is conducted to evaluate the potential of a brassica water extract (BWE) in enhancing drought tolerance in wheat. The experiment was based on three factors, viz, drought with three levels (100%, 60% and 30% field capacity; FC), different concentrations of a brassica water extract (control, water spray, 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%) and two wheat cultivars, Ihsan-2016 (drought tolerant) and Galaxy-2013 (drought-sensitive). Drought stress, particularly at 30% FC, decreased the morpho-physiological attributes of both wheat cultivars; nevertheless, the application of brassica water extract, particularly at 2.0%, effectively enhanced tolerance against drought stress. Compared with the control, the application of 2.0% brassica water extract increased the morphological attributes, such as seedling length and the fresh and dry weights of both wheat cultivars in the range of 2–160% under 30% field capacity. In addition, the 2.0% brassica water extract triggered the activities of antioxidant enzymes, including superoxide dismutase, catalase and peroxidase (11–159%), decreased the hydrogen peroxide content (14–30%) and enhanced chlorophyll a and b and carotenoid contents (19–154%), as compared to the control, in both wheat cultivars under 30% field capacity. The vigorous growth and higher drought tolerance in wheat cultivars with brassica water extract application were related to improved chlorophyll contents and physiological attributes, a better antioxidant defense system and a reduced H2O2-based damaging effect.
1. Introduction
Among abiotic stresses, drought is one of the major factors that cause injurious effects to wheat growth and productivity by manipulating its physiological pathways [1]. Wheat (Triticum aestivum L.) is considered as an important food and cereal crop in the world. Its cultivation has been extended to arid regions to possibly assist in addressing future food security issues in problematic zones where water shortage is the greatest predicament in diminishing wheat crop [2].
Drought stress causes oxidative stress and cellular damage [3], diminished carbon assimilation [4], reduced photosynthesis through the lessening of the synthesis of photosynthetic pigments [5] and limits water retention at the cellular, tissue, and organ levels [1], thereby reducing dry matter production and seed yield [6,7]. It also disturbs the balance between reactive oxygen species (ROS) and antioxidant enzymes [8], which subsequently leads to electrolyte leakage, the deactivation of various enzymes and membrane damage [9]. Additionally, it has been reported to affect metabolic processes, such as carbon assimilation, turgor pressure and gas exchange, and ultimately cause crop failure [10].
In recent years, different agronomic and physiological approaches have been used to alleviate the adverse effect of drought stress, which include the application of osmoprotectants, growing drought-tolerant varieties and practicing sustainable tillage using natural and synthetic mulches as well as allelopathic crop water extracts. The application of plant hormones has been reported as an emerging and easy-to-adapt strategy for enhancing crop performance under stress conditions [11]. In addition, the use of allelopathic crop water extracts also provides a bio-safe and eco-sustainable alternative for crop enhancement under stress [12]. The effect of the application of allelochemicals, either for growth stimulation or retardation, is dose-dependent. Hormesis is described as the low-dose stimulation and high-dose inhibition effect of allelochemicals [13]. Brassica napus, a well-known and widely grown species of the brassica family, is considered as a potential allelopathic crop owing to the presence of potent allelochemicals, such as brassinosteroids, allyl isothiocyanates and glucosinolates [14]. Brassinosteroids (BRs) are polyhydroxy steroidal phytohormones that have a great potential to enhance plant growth [15]. Brassinolide (BL), 28-homobrassinolide (28-HomoBL) and 24-epibrassinolide (24-EpiBL) are the most important classes of BRs [16]. Brassinosteroids (BRs), a discovered class of plant hormones, can play a vital role in various physiological and biochemical processes in plants, such as cell division, cell elongation and expansion, photosynthesis, the synthesis of nucleic acid and proteins, membrane polarization, proton pump activation, ions uptake and transport into the cells and enzyme activation [17]. The exogenous application of BRs through a foliar spray is reported to enhance tolerance to abiotic stresses, including heat, salinity and drought [18,19]. Under abiotic stresses, the foliar application of BRs modulates physiological and biochemical functions and allows plants to withstand oxidative stress [20]. The authors of [21] documented that BRs improve the developmental and physiological traits under stress. According to [22], BRs also ameliorate oxidative damage through reducing the accumulation of ROS and enhancing the activity of antioxidant enzymes. The authors of [23] reported that the application of brassinolide improves the growth of wheat under moisture deficit conditions.
The exogenous application of 24-epibrassinolide, an active form of BRs, ameliorates the drought-induced inhibition of photosynthesis through positively regulating the activity of chloroplast, chlorophyll fluorescence and photosynthetic pigments; the accumulation of osmoprotectants; and the increase in photosynthetic enzyme activity [24]. Under drought stress, EBL application can boost plant photosynthetic pigments, osmolyte production, antioxidant enzyme activities and seed yield [25]. During the past few years, it has been well established that BWE application improves seedling growth and physiological and biochemical traits under stress conditions; however, the positive influence was highly dose-dependent [26]. Therefore, the present study is conducted to optimize the concentration of brassica water extracts for hormetic promotion as well as to establish the morphological, physiological and biochemical bases for drought tolerance under the influence of brassica water extract hormesis.
2. Materials and Methods
2.1. Experimental Site and Plant Material
The pot study was conducted in the warehouse of the Faculty of Agriculture, University of Agriculture, Faisalabad (Latitude = 31°44′ N, longitude = 73°06′ E, Altitude = 184.4 m), during the winter season in 2018. A sandy loam soil, with pH of 8.4, 0.43 dSm−1 Ec, 0.77% nitrogen, 104 ppm exchangeable K, 120 ppm phosphorus and 0.9% of organic matter, was used as pot filling. The seeds of the chosen wheat cultivars, Ihsan-2016 (a commonly cultivated variety in arid areas) and Galaxy-2013 (cultivar used in irrigated areas) were collected from Barani Agricultural Research Institute, Chakwal and Ayub Agricultural Research Institute, Faisalabad, respectively.
2.2. Preparation of the Brassica Water Extract
The brassica water extract was prepared following the protocol of [27]. Firstly, brassica herbage was harvested at maturity, chopped with a fodder cutter into 2–3 cm pieces and then shade-dried to avoid rainwater-induced leaching losses. Next, the chopped material was soaked in distilled water (with 1:10 w/v ratio) for 24 h at room temperature (21 ± 2 °C) and then boiled at 100 °C to reduce the volume (up to 95%) for easy handling and application. The extract was then sieved and packed as 100% (stock solution) of brassica water extract, which was further used to prepare the various concentrations as per treatments. Foliar application was conducted at 45 days after sowing (DAS) with the help of a knapsack hand sprayer fitted with a T-jet nozzle with 207 kPa of pressure.
2.3. Experimental Design and Treatments
The study was based on three factors: three levels of drought stress (100%, 60% and 30% field capacity), various concentrations of brassica water extract (control, water spray, 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%) and two contrasting wheat cultivars, drought-tolerant Ihsan-2016 and the drought-sensitive Galaxy-2013. The sowing of wheat was performed under uniform soil moisture conditions and the foliar application of brassica water extract and drought stress were imposed at 45 DAS. After treatment implementation, soil moisture was determined on a daily basis with the help of a soil moisture meter (TZS-W, Zhejiang, China), and water losses were compensated by adding water to achieve the described level of field capacity in the respective treatment. Leaf samples from each pot were collected after 15 days of stress imposition for the determination of the biochemical and physiological attributes. The concentration of brassica water extract that revealed significantly higher morphological growth attributes was compared with the control in terms of photosynthetic pigments and oxidative metabolism in the wheat seedlings. The study was conducted in a glasshouse where the treatments were arranged under a complete randomized design with a factorial arrangement and each treatment was repeated three times.
2.4. Crop Husbandry
The seeds of both wheat cultivars were sown during the first week of October 2018 in plastic pots that measured 20 cm × 20 cm, and 5 kg of soil was used to fill each pot. The recommended dose of fertilizers (80:58:35 NPK mg kg−1) in the form of urea, DAP and SOP was used against the recommendation rate (160:100:60 kg ha−1) of wheat crop at the time of sowing. Seeds (15 per pot) of both wheat cultivars were sown at an equal distance, and a uniform stand was maintained by keeping ten seedlings per pot after the completion of emergence. All other practices, except for treatments, were kept uniform throughout the experimentation.
2.5. Data Recorded
2.5.1. Morphological Growth
Plant morphological characteristics were recorded at 60 DAS (15 days after stress applied). Five plants from each pot were uprooted to measure the seedling length and their fresh and dry weights. Shoot and root lengths were measured with the help of a meter rod, and the fresh weights were recorded using a digital weighing balance (TX323L, Shimadzu, Japan). The samples were oven-dried at 70 °C for 3 days and the dry weights were recorded.
2.5.2. Photosynthetic Pigments
The chlorophyll a and b and carotenoid contents were determined following the procedure of [28]. Leaf sample (0.5 g) from each treatment was homogenized with 8 mL of 80% acetone (v/v) and then filtered using filter paper. The absorbance of the filtrate solution was recorded using a spectrophotometer (UV-4000, ORI, Hille, Germany) at 663, 645 and 480 nm wavelengths for the chlorophyll a and b and carotenoid contents, respectively.
2.5.3. Physiological Attributes
Physiological attributes, i.e., the rate of photosynthesis (A), transpiration rate (E), internal carbon dioxide concentration (Ci) and stomatal conductance (gs), were recorded at 60 DAS using a photosynthesis system (Lci, 4225).
2.5.4. Enzymatic Antioxidants and H2O2 Content
Leaf samples were harvested at 60 DAS, kept in a disposable zipper bag and stored at −80 °C for the determination of antioxidant enzyme activity. The activity of SOD was measured by following the procedure of [29]. For this, 200 mg leaf sample was homogenized in 2 mL of extraction buffer (0.5 mM EDTA + 0.1 M phosphate pH 7.5) with precooled mortar and pestle. The homogenate was centrifuged at 10,000 rmp at 4 °C and the supernatant was stored at 4 °C. SOD activity in the supernatant was assayed by its ability to inhibit the photochemical reduction of nitrobluetetrazolium. A 3 mL assay mixture, containing 0.2 mL of 200 mM methionine + 1.5 M sodium carbonate + 0.1 mL 3 mM EDTA + 0.1 mL 2.25 mM NBT + 0.1 mL riboflavin (60 µM) + 1.5 mL 100 mM potassium phosphate buffer + 1 mL distilled water and 0.1 mL of enzyme, was incubated under two 15 W inflorescent lamps for 15 min; illuminated and nonilluminated reactions without supernatant served as calibration. The absorbance of the samples along with the blank were recorded at 560 nm wavelength in a spectrophotometer (UV-4000, ORI, Hille, Germany). One unit of enzyme activity was defined as the quantity of enzyme that reduced the absorbance reading of samples to 50% in comparison with tubes lacking enzymes (supernatant) and was calculated as follows.
where ∗ sample without enzyme, # sample with enzyme.
Fresh leaf samples (0.5 g) were ground in a 5 mL of 50 mM phosphate buffer (pH 7.8) with the help of a pestle and mortar. The homogenates were centrifuged at 15,000 rpm for 20 min at 4 °C. The supernatant was used to assess the peroxidase and catalase activity. POD activity was determined by following procedure of [30] with slight modifications. The reaction mixture contained 10 mM guaiacol + 5 mM H2O2 and 50 mM phosphate buffer (pH 7.0). The reaction mixture was preheated at 20 °C in a water bath. Then, 2.8 mL reaction solution + 0.2 Ml enzyme was added in 10 mL centrifuged tube and mix thoroughly. Absorbance was recorded with blank and with reaction mixture. Four absorbance readings were recorded at 470 nm wavelength with 1 min time interval using spectrophotometer (UV-4000, ORI, Hille, Germany).
where V, Vt, T and W are the volume of the sample solution (mL), the measured liquid volume (mL), the measurement time and the fresh weight of the sample, respectively.
Catalase activity in fresh leaves was measured following the method of [31] with slight modification. A total of 100 Mm H2O2 + 50 mM phosphate buffer (pH 7.8) solution was preheated in water bath at 25 °C. In a 10 mL tube, 0.2 mL phosphate buffer + 0.2 mL enzyme solution was added and preheated in water bath for 3 min. Then, 0.3 mL (100 mM H2O2) solution was added in 10 mL tube. The control tube was heated in a boiling water bath for 5 min to kill the enzyme solution. After mixing, the absorbance at the 240 nm wavelength was calculated at interval of 1 min. With continuous determination for 4 min, 1 unit of enzyme activity (U) was considered as a decrease of 0.1 of A240 within 1 min.
where V, Vt, T and W are the volume of the sample solution (mL), the measured liquid volume (mL), the measurement time and the fresh weight of the sample, respectively. H2O2 contents were determined with the help of the procedure described by [32]. A total of 500 mg of fresh leaves were homogenized with 5 mL of 0.1% (w/v) trichloroacetic acid in a pre-chilled pestle and mortar. The homogenate was centrifuged at 12,000 rpm for 15 min. Then, 0.5 mL supernatant was mixed in 0.5 mL of 0.05 M phosphate buffer (pH 7.0) + 1 mL of 1 M potassium iodide. The mixture vortexed and absorbance was recorded at 390 nm using water as blank. The same steps were followed to make a standard curve by preparing different dilutions of hydrogen peroxide.
2.6. Statistical Analysis
Data collected were analyzed statistically using the analysis of variance technique with the help of SPSS (Analytical Software, Tallahassee, FL, USA), and differences among treatment means were compared using Tukey’s Honest Significant Difference (HSD) test at 5% probability level [33]. The calculation, graphical representation and correlation matrix of data were performed with Microsoft Excel, 2016, (Redmond, Washington, DC, USA), SigmaPlot version 10.0 (Systat Inc., San Jose, CA, USA), and R-software version 4.0.3 (R Studio, Boston, MA, USA), respectively.
3. Results
3.1. Morphological Growth
Shoot and root length as well as the fresh and dry weights of both wheat cultivars decreased with increasing drought levels (Table 1 and Table 2). The interactive effect of drought and brassica water extracts (BWE) on all morphological attributes, except for the shoot dry weight, was non-significant (p ≤ 0.05) for both wheat cultivars. Ihsan-2016 showed a decrease of 24–12% in shoot length, 43–19% in root length, 44–19% in shoot fresh weight, 56–23% in root fresh weight, 50–14% in shoot dry weight and 54–26% in root dry weight when grown under 30% and 60% FC, respectively, as compared with 100% FC. The respective decrease in these attributes of Galaxy-2013 were 24–10%, 46–21%, 44–18%, 62–30%, 49–23% and 58–28% under 30% and 60% FC as compared with 100% FC (Table 1 and Table 2). Nevertheless, the foliar application of BWE was effective in alleviating the detrimental effect of drought stress on the seedling growth of wheat cultivars, where maximum effectiveness was recorded for 2.0% BWE application (Table 1). Averaging both cultivars, the application of 2.0% BWE enhanced shoot length by 16, 18 and 19%, root length by 39, 45 and 74%, shoot fresh weight by 24, 31 and 29%, root fresh weight by 50, 83 and 151%, shoot dry weight by 39, 62 and 46% and root dry weight by 34, 38 and 73% under 100, 60 and 30% FC, respectively, as compared with the control (no BWE application). Nonetheless, such effects of BWE were more evident on the shoot growth of Ihsan-2016 and the root growth of Galaxy-2013 (Table 2).

Table 1.
Summary of c regarding the effect of foliar application of the brassica water extracts on morphological, physiological and biochemical attributes in the two wheat cultivars under different drought levels.

Table 2.
Effect of drought and foliar application of the brassica water extracts on the morphological traits of the wheat cultivars.
3.2. Photosynthetic Pigments
The chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents of both wheat cultivars decreased with increasing drought levels (Figure 1; Table 1). The interactive effect of drought and BWE for all photosynthetic traits were non-significant (p ≤ 0.05) for both wheat cultivars (Table 1). Compared to 100% FC, the chlorophyll a and b, total chlorophyll and carotenoid contents decreased by 59 and 30%, 46 and 19%, 55 and 27%, and 57 and 27% at 30% and 60% FC, respectively, in Ihsan-2016 (Figure 1; Table 1). In Galaxy-2013, the chlorophyll a content decreased by 51 and 37%, chlorophyll b by 44 and 21%, total chlorophyll by 49 and 33% and carotenoid by 61 and 30% at 30% and 60% FC, respectively, as compared to 100% FC. Averaging both cultivars, the application of 2% BWE enhanced the chlorophyll a content by 54, 90 and 139%; chlorophyll b by 90, 108 and 141%; total chlorophyll by 41, 58 and 82%; and carotenoid by 47, 69 and 112% at 100, 60 and 30% FC, respectively, as compared with the control (no BWE application) (Figure 1; Table 1). The results reveal that BWE was more effective in enhancing chlorophyll a and b and the total chlorophyll contents under severe drought (30% FC) as compared with mild-drought (60% FC) and well-watered conditions (100% FC). The foliar spray of 2.0% BWE enhanced the chlorophyll content a by 55 and 59%, chlorophyll b by 94 and 86%, total chlorophyll by 64 and 65%, and carotenoid by 48 and 47% at 100% FC in Ihsan-2016 and Galaxy-2013, respectively (Figure 1).
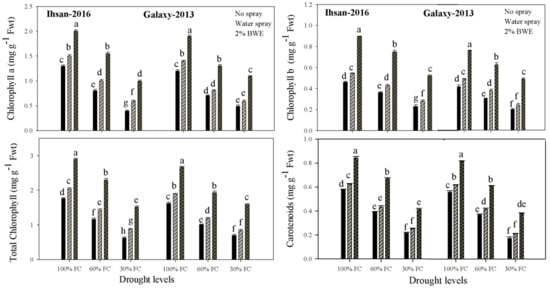
Figure 1.
Effect of drought stress and foliar application of the brassica water extracts on chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents of the two wheat cultivars. Bars with different small letters differ significantly at 5% probability, according to Tukey’s HSD test.
3.3. Physiological Attributes
Drought treatments significantly (p ≤ 0.05) altered the photosynthetic rate (A), transpiration rate (E), internal carbon dioxide concentration (Ci) and stomatal conductance (gs) of both wheat cultivars (Table 1 and Table 3). The interactive effect of drought and BWE on these attributes, except for Ci, was significant for both cultivars (p ≤ 0.05). There was a significant decline of 53 and 21% in PN, 49 and 19% in E, 38 and 18% in Ci and 65 and 16% in gs in the leaves of Ihsan-2016 when grown at 30 and 60% FC, respectively, as compared to 100% FC. The corresponding decrease in these attributes for Galaxy-2013 was 57 and 21%, 46 and 21%, 42 and 20%, and 53 and 23% at 30 and 60% FC, as compared to 100% FC (Table 3). Nonetheless, the foliar application of BWE significantly (p ≤ 0.05) enhanced the gaseous exchange attributes in both wheat cultivars exposed to drought stress, where the maximum increase was recorded for 2% BWE application. Averaged across both cultivars, the application of 2.0% BWE enhanced PN by 73, 103 and 71%, E by 61, 82 and 59%, Ci by 45, 59 and 57%, and gs by 70, 120 and 83%, at 100, 60 and 30% FC, respectively, as compared with the control, indicating that the effect of BWE was more pronounced when the seedlings were subjected to severe drought (30% FC). The BWE-induced enhancement of these attributes, except for E, were more prominent in Ihsan-2016 as compared to Galaxy-2013 (Table 3).

Table 3.
Effect of drought and foliar application of the brassica water extracts on physiological attributes.
3.4. Antioxidant Enzymes
Drought stress and BWE application significantly (p ≤ 0.05) affected the activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) of both wheat cultivars (Figure 2; Table 1). In addition, their interactions also significantly (p ≤ 0.05) influenced the activities of antioxidant enzymes (except for SOD) in both wheat cultivars. The exposure of Ihsan-2016 and Galaxy-2013 to 60% FC enhanced the activity of SOD by 37 and 41%, POD by 41 and 51% and CAT by 31 and 33%, respectively, as compared with activities under 100% FC. A further increase in water deficit (30% FC) resulted in a significant decrease in the activities of SOD by 48 and 53%, POD by 39 and 46% and CAT by 43 and 58% in Ihsan-2016 and Galaxy-2013, respectively, as compared to 100% FC (Figure 2).
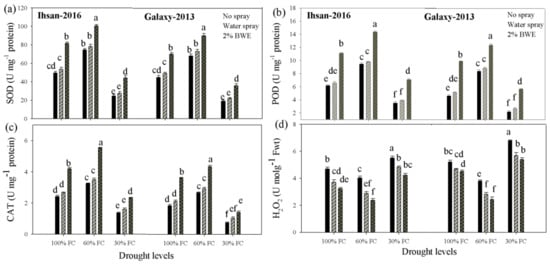
Figure 2.
Effect of drought and foliar application of the brassica water extracts on the activity of superoxide dismutase (a), peroxidase (b), catalase (c) and hydrogen peroxide (d) in the two wheat cultivars. Bars with different small letters differ significantly at 5% probability according to Tukey’s HSD test.
Nonetheless, the foliar application of BWE enhanced the activities of antioxidant enzymes under well-watered (100% FC) and drought stress conditions. Averaging both cultivars, the application of 2% BWE enhanced the activity of SOD by 61, 33 and 84%, POD by 96, 49 and 130% and CAT by 85, 67 and 81% at 100, 60 and 30% FC, respectively, as compared with the control treatment without BWE (Figure 2).
3.5. Hydrogen Peroxide (H2O2)
Hydrogen peroxide (H2O2) is produced under drought stress due to the incomplete reduction of oxygen to form water. In this experiment, the interactive effect of drought stress and BWE on H2O2 content was non-significant (p ≤ 0.05) in both wheat cultivars (Figure 2; Table 1). The H2O2 content in leaves of both wheat cultivars increased by 25 and 24% at 30% FC as compared with 100% FC. The foliar application of 2% BWE was helpful in reducing the H2O2 content under normal as well as drought stress, and the maximum decrease was recorded for 2.0% BWE, which decreased the H2O2 content by 30, 62 and 28% at 100, 60 and 30% FC, respectively, as compared with the control treatment without BWE application (Figure 2).
3.6. Correlation Analysis
The Pearson’s correlation analysis was conducted to assess the possible relationship/s among the different morphological, physiological and biochemical attributes of both wheat cultivars when grown under varying drought levels and subjected to the foliar application of the BWEs (Figure 3). All observed traits were positively correlated, except H2O2, which was negatively associated with the scavenging enzymatic and seedling growth attributes. Nonetheless, the activities of antioxidant enzymes were positively correlated with morphological as well as physiological attributes (Figure 3).
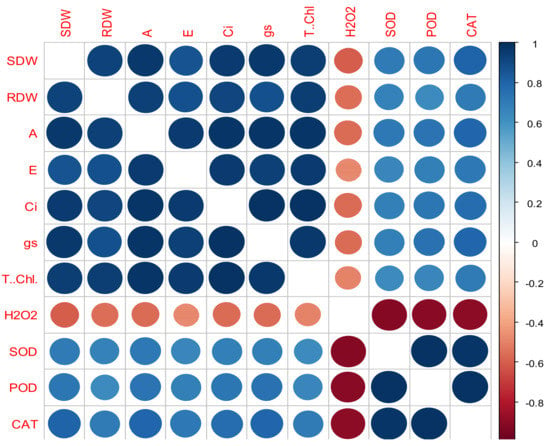
Figure 3.
Correlation between the morpho-physiological and biochemical traits of the wheat cultivars under different drought levels. SDW: shoot dry weight; RDW: root dry weight; A: photosynthesis rate; E: transpiration rate; Ci: internal carbon dioxide concentration; gs: stomatal conductance; T. Chl.: total chlorophyll; H2O2: hydrogen peroxide; SOD: superoxide dismutase; POD: peroxidase; CAT: catalase.
4. Discussion
4.1. Drought Stress and Plant Performance
Drought is one of the major abiotic stresses that causes injurious effects on the growth and productivity of wheat crops throughout the world [34]. Water deficit conditions induce changes in the morpho-physiological and yield-related traits of crop plants by increasing the production of ROS and causing oxidative damage and hormonal imbalance [35,36]. It also damages the cellular membrane [37] and encourages ABA biosynthesis and its binding to the receptor to initiate the signal transduction that may lead to the closure of stomata [38]. Drought inhibits chlorophyll synthesis and mineral uptake by the roots and their transport to above ground parts, which ultimately results in reducing seed yield, owing to less assimilate portioning [4]. At 30 and 60% FC, drought stress caused a marked reduction in morphological traits, mainly attributed to a reduction in cell division, elongation and expansion [39]. Similar to these findings, our results also showed a severe decline in shoot fresh and dry weights under moderate- to severe-drought stress (60% and 30% FC); this decline may also cause injurious effects to physiological traits. Moreover, similarly, a significant decline in photosynthetic rate and the activity of photosynthetic enzymes and pigments under drought stress was also observed by [40].
The production of ROS is a fundamental process to transmit cellular signals in plants upon exposure to stress [41]. However, under drought stress, the equilibrium between ROS and the antioxidant defense system is disturbed, inducing oxidative damage to cellular organelles [42]. In our study, under severe and moderate drought (30 and 60% FC), a significant reduction in antioxidant enzyme activities may lead to the enhanced over-accumulation of ROS (i.e., H2O2) that damages cellular organelles. Moreover, drought stress is also reported to hamper the shoot biomass that might lead to oxidative damage and curtails the efficiency of the photosynthetic system [43].
4.2. Foliar Application of the Brassica Water Extracts Lessened the Detrimental Effects of Drought
The application of 2.0% BWE reduced the damaging effect of drought by promoting morpho-physiological and antioxidant activities. Brassinosteroids, as a group of plant steroid hormones, can mediate various physiological processes in plants [44] and mitigate the adverse effects of drought stress [45]. The results of the present study reveal that BWE, particularly when applied with 2% concentration, augmented the morphological attributes in wheat; however, growth traits in both cultivars were reduced when BWE was applied at a concentration of above 2.0%. Under drought conditions, the improved morphological attributes due to BWE application could be linked with the brassinosteroid-mediated regulation of cell division, elongation, photomorphogenesis and xylem differentiation [46]. Moreover, our results also show that the foliar application of BWE enhanced shoot biomass in wheat, showing that these findings are in line with a previous report by [47], in which the authors reported that BRs, under BWE application, modulated the growth and development of crop plants.
The brassica water extracts modulated physiological processes by enhancing the activity of photosynthetic pigments. The chlorophyll contents decreased as the thylakoid membrane disintegrates upon cellular dehydration [48]. Under drought conditions, higher photosynthetic pigments, through the application of 24-eppibrassinolide, were reported previously by [49]. The results of the current study depict that drought stress hindered physiological functioning, such as the photosynthetic rate, internal carbon dioxide concentration and stomatal conductance, which could be linked to a reduced uptake of CO2 and a restricted chloroplast activity in the modulation of the photosynthetic process [50]; however, the foliar application of 2% BWE augmented physiological functioning, owing to the 24-eppibrassinolide-mediated improvement of leaf water status, photosynthetic activity, and the antioxidant defense system [51]. BWE reduced the damaging effect of ROS through accelerating the activity of antioxidant enzymes, including SOD, POD and CAT. Similarly, the foliar application of BWE has also been reported to enhance the activities of antioxidant enzymes, reduce H2O2 contents, mainly due to the BR-based scavenging action of ROS [52], and reduced drought-induced oxidative stress, due to the presence of 24-eppibrassinolide [53].
4.3. Drought Tolerance through Enhanced Physiological Attributes under the Foliar Application of Brassica Water Extracts
Water deficit conditions hinder the rate of carbon assimilation, reduce cell turgidity and cause oxidative damage [10,54]. Drought stress also caused a marked reduction in the photosynthesis process due to stomatal closure under the limited intake of CO2 to the mesophyll tissues [55]. Nevertheless, the exogenous application of BWE improved the gaseous exchange parameters, including net photosynthesis, under drought stress. These findings are in line with the previously reported results of [56], who showed that the application of BRs significantly and positively mediated intercellular CO2 concentrations and net photosynthetic rate under stress conditions. Transpiration rates are influenced negatively under water deficit conditions through the reduction in leaf size and increase in leaf dropping [57]. However, BWE application significantly increased the plant growth due to BR modulation under stress conditions as reported by [56]. In our work, enhanced drought tolerance was not only associated with more stomatal conductance, but also with modulated CO2 fixation, which ultimately causes increased photosynthetic rates.
Reduction in stomatal conductance is one of the main leaf-scale, foremost and short-term physiological responses that appear under drought conditions [58]. BWE application significantly promoted physiological functioning through the BRs-modulated delayed time of senescence under stress [59]. Moreover, the exogenous application of BRs also alleviates photoinhibition by triggering the efficiency of photosystem II (PSII) under stress conditions [60].
4.4. Brassica Water Extracts Strengthened the Antioxidant Defense System in Wheat under Drought Stress
A balance between ROS and antioxidant enzymes stabilizes the redox homeostasis through an efficient defense system [61]. Under drought stress, oxidative stress causes enhanced ROS production and restricted photosynthetic rates [8], retards the synthesis of nucleic acids and proteins, produces lipid peroxidation and finally cell death [62]. In this study, severe drought stress (30% FC) reduced the activity of antioxidant enzymes and enhanced the production of H2O2. However, the foliar application of 2% BWE enhanced antioxidant enzyme activities under moderate drought, which showed a higher drought tolerance. However, reduced antioxidant enzyme activities in the control treatment without BWE application in wheat seedlings suggested a reduced capability of the wheat plants to combat drought-induced oxidative damage. In our work, improved antioxidant activities reduce ROS-induced damages as evident from the reduced H2O2 concentration under BWE application. The better efficiency of biochemical attributes was recognized with the presence of BRs under BWE application. These findings are consistent with the previous report of [63], who showed that 24-epibrassinolide, under BWE application, enhanced the activity of antioxidant enzymes and reduced oxidative damage in plants.
4.5. Performance of Wheat Cultivars in Response to Drought and Brassica Water Extract Application
Severe drought stress caused a prominent and significant reduction in the morpho-physiological and biochemical attributes of the drought-sensitive cultivar (Galaxy-2013) than in those of the tolerant one (Ihsan-2016). However, the effect of the foliar application of BWE was more evident in the drought-tolerant cultivar (Ihsan-2016) of wheat. Higher tolerance was associated with accelerated antioxidant enzyme activities due to the inherent ability of the ROS-based scavenging system under the foliar application of the brassica water extract. This indicates that the wheat cultivar Ihsan-2016 has more potential to accelerate growth and antioxidant production for drought tolerance.
5. Conclusions
Drought stress, particularly at 30% FC, negatively affected the seedling growth, photosynthetic pigments, gaseous exchange parameters and biochemical attributes of wheat. Nevertheless, the hormetic effect of the foliar application of the brassica water extracts, particularly at a 2.0% concentration, significantly alleviated the negative effects of drought stress through the modulation of physiological functioning. Moreover, the enhanced drought tolerance through the foliar application of the brassica water extract was associated with a higher seedling biomass, photosynthetic pigments, better physiological functioning and improved activities of antioxidant enzymes.
Author Contributions
Conceptualization, A.K., M.U.I., M.Z.U.H. and S.H.; methodology and software, A.K., M.U.I., A.A.A.-H. and M.H.S.; formal analysis, validation and data curation, S.H., A.A.A.-H., M.N. and M.H.S.; investigation, A.K., M.U.I. and M.H.S.; writing—original draft, A.K., M.U.I., M.H.S., M.Z.U.H. and S.H.; writing—review and editing A.A.A.-H., F.K., M.H.S., B.A. and H.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Researchers Supporting Project number (RSP-2021/186), King Saud University, Riyadh, Saudi Arabia.
Informed Consent Statement
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2021/186), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Ahmed, H.G.M.D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef]
- Kheir, A.M.; Alrajhi, A.A.; Ghoneim, A.M.; Ali, E.F.; Magrashi, A.; Zoghdan, M.G.; Abdelkhalik, S.A.; Fahmy, A.E.; Elnashar, A. Modeling deficit irrigation-based evapotranspiration optimizes wheat yield and water productivity in arid regions. Agric. Water Manag. 2021, 256, 107122. [Google Scholar] [CrossRef]
- Liu, H.; Sultan, M.A.R.F.; Liu, X.L.; Zhang, J.; Yu, F.; Zhao, H.X. Physiological and comparative proteomic analysis reveals different drought responses in roots and leaves of drought-tolerant wild wheat (Triticum boeoticum L.). PLoS ONE 2015, 10, 121–852. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, D.; Li, X.; Shi, Y.; Shao, Y.; Fang, B.; Cheng, H. Drought effects on photosynthetic performance of two wheat cultivars contrasting in drought. N. Z. J. Crop Hortic. Sci. 2021, 49, 17–29. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef] [Green Version]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navab, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Developing Climate-Resilient Chickpea Involving Physiological and Molecular Approaches with a Focus on Temperature and Drought Stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef]
- Talbi, S.; Romero-Puertas, M.C.; Hernández, A.; Terrón, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Farooq, M.; Nawaz, A. Seed priming with sorghum extracts and benzyl aminopurine improves the tolerance against salt stress in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2018, 24, 239–249. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Li, P. Utilizing the allelopathic potential of Brassica species for sustainable crop production: A review. J. Plant Growth Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Latha, P.; Vardhini, B.V. Effect of homobrassinolide on bio-chemical activities and chlorophyll pigments of mustard plants grown in semi-arid tropics of Nizamabad. Eur. J. Biomed. Pharm. Sci. 2017, 4, 613–618. [Google Scholar]
- Kahlaoui, B.; Misle, E.; Khaskhoussy, K.; Jaouadi, I.; Hachicha, M. Brassinosteroids and drought tolerance in plants. Water Stress Crop Plants Sustain. Approach 2016, 2, 600–607. [Google Scholar]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Zheng, B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chen, Z.Y.; Jiang, Y.; Duan, B.B.; Xi, Z.M. Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Brassinosteroids as a multidimensional regulator of plant physiological and molecular responses under various environmental stresses. Environ. Sci. Pollut. Res. 2021, 28, 44768–44779. [Google Scholar] [CrossRef]
- Ribeiro, D.S.D.G.; da Silva, B.R.S.; da Silva, L.A.K. Brassinosteroids induce tolerance to water deficit in soybean seedlings: Contributions linked to root anatomy and antioxidant enzymes. Acta Physiol. Plant. 2019, 41, 82. [Google Scholar] [CrossRef]
- Dehghan, M.; Balouchi, H.; Yadavi, A.; Zare, E. Improve wheat (Triticum aestivum L.) performance by brassinolide application under different irrigation regimes. S. Afr. J. Bot. 2020, 130, 259–267. [Google Scholar] [CrossRef]
- Talaat, N.B. 24-Epibrassinolide and Spermine combined treatment sustains maize (Zea mays L.) drought tolerance by improving photosynthetic efficiency and altering phytohormones profile. J. Soil Sci. Plant Nutr. 2020, 20, 516–529. [Google Scholar] [CrossRef]
- Zafari, M.; Ebadi, A.; Sedghi, M.; Jahanbakhsh, S.; Miransari, M.J. Alleviating effect of 24-epibrassinolide on seed oil content and fatty acid composition under drought stress in safflower. J. Food Compos. Anal. 2020, 92, 103544. [Google Scholar] [CrossRef]
- Farooq, O.; Ali, M.; Sarwar, N.; Rehman, I.M.M.; Naz, T.; Asghar, M.; Ehsan, F.; Nasir, M.; Hussain, Q.M.; Afzal, S. Foliar applied brassica water extract improves the seedling development of wheat and chickpea. Asian J. Agric. Biol. 2021, 1. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Khaliq, A. Use of sorghum allelopathic properties to control weeds in irrigated wheat in semi-arid region of Punjab. Agric. Ecosyst. Environ. 2000, 79, 105–112. [Google Scholar] [CrossRef]
- Arnon, D.T. Copper enzyme in isolated chloroplasts polyphenols oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.A.; Plumb-Dhindsa, P.; Thrope, T.A. Leaf senescence Correlated with increased permeability and lipid peroxidation and decreased levels of superoxide dimutase and catalase. J. Exp. Bot. 1981, 126, 93–101. [Google Scholar] [CrossRef]
- Putter, J. Peroxidases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie, Weinheim. Rade and Markets Division (EST); Elsevier: Rome, Italy, 1974; pp. 685–690. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co. Inc.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- He, J.X.; Wang, J.; Liang, H.G. Effects of water stress on photochemical function and protein metabolism of photosystem II in wheat leaves. Physiol. Plant. 2010, 93, 771–777. [Google Scholar] [CrossRef]
- Asghar, H.N.; Zahir, Z.A.; Akram, M.A.; Ahmad, H.T.; Hussain, M.B. Isolation and screening of beneficial bacteria to ameliorate drought stress in wheat. Soil Environ. 2015, 34, 100–110. [Google Scholar]
- Miao, Y.; Zhu, Z.; Guo, Q.; Ma, H.; Zhu, L. Alternate wetting and drying irrigation-mediated changes in the growth, photosynthesis and yield of the medicinal plant Tulipa edulis. Ind. Crop. Prod. 2015, 66, 81–88. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.H.P.J.C.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Jourdan, N.F.; Martino, C.; El-Esawi, M.; Witczak, J.; Bouchet, P.E.; d’Harlingu, A.; Ahmad, M. Blue-light dependent ROS formation by Arabidopsis cryptochrome-2 may contribute toward its signaling role. Plant Signal. Behav. 2015, 10, 1042647. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Ashraf, M.A.; Anwar, F.; Rasheed, R.; Niaz, M.; Wahid, A. Biochemical characterization of maize (Zea mays L.) for salt tolerance. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2014, 148, 1016–1026. [Google Scholar]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Peres, A.L.G.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef]
- Liu, S.; Che, Z.; Chen, G. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Da Fonseca, S.S.; da Silva, B.R.S.; Lobato, A.K.D.S. 24-Epibrassinolide positively modulate leaf structures, antioxidant system and photosynthetic machinery in rice under simulated acid rain. J. Plant Growth Regul. 2020, 39, 1559–1576. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef] [Green Version]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Aarrouf, J.; Bidel, L.P. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ. Sci. Pollut. Res. 2022, 29, 10183–10197. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef]
- De Assis-Gomes, M.D.M.; Pinheiro, D.T.; Bressan-Smith, R.; Campostrini, E. Exogenous brassinosteroid application delays senescence and promotes hyponasty in Carica papaya L. leaves. Theor. Exp. Plant Physiol. 2018, 30, 193–201. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Paciolla, C.; Paradiso, A.; De Pinto, M.C. Cellular redox homeostasis as central modulator in plant stress response. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer: Cham, Switzerland, 2016; pp. 1–23. [Google Scholar]
- Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defense system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).