Self-Reporting Technique-Based Clinical-Trial Service Platform for Real-Time Arrhythmia Detection
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Performance Evaluation
3. Results
3.1. Wearable ECG Device Selection
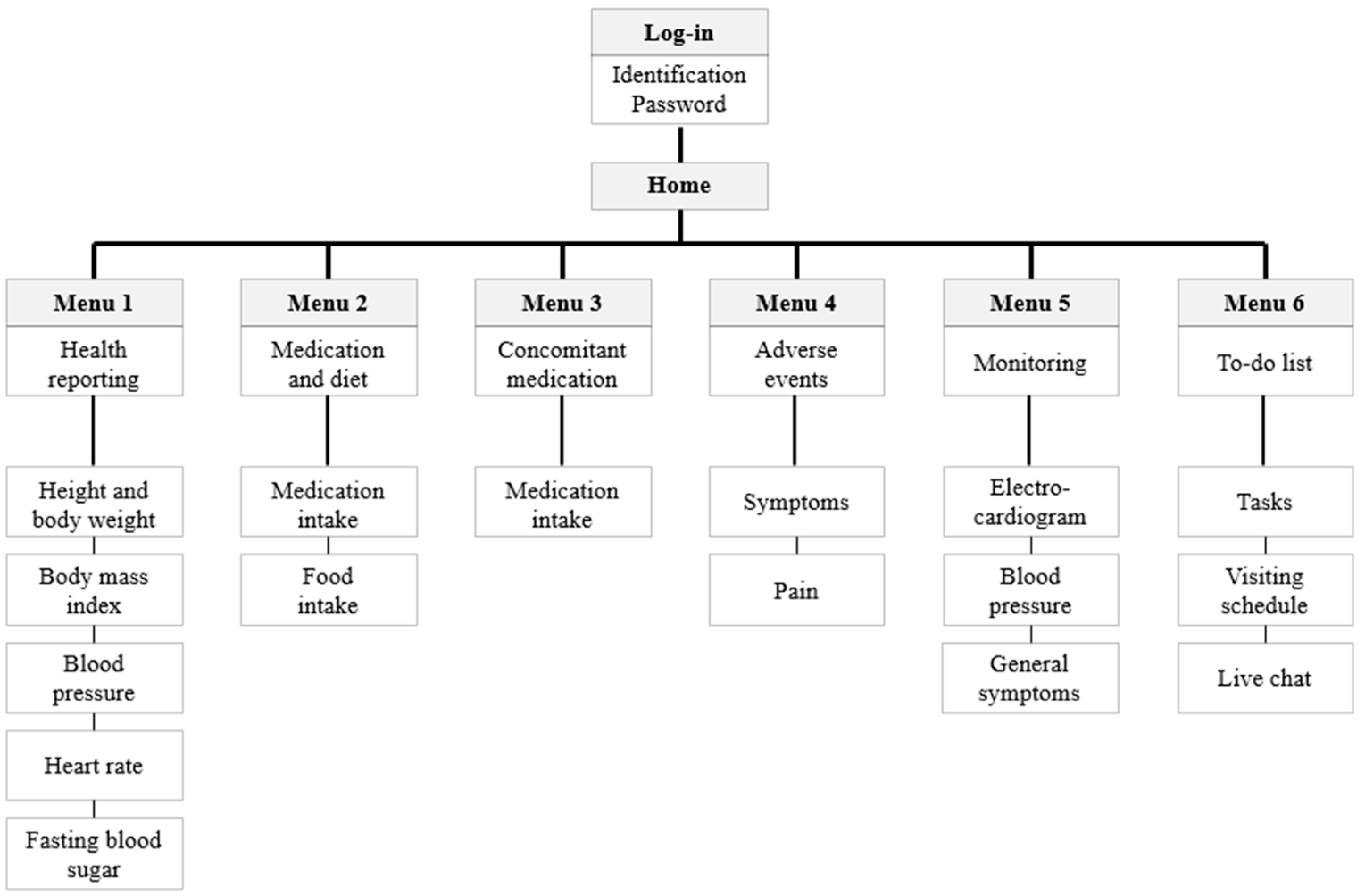
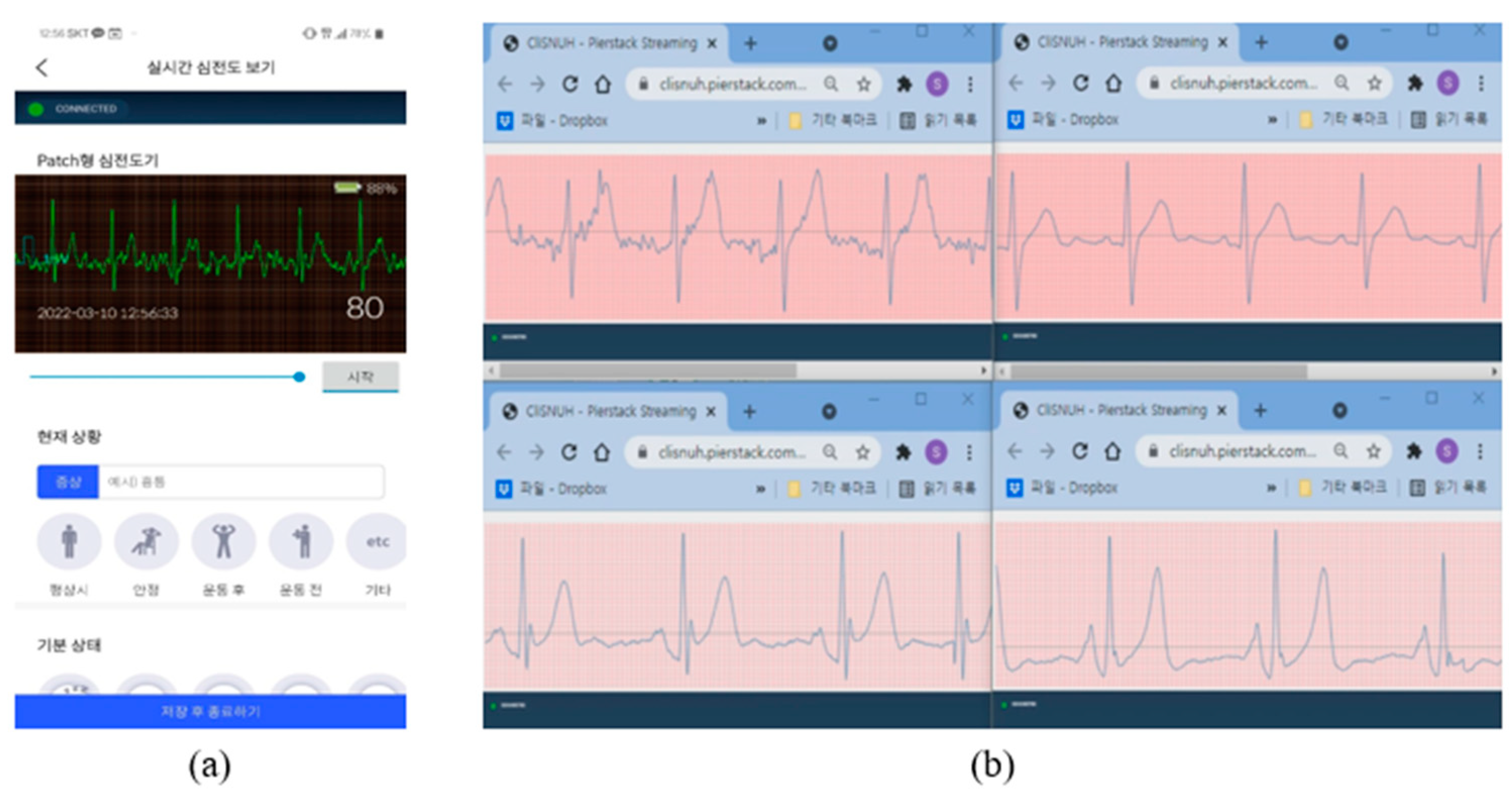
3.2. Developed Mobile App (External Network)
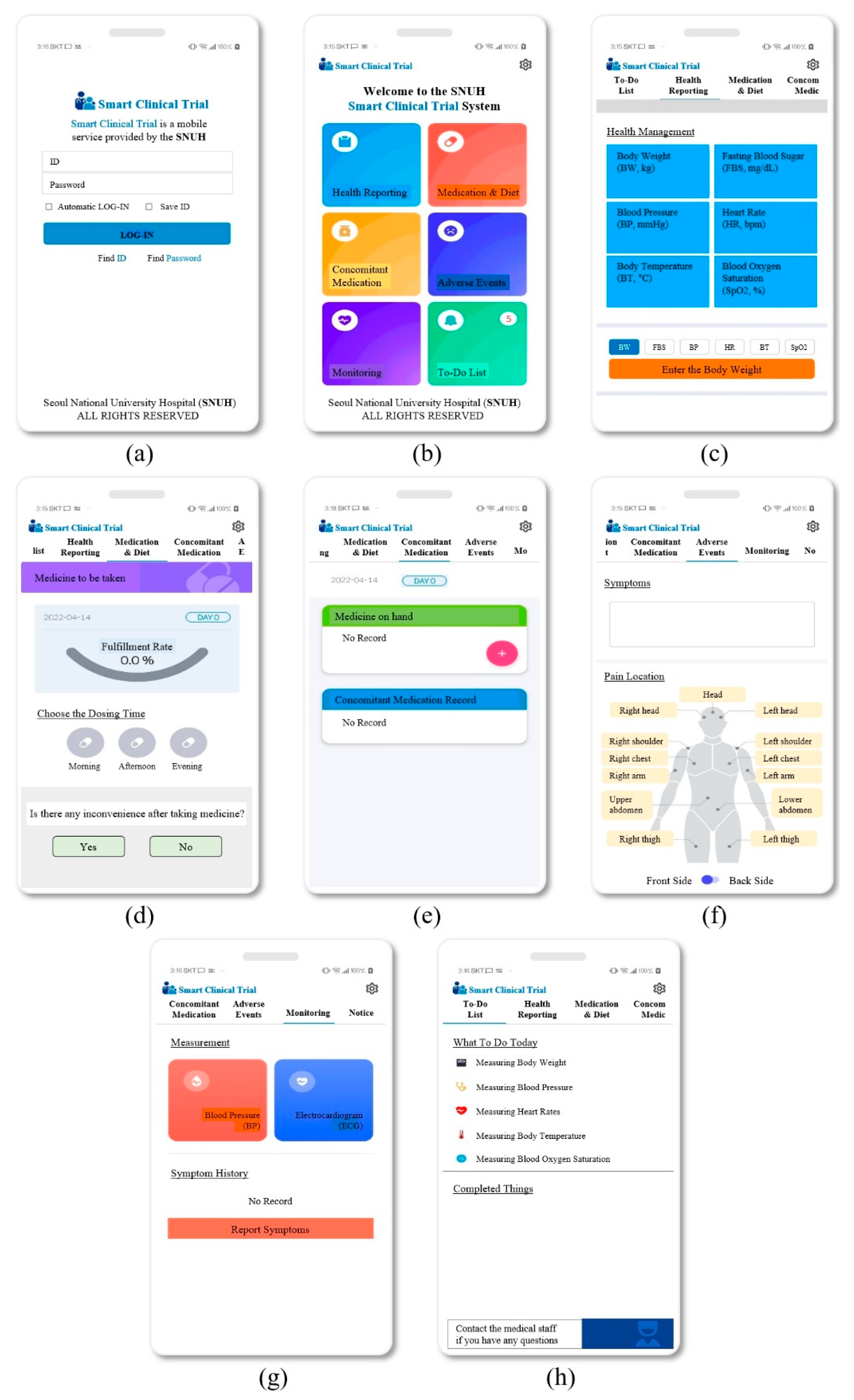
3.2.1. Health Reporting
3.2.2. Medication and Diet
3.2.3. Concomitant Medication
3.2.4. Adverse Events
3.2.5. Monitoring
3.2.6. To-do List
3.3. Developed DMZ
3.3.1. App Server
3.3.2. Push Server
3.4. Developed Internal Network
3.4.1. API Server
3.4.2. Batch Server
3.4.3. MySQL Database
3.4.4. Contents Server
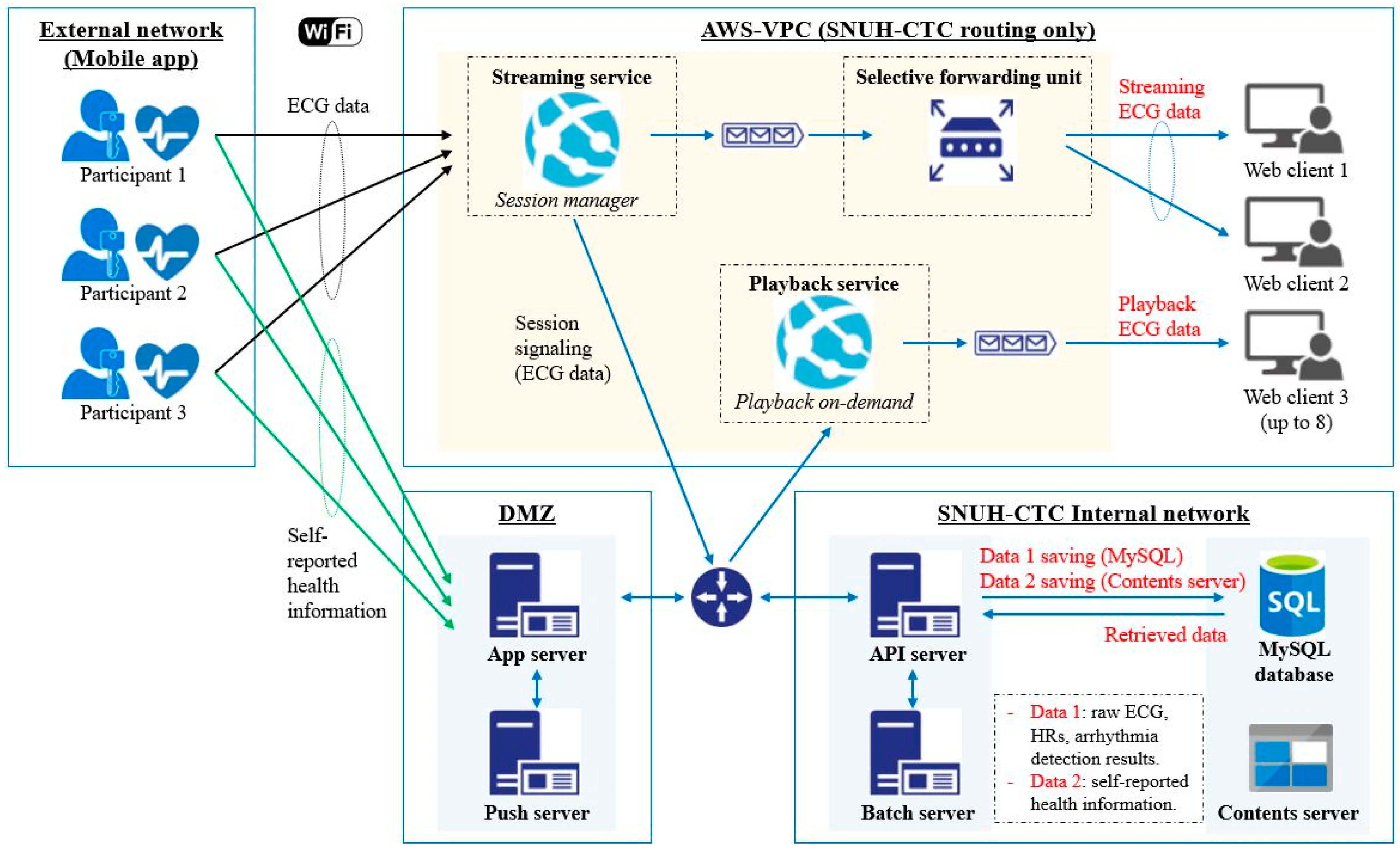
3.5. Developed AWS-VPC
3.5.1. Live-Streaming System
3.5.2. Playback-Reviewer System
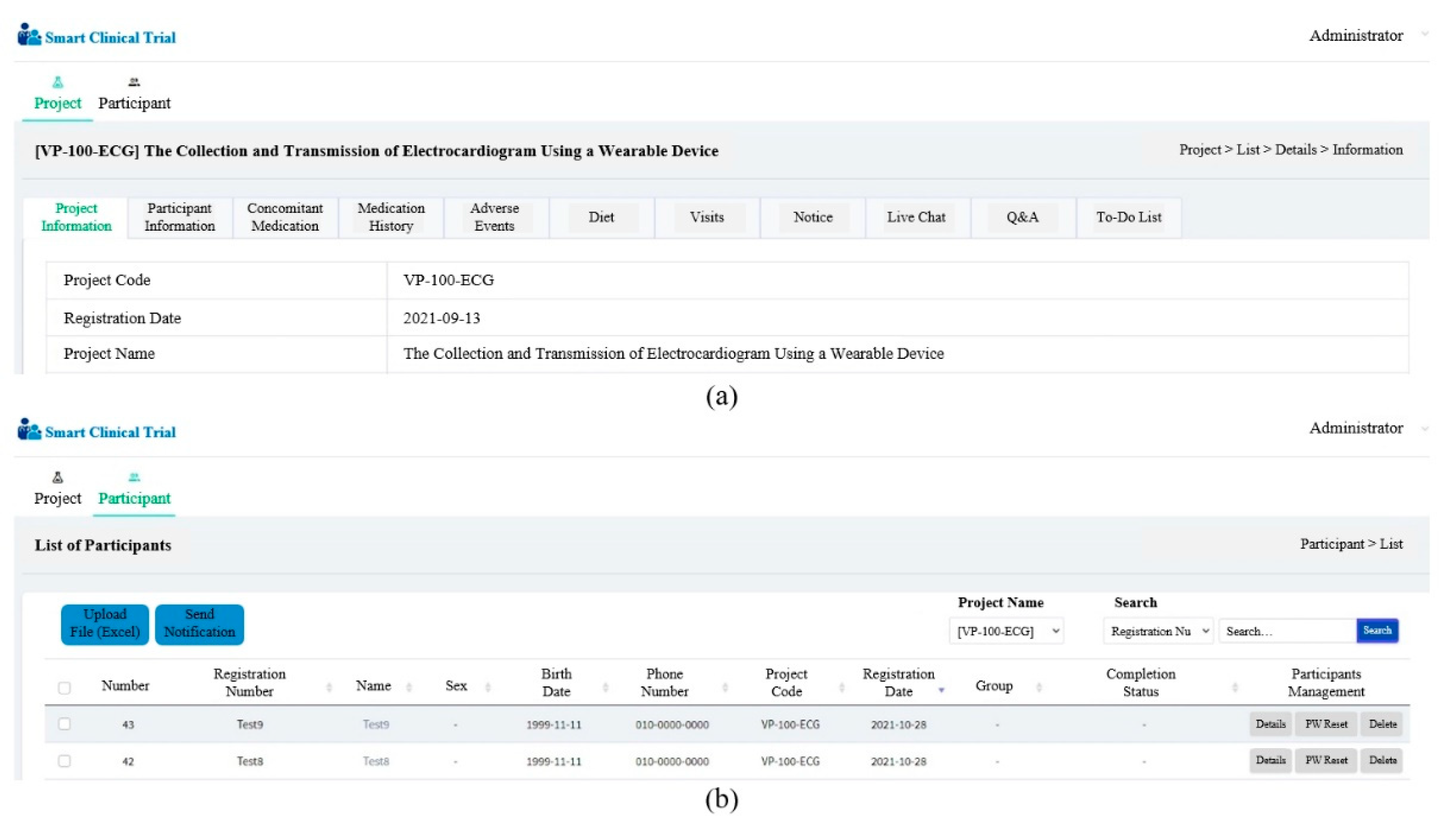
3.6. Developed Web Client
3.7. Interworking Experiments
3.8. Design of Case Study
4. Discussion
4.1. Principal Achievements
4.2. Platform Strengths
4.3. Clinical Potential
4.4. Performance Review of Potential Increased Use
4.5. Consideration of the Algorithm Performance
4.6. Benefit–Risk Assessment
4.7. Limitations
4.8. Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. UI Design Images of the Mobile App
Appendix B. Developed Web Client
References
- O’Rourke, K.; VanderZanden, A.; Shepard, D.; Leach-Kemon, K. Cardiovascular disease worldwide, 1990–2013. JAMA 2015, 314, 1905. [Google Scholar] [CrossRef]
- Auer, R.; Bauer, D.C.; Marques-Vidal, P.; Butler, J.; Min, L.J.; Cornuz, J.; Satterfield, S.; Newman, A.B.; Vittinghoff, E.; Rodondi, N. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA 2012, 307, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.J.D.S.; Schwart, W.R.; Cámara-Chávez, G.; Menott, D. ECG-based heartbeat classification for arrhythmia detection: A survey. Comput. Meth. Progr. Biomed. 2016, 127, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.H.; Gilligan, A.M.; Romero, K. Economic burden and disparities in healthcare resource use among adult patients with cardiac arrhythmia. Appl. Health Econ. Health Policy 2014, 12, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Ghassemi, M.M.; Ambai, V.; Isakadze, N.; Levantsevych, O.; Shah, A.; Clifford, G.D. Monitoring and detecting atrial fibrillation using wearable technology. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3394–3397. [Google Scholar] [CrossRef]
- Smisek, R.; Hejc, J.; Ronzhina, M.; Nemcova, A.; Marsonova, L.; Kolarova, J.; Smital, L.; Vitek, M. Multi-stage SVM approach for cardiac arrhythmias detection in short single-lead ECG recorded by a wearable device. Physiol. Meas. 2018, 39, 094003. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Friedman, R.A.; Kligfield, P.; Levine, B.D.; Viskin, S.; Chaitman, B.R.; Okin, P.M.; Saul, J.P.; Salberg, L.; Hare, G.F.A.; et al. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age) a scientific statement from the American Heart Association and the American College of Cardiology. Circulation 2014, 130, 1303–1334. [Google Scholar] [CrossRef]
- Schlant, R.C.; Adolph, R.J.; DiMarco, J.P.; Dreifus, L.S.; Dunn, M.I.; Fisch, C.; Garson, A.; Haywood, L.J.; Levine, H.J.; Murray, J.A.; et al. Guidelines for electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Electrocardiography). Circulation 1992, 85, 1221–1228. [Google Scholar] [CrossRef]
- Priori, S.G. Survivors of out-of-hospital cardiac arrest with apparently normal heart: Need for definition and standardized clinical evaluation. Circulation 1997, 95, 265–272. [Google Scholar] [CrossRef]
- Holter, N.J. New method for heart studies: Continuous electrocardiography of active subjects over long periods is now practical. Science 1961, 134, 1214–1220. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Xu, L.; Gao, Z.; Zhang, H.; Liu, H.; Li, S. An automatic cardiac arrhythmia classification system with wearable electrocardiogram. IEEE Access 2018, 6, 16529–16538. [Google Scholar] [CrossRef]
- Martin, T.; Jovanov, E.; Raskovic, D. Issues in wearable computing for medical monitoring applications: A case study of a wearable ECG monitoring device. In Proceedings of the Digest of Papers. Fourth International Symposium on Wearable Computers, Atlanta, GA, USA, 16–17 October 2000; pp. 43–49. [Google Scholar] [CrossRef]
- Subramanian, V.B. Clinical and research applications of ambulatory Holter ST-segment and heart rate monitoring. Am. Cardiol. 1986, 58, B11–B20. [Google Scholar] [CrossRef]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.F.; Nault, I.; Blier, L.; Roy, K.; et al. A novel wearable device for continuous ambulatory ECG recording: Proof of concept and assessment of signal quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.A., III; Topol, E.J.; Steinhubl, S.R. Novel wireless devices for cardiac monitoring. Circulation 2014, 130, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Voorsluys, W.; Niu, S.; Khandoker, A.; Buyya, R. An autonomic cloud environment for hosting ECG data analysis services. Future Gener. Comput. Syst. 2012, 28, 147–154. [Google Scholar] [CrossRef]
- De Chazal, P.; O’Dwyer, M.; Reilly, R.B. Automatic classification of heartbeats using ECG morphology and heartbeat interval features. IEEE Trans. Biomed. Eng. 2004, 51, 1196–1206. [Google Scholar] [CrossRef]
- Hickey, K.T.; Angelo, B.B.; Garan, H.; Sciacca, R.R.; Riga, T.; Warren, K.; Frulla, A.P.; Hauser, N.R.; Wang, D.Y.; Whang, W. Evaluating the utility of mHealth ECG heart monitoring for the detection and management of atrial fibrillation in clinical practice. J. Atr. Fibrillation 2017, 9, 1546. [Google Scholar] [CrossRef]
- McManus, D.D.; Lee, J.; Maitas, O.; Esa, N.; Pidikiti, R.; Carlucci, A.; Harrington, J.; Mick, E.; Chon, K.T. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 2013, 10, 315–319. [Google Scholar] [CrossRef]
- Fensli, R.; Gunnarson, E.; Gundersen, T. A wearable ECG-recording system for continuous arrhythmia monitoring in a wireless tele-home-care situation. In Proceedings of the 18th IEEE Symposium on Computer-Based Medical Systems (CBMS’05), Dublin, Ireland, 23–24 June 2005; pp. 407–412. [Google Scholar] [CrossRef]
- Castrejón, I.; Pincus, T. Patient self-report outcomes to guide a treat-to-target strategy in clinical trials and usual clinical care of rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30 (Suppl. S73), S50–S55. [Google Scholar]
- Iskandar, A.; Virma, E.; Ahmar, A.S. Implementing DMZ in improving network security of web testing in STMIK AKBA. Int. J. Eng. Technol. 2018, 7, 99–104. [Google Scholar] [CrossRef][Green Version]
- Bae, T.W.; Kwon, K.K. Efficient real-time R and QRS detection method using a pair of derivative filters and max filter for portable ECG device. Appl. Sci. 2019, 9, 4128. [Google Scholar] [CrossRef]
- Bae, T.W.; Lee, S.H.; Kwon, K.K. An adaptive median filter based on sampling rate for R-peak detection and major-arrhythmia analysis. Sensors 2020, 20, 6144. [Google Scholar] [CrossRef] [PubMed]
- Senhadji, L.; Carrault, G.; Bellanger, J.J.; Passariello, G. Comparing wavelet transforms for recognizing cardiac patterns. IEEE Eng. Med. Biol. 1995, 14, 167–173. [Google Scholar] [CrossRef]
- Sredojev, B.; Samardzija, D.; Posarac, D. WebRTC technology overview and signaling solution design and implementation. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 25–29 May 2015; pp. 1006–1009. [Google Scholar] [CrossRef]
- Petrangeli, S.; Pauwels, D.; Van der Hooft, J.; Wauters, T.; Turck, F.D.; Slowack, J. Improving quality and scalability of WebRTC video collaboration applications. In Proceedings of the 9th ACM Multimedia Systems Conference, Amsterdam, The Netherlands, 12–15 June 2018; pp. 533–536. [Google Scholar]
- Kohden, N. Life Scope TR BSM-6000 Series. Available online: https://ae.nihonkohden.com/en/products/patientmonitoring/bsm6000.html (accessed on 11 March 2022).
- Draeger. Infinity M300. Available online: https://www.draeger.com/ko_kr/Products/Infinity-M300 (accessed on 11 March 2022).
- Bae, T.W.; Kwon, K.K.; Kim, K.H. Vital block and vital sign server for ECG and vital sign monitoring in a portable u-vital system. Sensors 2020, 20, 1089. [Google Scholar] [CrossRef]
- Van Norman, G.A. Decentralized clinical trials: The future of medical product development? Basic Transl. Sci. 2021, 6, 384–387. [Google Scholar] [CrossRef]
- Xue, J.Z.; Smietana, K.; Poda, P.; Webster, K.; Yang, G.; Agrawal, G. Clinical trial recovery from COVID-19 disruption. Nat. Rev. Drug Discov. 2020, 19, 662–663. [Google Scholar] [CrossRef]
- Ministry of Foreign Affairs of the Republic of Korea. Current Status of Response to COVID-19 and Future Plans. 2020. Available online: http://www.mofa.go.kr/eng/brd/m_22591/view.do?seq=10 (accessed on 27 April 2022).
- Bae, Y.S.; Kim, K.H.; Choi, S.W.; Ko, T.; Lim, J.S.; Piao, M. Satisfaction and usability of an information and communications technology–based system by clinically healthy patients with COVID-19 and medical professionals: Cross-sectional survey and focus group interview study. JMIR Form. Res. 2021, 5, e26227. [Google Scholar] [CrossRef]
- Bae, Y.S.; Kim, K.H.; Choi, S.W.; Ko, T.; Jeong, C.W.; Cho, B.; Kim, M.S.; Kang, E. Information technology–based management of clinically healthy COVID-19 patients: Lessons from a living and treatment support center operated by Seoul National University Hospital. J. Med. Internet Res. 2020, 22, e19938. [Google Scholar] [CrossRef]
- Food and Drug Administration. Factors to Consider Regarding Benefit-Risk in Medical Device Product Availability, Compliance, and Enforcement Decisions: Guidance for Industry and Food and Drug Administration Staff. 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/factors-consider-regarding-benefit-risk-medical-device-product-availability-compliance-and (accessed on 27 April 2022).
- Health Insurance Review and Evaluation Center of the Republic of Korea. Statistical Analysis of Heart Diseases in Korea. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=10428&pageIndex=1#none (accessed on 27 April 2022).
- Food and Drug Administration. Unique Device Identification System (UDI System). Available online: https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/unique-device-identification-system-udi-system (accessed on 27 April 2022).
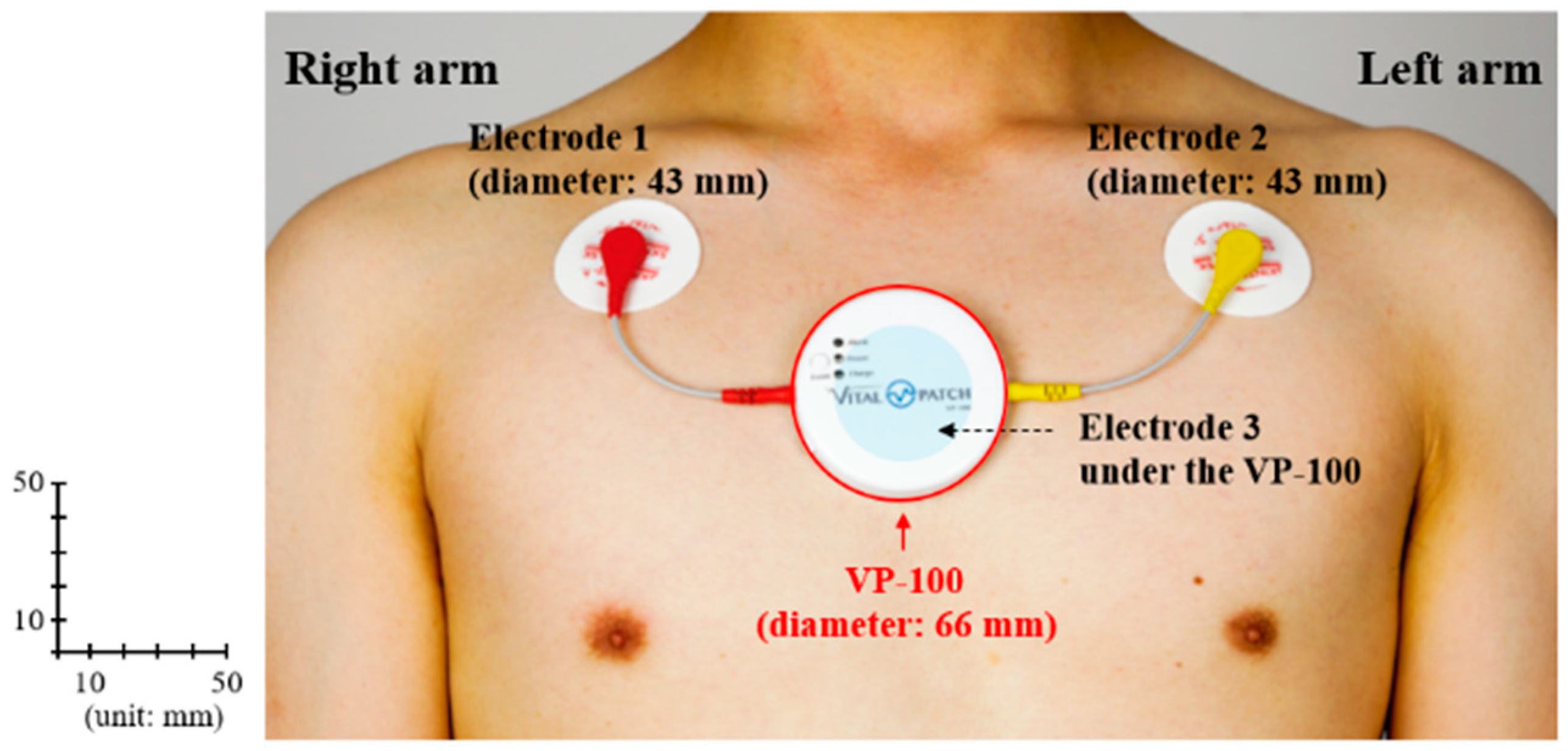
| Sampling rate | 250 Hz |
| Bandwidth | 1–30 Hz |
| Resolution | 24-bit |
| Common mode rejection ratio | 105 dB |
| Mode of operation | Continuous |
| Interface type | Wireless (Bluetooth V2.1) |
| Data-transmission distance | Up to 10 m |
| Power source | Li/Po rechargeable battery 3.7 V/800 mAh |
| Operating time | Up to 16 h with a fully charged battery |
| Internal storage | 4 GB secure digital card |
| Features | Life Scope TR BSM-6301 (Nihon Kohden) | Infinity M300 Telemetry (Draeger) | u-Vital System (ETRI) | Proposed Platform (SNUH-CTC) a | |
|---|---|---|---|---|---|
| Interface Type | Wired | Wireless | Wireless | Wireless | |
| Objective vital sign measurements | ECG a | O | O | O | O |
| HRs a | O | O | O | O | |
| SpO2 a | O | O | O | O | |
| BP a | O | X | O | O | |
| BT a | O | X | O | O | |
| Subjective self-reports | X | X | X | O | |
| Communication between clinicians and patients | X | X | X | O | |
| Detection of arrhythmia | X | O | O | O | |
| Real-time display | O | O | O | O | |
| Storage of full data | O | O | O | O | |
| Playback review | X | X | X | O | |
| Market approval | FDA a, CE a | FDA, CE | X | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Huh, K.Y.; Piao, M.; Ryu, H.; Yang, W.; Lee, S.; Kim, K.H. Self-Reporting Technique-Based Clinical-Trial Service Platform for Real-Time Arrhythmia Detection. Appl. Sci. 2022, 12, 4558. https://doi.org/10.3390/app12094558
Kim H, Huh KY, Piao M, Ryu H, Yang W, Lee S, Kim KH. Self-Reporting Technique-Based Clinical-Trial Service Platform for Real-Time Arrhythmia Detection. Applied Sciences. 2022; 12(9):4558. https://doi.org/10.3390/app12094558
Chicago/Turabian StyleKim, Heejin, Ki Young Huh, Meihua Piao, Hyeongju Ryu, Wooseok Yang, SeungHwan Lee, and Kyung Hwan Kim. 2022. "Self-Reporting Technique-Based Clinical-Trial Service Platform for Real-Time Arrhythmia Detection" Applied Sciences 12, no. 9: 4558. https://doi.org/10.3390/app12094558
APA StyleKim, H., Huh, K. Y., Piao, M., Ryu, H., Yang, W., Lee, S., & Kim, K. H. (2022). Self-Reporting Technique-Based Clinical-Trial Service Platform for Real-Time Arrhythmia Detection. Applied Sciences, 12(9), 4558. https://doi.org/10.3390/app12094558






