Abstract
Tooth whitening is one of the most conservative procedures for increasing the aesthetics of patients, but the effect of bleaching on ceramic restorations has not been extensively studied. In this study, the bleaching effect on three dental restoration materials (polished/glazed lithium disilicate glass ceramic, leucite reinforced glass ceramic and zirconium dioxide ceramic) has been investigated in terms of surface roughness changes of the exposed samples. Philips Zoom NiteWhite 16% carbamide peroxide, Philips Zoom 6% hydrogen peroxide with following LED illumination and Pola Office 6% hydrogen peroxide have been used for ceramic bleaching. The experimental investigation and performed statistical analysis revealed that the highest surface roughness changes of all investigated ceramics were caused by the hydrogen peroxide and the lowest by carbamide peroxide. These findings correlated well with the colour changes observed in the same bleached dental ceramic samples indicating potential of carbamide peroxide as the most prospective bleaching agent.
1. Introduction
An aesthetically pleasing smile is the aspiration of many patients and dentists, and this is determined by the colour, shape, size, texture, and position of natural or restored teeth. The most common aesthetic and restorative dental procedures are teeth whitening, composite bonding, and restoring teeth with veneers and inlays, overlays, and crowns. Silicon dioxide (silica) containing (feldspathic porcelains, leucite-reinforced ceramics, and lithium disilicate ceramics) and alumina and zirconia core-based ceramics were mostly favoured restorative materials in dentistry during the last few decades due to their well-controlled manufacturing process, great aesthetics, high fracture strength and optimal biocompatibility [1]. The teeth whitening effect is achieved using different bleaching agents, such as hydrogen peroxide (HP) or carbamide peroxide (CP). The bleaching effect on restorative ceramics in the mouth depends on restorative material’s properties and type of whitening agent, its concentration, pH, and exposure time [2,3,4,5].
Although the teeth whitening procedure has been shown to be safe and effective for hard tooth tissues [6], increased surface roughness of the tooth with and without restoration is often observed as a side effect of bleaching. Rough surfaces can cause discolorations, secondary caries, and soft tissue inflammation due to increased oral microorganisms’ adhesion to the restoration [7]. The threshold for clinically acceptable surface roughness value is 0.2 μm [8]. In some cases, the tooth whitening procedure may lead to erosion of restorative materials [9]. On the other hand, the surface roughness is responsible for diffuse scattering of light incident on the tooth and inversely correlates with the brightness of the tooth enamel [10].
The concentration of bleaching materials can range from 5% to 40% [11] and can be used with additional light source such as light emitting diode (LED), LED plus lasers, lasers, ultraviolet lamp, halogen curing lights, and non-thermal atmospheric pressure plasmas (NAPP) [12,13,14,15].
Hydrogen peroxide (HP) reacts directly with discolorations and releases active carbon molecules to break the double carbon bonds between chromophore molecules, leaving smaller and colourless fragments that are converted into water and carbon dioxide [11,16,17]. Though the concentration of HP varies, the highest concentration allowed for treatment of patients in Europe is 6%. HP concentration > 15% does not improve whitening efficiency but may lead to significant changes in tooth surface morphology [18].
Contacting with tooth surface carbamide peroxide (CP), also known as urea hydrogen peroxide [19], slowly breaks down into HP and urea [20] and then follows the same reaction route as HP. Due to slow decomposition, in order to achieve the same bleaching effect CP should interact with the surface significantly longer than HP [21]. The authors of this article concluded that bleaching for 126 h with 10% and 16% carbamide peroxide had no effect on the surface roughness of fluorapatite glass-ceramic and feldspathic ceramics. In a study by Karakaya et al. [9], bleaching polished polymer-infiltrated ceramic network and polished resin nano-ceramics with 35% and 40% hydrogen peroxide, 10% and 16% carbamide peroxide showed no changes in surface roughness and topography. However, these bleaching materials had a remarkable effect on the optical properties and the colour of the investigated ceramics [22].
The opposite results were observed in investigation performed by Bahannan et al. [23], where it was shown that bleaching feldspathic porcelain with 10%, 20%, and 35% carbamide peroxide, the strongest effect on surface roughness was achieved with 20% and 35% bleaching agents. The study conducted by Demir et al. [7] revealed that bleaching of glazed lithium disilicate and leucite-reinforced glass-ceramics samples with 16% carbamide peroxide for 6 h per day for seven days increased surface roughness of both types of ceramics.
Conventional dental ceramics are considered the most inert among all dental restorative materials however it was found that their surfaces can exhibit surface deterioration when interacting with different bleaching materials [24]. The contact and possible diffusion of extremely unstable and reactive free radicals of H+ or H3O+, released by bleaching agents [18,21] may selectively leach alkaline ions and cause dissolution in ceramic glass networks [19,25,26]. Degradation of restorative ceramics was observed in a study performed by Karaokutan et al. [20], which revealed that bleaching glass-ceramics, zirconium substructures, and feld-spathic ceramics with 16% carbamide peroxide releases the most sodium ions, moderately potassium, calcium, phosphorus, aluminium, copper ions, and the least zinc and lithium ions.
Despite of controversial results provided by different authors regarding bleaching caused effects in restorative ceramics, surface roughness, colour, gloss and hardness variation, as well as leakage of ions, is commonly observed [24] after teeth whitening procedure. This indicates the need for more extensive studies on bleaching caused variations of restorative ceramics properties. Due to the fact that the search for bleaching methods and materials that do not significantly increase the roughness of the tooth surface is one of key issues and challenges in modern aesthetic dentistry, the main aim of the performed research was evaluation of hydrogen peroxide and carbamide peroxide bleaching impact on surface roughness of lithium disilicate, leucite-reinforced glass-ceramics, and zirconium dioxide ceramics.
2. Materials and Methods
2.1. Preparation of Dental Ceramics Samples
Ninety round-shaped (Ø11 mm × 2 mm) dental ceramics specimens have been prepared for the investigation of bleaching caused variations of surface roughness: 30 lithium disilicate reinforced glass ceramic (Li2O5Si2, Ivoclar Vivadent IPS E.max) discs, 30 leucite reinforced glass ceramic (SiO2-Al2O3-K2O, Ivoclar Vivadent IPS Empress) discs and 30 zirconium dioxide (ZrO2, Dental Direkt BIO ZW ISO) disks. The surface of every sample was divided in two areas: polished and glazed. Polished and glazed samples were used to simulate clinical situations and to investigate the impact of different bleaching materials on the surface roughness of three selected ceramics. Glass ceramics specimens were manufactured in dental laboratory using CAD/CAM and press technologies. Zirconium dioxide specimens were manufactured using CAD/CAM and synthesis technologies. Glazed parts of glass ceramics specimens were firstly covered with paint (Ivoclar Vivadent IPS E.max and Ivoclar Vivadent IPS Empress essence correspondingly) and then with glazing paste (Ivoclar Vivadent IPS E.max and Ivoclar Vivadent IPS Empress). Glazed parts of zirconium dioxide specimens were firstly covered with paint (Ivoclar Vivadent IPS E.max essence) and then with glazing paste (Ivoclar Vivadent IPS E.max). Lastly surfaces of all ceramic’s specimens were polished with a “bison” type brush and diamond paste and then polished with a fluffy brush.
2.2. Bleaching of Samples
Three bleaching materials: (1) 16% carbamide peroxide (CH6N2O3, Philips Zoom NiteWhite), (2) 6% hydrogen peroxide + LED (H2O2, Philips Zoom) and (3) 6% hydrogen peroxide (H2O2, Pola Office + 6%) have been selected for the investigation. Bleaching of specimens was performed by strictly following instructions of the manufacturers.
10 lithium disilicate, 10 leucite-reinforced glass ceramic and 10 zirconium dioxide samples with and without glaze were bleached with Philips Zoom Nitewhite system using 16% carbamide peroxide (CP). A thin layer of bleaching gel was deposited manually on the surface of samples (Figure 1a) and stored for 8 h per day for 14 days. After bleaching the material was removed with a damp cloth and the surfaces were dried.
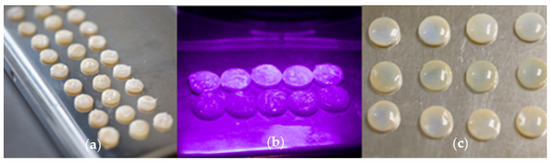
Figure 1.
Bleaching of dental ceramics: (a) samples bleached with 16% carbamide peroxide, (b) samples bleached with 6% hydrogen peroxide LED light, (c) samples bleached with 6% hydrogen peroxide.
10 lithium disilicate, 10 leucite-reinforced glass ceramic, and 10 zirconium dioxide samples with and without glaze were bleached with Philips Zoom system using 6% hydrogen peroxide (HP) and LED light. pH-booster material (consisting of water, PVP, glycine, potassium hydroxide) was applied to the specimens and the entire surface was completely covered with bleaching material. The LED light was directed to the samples (Figure 1b) maintaining the distance between the light source and the disks similar as during in-office bleaching. The highest light intensity was selected. A 15 min bleaching procedure was repeated four times. After each session, HP was wiped off with cotton balls and reapplied with pH-booster and HP. After bleaching specimens were wiped with cotton balls, washed with a stream of water and then dried.
10 lithium disilicate, 10 leucite-reinforced glass ceramic, and 10 zirconium dioxide samples with and without glaze were bleached with Pola Office + 6% system using 6% of hydrogen peroxide (HP). A thin layer of bleaching gel was deposited on the specimens’ surfaces (Figure 1c), which remained for 15 min. The material was removed with a suction system and HP was reapplied. There was a total of four sessions. After bleaching samples were washed with water and wiped dry.
2.3. Evaluation of Surface Topography of Specimens
Surface topography and roughness of bleached dental ceramics specimens were assessed performing measurements with atomic force microscope (AFM) (NT-206, Microtestmachines Co., Gomel, Belarus) working in tapping mode. 16 μm high pyramidal shape Si probe tip with a 6 mm diameter was used for samples surface area (20 μm × 20 μm) scanning. The scanning was repeated three times for each sample. Different centrally situated scanning locations on the sample’s surface were selected, which represented best the average surface roughness variations over the whole sample area, trying to avoid areas with extremely low or extremely high surface roughness gradients. The arithmetic surface roughness (Ra) and root mean square roughness (Rq) was calculated from topographic images using open access Gwyddion software. It should be noted that surface topography and roughness of each initial ceramic sample (90 samples in total) was tested before the bleaching procedure using the same AFM equipment as is indicated above. Polished and glazed parts of the samples were investigated separately.
2.4. Evaluation of Colour of Specimens
Prior to bleaching colour of all specimens (with and without glaze) were determined using spectrophotometer VITA Easyshade V (VITA Zahnfabrik). A single tooth colour evaluation setting mode was selected to obtain CIELAB data (L, a, b), where L indicates the brightness within the interval from 0 (black) to 100 (white); a indicates a point on the red-green colour scale; b indicates a point on the yellow-blue colour scale. Colour changes of dental ceramic samples after bleaching were calculated using following formula:
Colour differences (ΔE) of bleached samples were evaluated using the National Bureau of Standards (NBS) assessment criteria (Table 1)

Table 1.
National Bureau of Standards (NBS) criteria for the assessment of colour differences ∆E.
2.5. Statistical Analysis
Statistical analysis was performed with SPSS 21 for Windows. Study data were analysed with descriptive statistics and one-way analysis of variance ANOVA methods. The difference between the variables was considered statistically significant if p < 0.05. Data were normally distributed (Shapiro-Wilk); therefore, ANOVA, Tukey HSD and paired t-test were used.
3. Results
Independently of the actions which were undertaken, each dental ceramic sample was scanned in AFM facility selecting at least three specific scanning locations on the sample’s surface and the surface roughness Ra1 (arithmetic average roughness) and Rq1 (root mean square roughness) of initially prepared polished and glazed specimens as well as surface roughness Ra2 and Rq2 was obtained after ceramics bleaching with different agents. Typical 3D topography images of the AFM scanned samples and the evaluated mean surface roughness values are provided in the Table 2 and Table 3 correspondingly.

Table 2.
Typical 3D surface topography of dental ceramics before and after bleaching with different bleaching agents.

Table 3.
Mean surface roughness values of dental ceramics before and after bleaching with different bleaching agents.
The impact of bleaching agents on surface roughness of bleach materials was assessed using statistical analysis methods. The results of the performed statistical analysis of surface roughness alterations due to applied bleaching are provided in Figure 2.
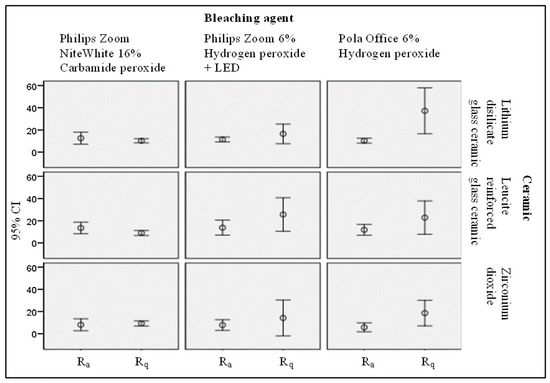
Figure 2.
Statistical evaluation of the bleaching agent impact on the polished surface roughness of the treated ceramic samples.
Since teeth whitening is a common aesthetic improving procedure, the results of surface morphology and roughness changes of dental ceramics caused by application of different bleaching agents were additionally compared with the results of colour changes of the same samples observed after bleaching. The details of the colour difference assessment procedure and some results could be found in our previous publication [27]. In this paper we have provided just a summary of colour difference evaluation results of all of the investigated samples. The results of colour changes of bleached dental ceramics with indicated NBS criteria are shown in Table 4.

Table 4.
Mean values ± standard deviations of ceramics colour alteration after bleaching (ΔE).
The results of the performed statistical analysis of colour changes of samples due to the bleaching are provided in Figure 3.
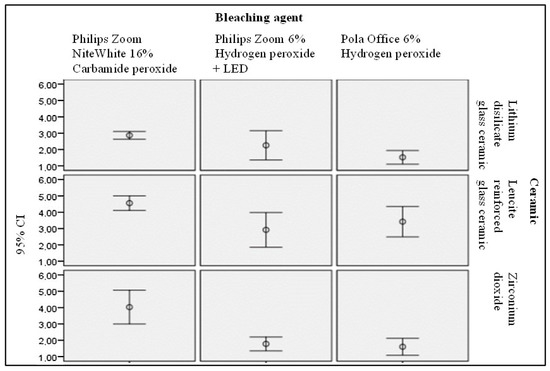
Figure 3.
Statistical evaluation of the bleaching agent impact on the polished surface colour alterations of the treated ceramic samples.
4. Discussions
Conventional dental ceramics being the most inert among all dental restorative materials can exhibit surface deterioration when interacting with different bleaching materials [24]. Contacting with extremely unstable and reactive free radicals of H+ or H3O+, released by bleaching agents [18,21], followed by possible radical diffusion may cause dissolution in ceramic glass networks [19,25,26]. Moreover, an increase in surface roughness greater than the threshold (Ra > 0.2 μm) may result in an increase of plaque accumulation, thereby increasing the risk of periodontal inflammation or affecting ceramic aesthetics by changing the ceramic texture [21]. The average surface roughness of no one of investigated samples before and after bleaching has exceeded the value of 0.2 μm which was clinically set as a level for possible bacteria accumulation.
The lowest surface roughness between all initially prepared ceramics (polished and glazed) was found for zirconium dioxide. Ra1 of polished zirconium dioxide samples was the lowest and varied between 6.00 and 8.31 nm, Rq1—between 7.30 and 11.39 nm. The roughness of initial leucite-reinforced glass ceramic samples and lithium disilicate samples was higher, as compared with zirconium dioxide samples, indicating the highest surface roughness of glazed lithium disilicate (Ra1 = 12.36 ± 2.76 nm and Rq1 =17.91 ± 4.85 nm) and of polished leucite reinforced glass (Ra1 = 13.15 ± 5.13 nm and Rq1 = 17.68 ± 6.72 nm). There was no significant roughness difference between polished and glazed parts of initial ceramics observed.
Bleaching of the experimental samples with carbamide peroxide (16% CP) was responsible for the reduction of surface roughness of lithium disilicate and leucite-reinforced glass ceramic independently from initial preparation of samples (polished or glazed). A small increase of roughness after CP treatment was observed in zirconium dioxide samples, while application of hydrogen peroxide (6% HP) as a bleaching agent was responsible for the significant increase of surface roughness in all of the investigated samples. Application of 6% HP with following LED illumination caused highest increase of surface roughness of polished leucite-reinforced glass ceramic.
Performed statistical analysis revealed that the surface roughness of polished lithium disilicate bleached with Pola Office + 6% HP was statistically significant rougher (mean Ra2 = 37.17 nm) (Sig. 0.005) (mean Rq2 = 50.69 nm) (Sig. 0.004) than bleached with Philips Zoom NiteWhite 16% CP or Philips Zoom 6% HP + LED. The reduction of Ra and Rq values of the polished lithium disilicate were observed after its treatment with 16% CP (mean Ra2 = 10.22 nm) (F = 7.765; p < 0.0) (Sig. 0.005) (mean Rq2 = 12.84 nm) (Sig. 0.004). However, significant difference according to paired t-test was not identified. Surface roughness of glazed leucite-reinforced glass-ceramic bleached with Pola Office + 6% HP was statistically significantly rougher (mean Ra2 = 21.76 nm, mean Rq2 = 32.97 nm) than bleached with Philips Zoom NiteWhite 16% CP or Philips Zoom 6% HP + LED. The surface roughness of the glazed leucite-reinforced glass ceramic was reduced after its treatment with 16% CP (mean Ra2 = 9.20 nm) (F = 4.714; p < 0.05) (Sig. 0.030) and (mean Rq2 = 11.88 nm) (Sig. 0.028), but there was no significant difference between the initial and bleached samples. The surface roughness of the polished leucite-reinforced glass-ceramic was reduced to a statistically significant level after its treatment with 16% CP (Ra2 = 9.12, Sig. 0.035) (Rq2 = 11.72 nm, Sig. 0.025). The surface roughness of polished lithium disilicate (Ra2 = 37.17 nm, Sig. 0.017) (Rq2 = 50.69 nm, Sig. 0.018) and glazed zirconium dioxide (Rq = 27.72, Sig. 0.050) became significantly rougher after bleaching with 6% HP. The surface roughness changes after bleaching of dental ceramics with 6% HP and 6% HP + LED were comparable with the results of other authors [1,9]. It was found that 6% HP had the most significant effect on surface roughness of the investigated samples, while the impact of 16% CP on the surface roughness was the most modest.
Only slightly increased surface roughness was observed for zirconium dioxide treated with 16%. This finding was in line with the results indicated by Zaki et al. [25], however evaluated surface roughness values were lower. There was no significant difference between behaviour of polished and glazed surfaces observed as it was discussed by Demir at al [7]. The lower bleaching effect observed for zirconium ceramic can be related to mechanical properties of this material, especially to its resistance to carbamide peroxide treatment. Observed small, however statistically significant reduction of surface roughness of lithium disilicate and leucite reinforced glass ceramic after bleaching with 16% CP complies with the results provided in [28,29], and approves the suggestion that the bleaching effect depends on dental material properties, as it was indicated in [30]. Despite of this, results of the performed investigation seem to be controversial as compared with the results provided by Demir et al. [7] and M. Silva et al. [31] who indicated increase of surface roughness after bleaching, or with the results of other authors [32,33] who reported no changes in surface morphology of ceramic after 10–16% CP bleaching.
It is known [6] that colour changes of treated dental ceramic mainly depend on the surface roughness of dental material, bleaching agent, its concentration, pH and exposure time. On the other hand, surface roughness changes may significantly reduce the whitening effect due to the changes of light dispersion on the surface. In order to have a complex assessment of bleaching agent’s impact on investigated dental ceramics, some additional investigations of colour changes of bleached materials have been performed.
Investigation has shown that bleaching affected differently polished and glazed surfaces. Analysis of freshly prepared polished samples revealed that: (1) Philips Zoom NiteWhite 16% urea (carbamide) peroxide (16% CP) treated lithium disilicate glass colour change (mean 2.8695) was significantly different from Pola Office + 6% HP-induced colour change (mean 1.5229) (F = 6.713; p < 0.05) (Sig. 0.003); (2) 16% CP treated leucite-reinforced glass-ceramic colour change (mean 4.5565) was significantly different from of Philips Zoom 6% HP + LED light-induced colour change (mean 3.421) (F = 4.904; p < 0.05) (Sig. 0.013) and (3) 16% CP treated zirconia colour (average 4.0287) was significantly different from Pola Office + 6% HP induced colour change (average 1.6035) and Philips Zoom 6% HP + LED -induced colour change (average 1.7788) (F = 18.438; p < 0.05) (Sig. 0.000).
Analysing glazed dental ceramic samples it was found that: 16% CP treated lithium disilicate glass ceramic colour change (average 3.0205) differed significantly from Pola Office + 6% HP- induced colour change (average 1.7726) (Sig. 0.018) and Philips Zoom 6% HP + LED caused colour change (mean 1.8951, nm) (F = 5.185; p < 0.05) (Sig. 0.036); 2) 16% CP treated leucite-reinforced glass-ceramic colour (average 4.4624) was significantly different as compared with Philips Zoom 6% HP + LED light induced colour change (mean 3.5188) (F = 3.709; p < 0.05) (Sig. 0.030). Performed statistical analysis revealed that the colour of all investigated ceramics was changing due to the bleaching. The response of each ceramic type was different depending on the bleaching material (chemical interaction between bleaching material and dental ceramic), bleaching conditions (exposure time, pH) and also was influenced by bleaching induced changes of surface roughness (light dispersion from the surface). Significant colour changes (from noticeable to visible) were observed after 16% CP treatment of all three types of dental ceramics. independently from the sample surface preparation (polished or glazed), but major effect was identified for zirconium ceramic [27]. It was suggested that the bleaching effectiveness of carbamide peroxide was related to its acidic pH, relative long period of sample’s exposure. Obtained results were in line with the results of Kara et al. [34] and Karakaya et al. [9], however were different from results reported by Rodrigues et al. [26] who has not observed any colour changes after bleaching of feldspar with 15% carbamide peroxide.
Based on the complex investigation results (surface colour changes and surface morphology changes), it was found that treatment of zirconium ceramic with Philips Zoom NiteWhite 16% CP resulted in the strongest whitening effect and the lowest surface roughness changes, thus indicating zirconium dioxide as the most conducive ceramic to aesthetic treatment.
5. Conclusions
Three types of dental ceramics (lithium disilicate glass ceramic, leucite reinforced glass ceramic and zirconium dioxide) have been investigated in order to assess the impact of aesthetic treatment on their properties.
It was found that the roughness of different dental ceramics was dependent on materials properties and were changing upon samples treatment with bleaching agents (Philips Zoom NiteWhite 16% carbamide peroxide (16% CP), Philips Zoom 6% hydrogen peroxide with following LED illumination (6% HP + LED)), and Pola Office + 6% hydrogen peroxide (6% HP)
Ceramics treated with Pola Office + 6% HP were in most cases significantly rougher as compared with a treatment using other bleaching materials.
Surface roughness of zirconium ceramic treated with Philips Zoom NiteWhite 16% carbamide peroxide was slightly increasing, but surface roughness of lithium disilicate and of leucite reinforced glass ceramic decreased.
The strongest bleaching effect was observed for all three types of different ceramics treated with Philips Zoom NiteWhite 16% CP independently from the sample surface preparation (polished or glazed).
Zirconium dioxide was identified as the most conductive ceramic to aesthetic treatment with 16% carbamide peroxide. Considering the investigation results, it is recommended that one prepare and implement teeth whitening procedures strictly following bleaching protocols. Treatment at clinics should be prioritized.
Author Contributions
Conceptualization, G.M., D.A., and R.O.; methodology, G.M. and V.Č.; formal analysis, G.M., S.T., and J.L.; investigation, G.M., D.A., and S.T.; writing—original draft preparation, G.M.; writing—review and editing, D.A. and J.L.; supervision, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by Lithuanian University of Health Sciences and the Bioethics Centre (Nr. BEC-OF-36).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the paper.
Conflicts of Interest
The authors declare no conflict of interest. The manuscript has not been published previously and is not being considered for publication elsewhere. The authors have obtained the necessary authority for publication.
References
- Mohammadibassir, M.; Rezvani, M.B.; Golzari, H.; Salehi, E.M.; Fahimi, M.A.; Fard, M.J.K. Effect of Two Polishing Systems on Surface Roughness, Topography, and Flexural Strength of a Monolithic Lithium Disilicate Ceramic. J. Prosthodont. 2017, 28, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Dawood, L.; Soliman, F.M.; El-Farag, S.A.A. Effect of In-Office Bleaching Techniques and Topical Fluoride Application on Color and Surface Roughness of Two Types of Dental Ceramics (In-Vitro Study). Egypt. Dent. J. 2020, 66, 1243–1251. [Google Scholar] [CrossRef]
- Mourouzis, P.; Koulaouzidou, E.A.; Helvatjoglu-Antoniades, M. Effect of in-office bleaching agents on physical properties of dental composite resins. Quintessence Int. 2013, 44, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mota, G.M.D.S.M.; Kury, M.; Tenório, C.P.D.S.B.; Amaral, F.L.B.D.; Turssi, C.P.; Cavalli, V. Effects of Artificial Staining and Bleaching Protocols on the Surface Roughness, Color, and Whiteness Changes of an Aged Nanofilled Composite. Front. Dent. Med. 2020, 1, 20. [Google Scholar]
- Wijetunga, C.L.; Otsuki, M.; Hiraishi, N.; Luong, M.N.; Tagami, J. Effect of pH of bleaching agent on tooth bleaching action in vitro. Dent. Mater. J. 2021, 40, 566–572. [Google Scholar] [CrossRef]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Demir, N.; Karci, M.; Ozcan, M. Effects of 16% Carbamide Peroxide Bleaching on the Surface Properties of Glazed Glassy Matrix Ceramics. BioMed Res. Int. 2020, 2020, 1864298. [Google Scholar] [CrossRef]
- Tinastepe, N.; Malkondu, O.; Iscan, I.; Kazazoglu, E. Effect of home and over the contour bleaching on stainability of CAD/CAM esthetic restorative materials. J. Esthet. Restor. Dent. 2021, 33, 303–313. [Google Scholar] [CrossRef]
- Karakaya, I.; Cengiz-Yanardag, E. Changes in Optical Characteristics and Surface Topography of CAD/CAM Materials after Bleaching Applications: An AFM Evaluation. J. Prosthodont. 2019, 29, 226–236. [Google Scholar] [CrossRef]
- Heintze, S.; Forjanic, V.; Rousson, V. Surface roughness and gloss of dental materials as a function of force and polishing time in vitro. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2006, 22, 146–165. [Google Scholar] [CrossRef]
- Kwon, S.R.; Wertz, P.W. Review of the Mechanism of Tooth Whitening. J. Esthet. Restor. Dent. 2015, 27, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, R.F.L.; Soares, A.F.; Pangrazio, E.G.K.; Wang, L.; Ishikiriama, S.K.; Bombonatti, J.F.S. Evaluation of temperature increase during in-office bleaching. J. Appl. Oral Sci. 2016, 24, 136–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Hassan, N.A. The effect of light-activation sources on tooth bleaching. Niger. Med. J. 2014, 55, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, R.F.L.; Azevedo, J.F.D.E.G.D.; Francisconi, A.C.; de Almeida, C.M.; Ishikiriama, S. Comparative clinical study of the effectiveness of different dental bleaching methods—Two year follow-up. J. Appl. Oral Sci. 2012, 20, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of Bleaching Products With Up to 6% and With More Than 6% Hydrogen Peroxide: Whitening Efficacy Using BI and WID and Side Effects—An in vitro Study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Coceska, E.; Gjorgievska, E.; Coleman, N.J.; Gabric, D.; Slipper, I.J.; Stevanovic, M.; Nicholson, J.W. Enamel alteration following tooth bleaching and remineralization. J. Microsc. 2016, 262, 232–244. [Google Scholar] [CrossRef]
- Grazioli, G.; Valente, L.L.; Isolan, C.P.; Pinheiro, H.A.; Duarte, C.G.; Münchow, E.A. Bleaching and enamel surface interactions resulting from the use of highly-concentrated bleaching gels. Arch. Oral Biol. 2018, 87, 157–162. [Google Scholar] [CrossRef]
- Anusavice, K.J. Degradability of dental ceramics. Adv. Dent. Res. 1992, 6, 82–89. [Google Scholar] [CrossRef]
- Karaokutan, I.; Aykent, F. Effect of a Home Bleaching Agent on the Ion Elution of Different Esthetic Materials. J. Prosthodont. 2020, 29, 805–813. [Google Scholar] [CrossRef]
- Ourique, S.A.M.; Arrais, C.A.G.; Cassoni, A.; Ota-Tsuzuki, C.; Rodrigues, J.A. Effects of different concentrations of carbamide peroxide and bleaching periods on the roughness of dental ceramics. Braz. Oral Res. 2011, 25, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Şahin, N.; Sahmali, S.M.; Uyar, A. Effect of Two Vital Bleaching Agents on the Color Properties of the Low-Fusing Dental Ceramics. Acta Stomatol. Cappadocia 2021, 1, 6–20. [Google Scholar] [CrossRef]
- Bahannan, S.A. Effects of different bleaching agent concentrations on surface roughness and microhardness of esthetic restorative materials. Saudi J. Dent. Res. 2015, 6, 124–128. [Google Scholar] [CrossRef]
- Rea, F.T.; Roque, A.C.C.; Macedo, A.P.; Almeida, R.P. Effect of carbamide peroxide bleaching agent on the surface roughness and gloss of a pressable ceramic. J. Esthet. Restor. Dent. 2019, 31, 451–456. [Google Scholar] [CrossRef]
- Zaki, A.A.; Fahmy, N.Z. The Effect of a Bleaching System on Properties Related to Different Ceramic Surface Textures. J. Prosthodont. 2009, 18, 223–229. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Oliveira, G.P.F.; Amaral, C.M. Effect of thickener agents on dental enamel microhardness submitted to at-home bleaching. Braz. Oral Res. 2007, 21, 170–175. [Google Scholar] [CrossRef]
- Morkūnaitė, G.; Ožiūnas, R.; Čeplauskas, V. Color changes of dental ceramics exposed to bleaching materials. In Proceedings of the Medical Physics in the Baltic States: 15th International Conference on Medical Physics, Kaunas, Lithuania, 4–6 November 2021; Adlienė, D., Ed.; Kaunas University of Technology: Kaunas, Lithuania, 2021; pp. 125–128, ISSN 1822-5721. [Google Scholar]
- Turker, B.; Biskin, T. Effect of three bleaching agents on the surface properties of three different esthetic restorative materials. J. Prosthet. Dent. 2003, 89, 466–473. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Yazici, R.; García-Godoy, F. Effect of home-use bleaching gels on fluoride releasing restorative materials. Oper. Dent. 2003, 28, 605–609. [Google Scholar]
- Attin, T.; Hannig, C.; Wiegand, A.; Attin, R. Effect of bleaching on restorative materials and restorations—A systematic review. Dent. Mater. 2004, 20, 852–861. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Davies, R.; Stewart, B.; DeVizio, W.; Tonholo, J.; Júnior, J.D.S.; Pretty, I. Effect of whitening gels on the surface roughness of restorative materials in situ. Dent. Mater. 2006, 22, 919–924. [Google Scholar] [CrossRef]
- Zavanelli, A.C.; Mazaro, V.Q.; Silva, C.R.; Zavanelli, R.A.; Mancuso, D.N. Surface roughness analysis of four restorative materials exposed to 10% and 15% carbamide peroxide. Int. J. Prosthodont. 2011, 24, 155–157. [Google Scholar] [PubMed]
- Duschner, H.; Götz, H.; White, D.J.; Kozak, K.M.; Zoladz, J.R. Effects of hydrogen peroxide bleaching strip gels on dental restorative materials in vitro: Surface microhardness and surface morphology. J. Clin. Dent. 2004, 15, 105–111. [Google Scholar] [PubMed]
- Kara, H.; Aykent, F.; Ozturk, B. The Effect of Bleaching Agents on the Color Stability of Ceromer and Porcelain Restorative Materials In Vitro. Oper. Dent. 2013, 38, E1–E8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).