1. Introduction
Carbon oxides, CO2 and CO (COx), are components of the atmosphere that play an important role in producing the so-called greenhouse effect. The carbon oxides are poisonous when accumulated to dangerous levels. At small concentrations, both gases are colorless, odorless and tasteless and can only be detected with a measuring instrument. Thus, reliable and fast control of the carbon oxides level in the atmosphere and process gases is of high importance. Therefore, there is considerable interest in developing proper analytical techniques to measure COx concentration.
Nowadays, different approaches for sensing CO
x are known, among them spectroscopic, catalytic, acoustic, electrochemical, metal oxide semiconductor (MOS)-based methods, etc. The spectroscopic sensors are based on the ability of gas molecules to absorb a specific wavelength of the light; the absorption level is related to the target gas concentration. Spectroscopic gas sensors exhibit high sensitivity and low cross-sensitivity to other gases. An optical CO sensor based on tunable diode laser spectroscopy was reported to possess the sensing resolution of 3 ppm for 1 s, averaging [
1]. A ppm-level CO sensor based on a 2
f wavelength modulation spectroscopy technique was described in [
2]. However, the spectroscopic methods are bulky, expensive and time consuming, which hinders their use for in situ monitoring.
The detection principle of the semiconducting type sensors is based on the change in electrical resistance of MOS (e.g., SnO
2, and WO
3) depending on the composition of the gaseous atmosphere [
3,
4,
5]. The MOS-based sensors were shown to possess good sensitivity, fast response time and performance stability; however, the sensor signal can be affected by the adsorption of different components of the gaseous atmosphere onto the semiconductor surface, which negatively influences the selectivity.
The operation mechanism of the catalytic-type sensors is based on using catalytic materials which are sensitive to combustible gases (CO, hydrocarbons, etc.) [
6,
7,
8]. The oxidation of combustible gas on the catalyst leads to an increase in temperature and, as a consequence, in resistance of the active element. The resistance change is proportional to the temperature change, and therefore, to the amount of the oxidized gas. A CO
2 sensor based on SnO
2 and reduced graphene oxide composite showed an excellent detection limit of 5 ppm at room temperature [
6]. A combustion-type CO gas sensor using a catalyst composed of 11 wt% Pt supported on the apatite-like oxide La
9.0Si
5.8Mn
0.2O
27−δ was reported to demonstrate high sensitivity (the relative resistance change reached a value of 0.0053 for 500 ppm CO) and a rapid response time of 10–20 s at 130 °C [
8]. The main drawbacks of the catalytic-type sensors are a need for oxygen for the oxidation process and low selectivity related with the possible oxidation of different combustible components of the gaseous atmosphere [
8].
Electrochemical sensors based on solid-oxide electrolytes are considered the most reliable way of sensing CO
x for industrial applications. These sensors operate at temperatures of 450 °C and above to ensure sufficient ionic conductivity in a solid-oxide electrolyte [
9,
10,
11,
12,
13,
14,
15]. Yttria-stabilized zirconia (YSZ) is typically used as an electrolyte. YSZ-based electrochemical sensors have such important advantages as good corrosion resistance in aggressive gaseous atmospheres, high stability and simplicity in design. Mixed potential, amperometric and impedancemetric electrochemical sensors were reported to be applicable for measuring CO. Impedancemetric sensors use the sensitivity of the sensor impedance to concentration of a target component in the gaseous atmosphere [
9,
10,
11,
12]. The sensor based on the Pt/YSZ/Au–Ga
2O
3 cell was reported to exhibit strong dependence of the impedance on CO concentration in the range of 100–800 ppm and small response time of 10 s at 550 °C [
12]. However, the impedance-type sensor signal can be affected by non-target gaseous species; further, these sensors require using a high-frequency AC signal source and a frequency response analyzer that leads to an increase in cost [
11].
The YSZ-based mixed potential sensor with NiO sensing electrode was proved to have good sensitivity to CO, showing a signal of 36 mV to 1000 ppm CO in the presence of 0.5−3% O
2 at 1000 °C and excellent selectivity, being insensitive to the presence of CO
2, CH
4 and H
2O in the gaseous atmosphere; however, the sensor signal and the response time were affected by the microstructure of the sensing electrode [
16]. Using ZnO as a sensing electrode was also shown to be justified; the sensor demonstrated good sensitivity, reaching a response of 68 mV to 400 ppm of CO at 700 °C [
17]. However, the mixed potential sensors, as a kind of potentiometric sensors, need a reference gas, which complicates their design; besides, they show low sensitivity to small changes of the target component concentration because of their logarithmic relation with the sensor signal [
17].
In the amperometric sensors, a signal is generated by a voltage applied across the oxide-ion conducting electrolyte. The generated oxide-ion current is determined by the availability of the oxygen-containing species in the gaseous atmosphere. Gas supply to the electrolyte/electrode interface is limited by a diffusion barrier that can be either a capillary connecting the outer and inner sensor compartments, or a porous electrolyte. An important advantage of the amperometric sensors over the mixed potential ones is that there is no need of a reference atmosphere that allows using a simple design. Further, these sensors do not require the presence of free oxygen for operation as the catalytic-type sensors do. A linear relationship between the sensor signal (the limiting current) and concentration of a target gaseous specie is another serious advantage of this type of sensors. Recently, we reported the possibility of measuring the concentrations of carbon oxides by using a solid oxide amperometric sensor [
13,
15]. A YSZ-based amperometric sensor with a couple of symmetrical Pt electrodes was shown to possess good sensitivity to CO in the concentration range of 1–6 vol.%, which was about 2 mA cm
−2 per 1 vol% CO at 450 °C in nitrogen atmosphere [
13]. A CO
2 sensor based on the proton-conducting La
0.9Sr
0.1YO
3–d electrolyte showed the linear dependence of the limiting current on CO
2 concentration in the range of 2–14 vol.% with a slope of ~0.7 mA cm
−2 per 1 vol% CO
2 at 500–600 °C in nitrogen atmosphere, which characterizes high sensor sensitivity [
15]. However, the sensor exhibited a rather slow response time of ~30 min under the stepwise change of CO
2 concentration in the range of 4.6–13.7 vol.%, which can be explained by insufficient proton conductivity of the electrolyte or poor electrode performance.
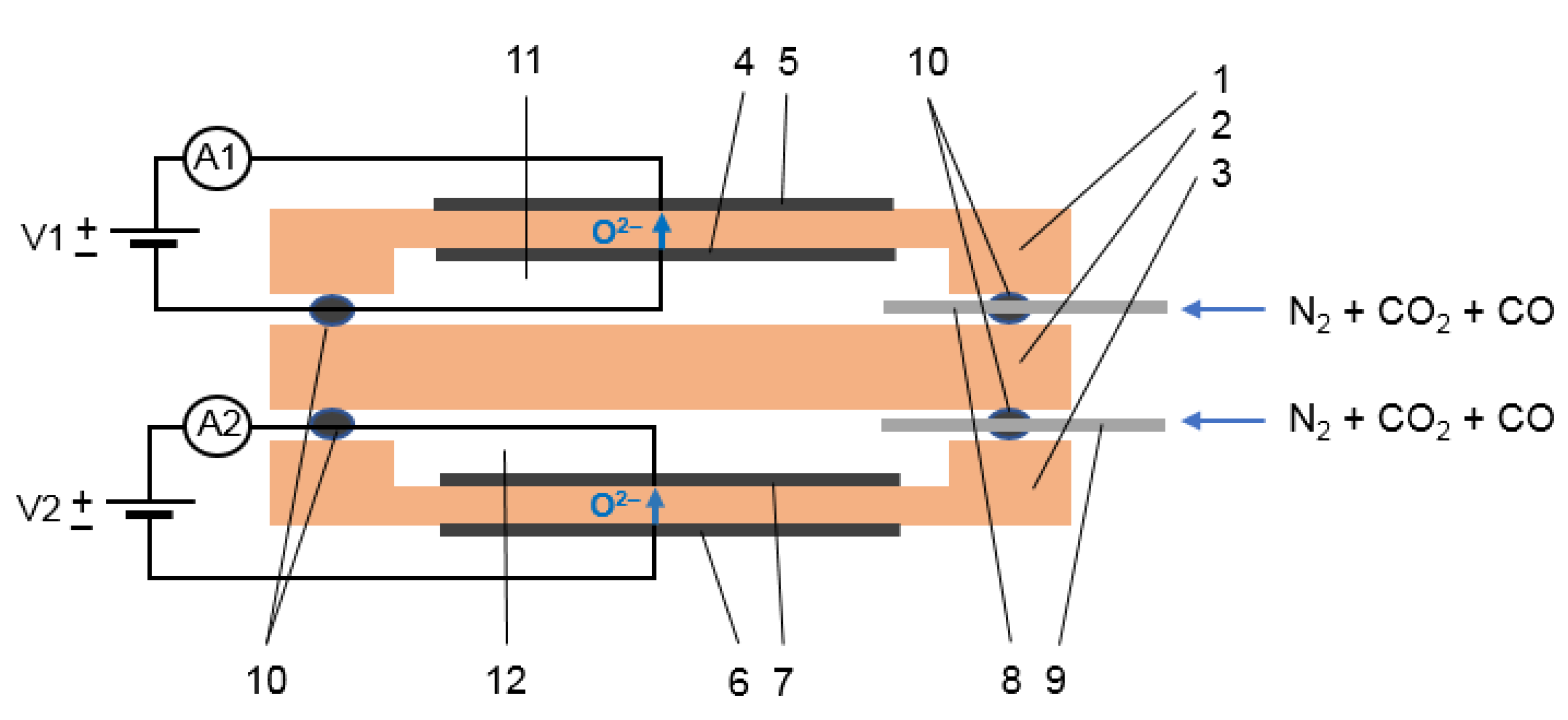
In the present study, we designed a dual-chamber amperometric sensor based on YSZ (ZrO2 + 8 mol% Y2O3) electrolyte and Pt electrodes for simultaneous measurement of CO and CO2 concentrations in inert gases. The simultaneous sensing of several gaseous species is relevant for many practical applications, e.g., in the medical and pharmaceutical industry, food preservation and storage industry, chemical manufacturing, etc. The use of solid-oxide amperometric sensors for simultaneous measurement of CO2 and CO in inert gases has not been reported to date, as far as we know. Nitrogen was used as a chemically inert gas for sensor testing. The sensor properties were studied in binary (N2 + CO and N2 + CO2) and ternary (N2 + CO + CO2) gaseous mixtures in the range of the carbon oxides concentration of up to 10 vol% at 600–750 °C. The effect of water vapor on the sensing properties was tested.
3. Results and Discussion
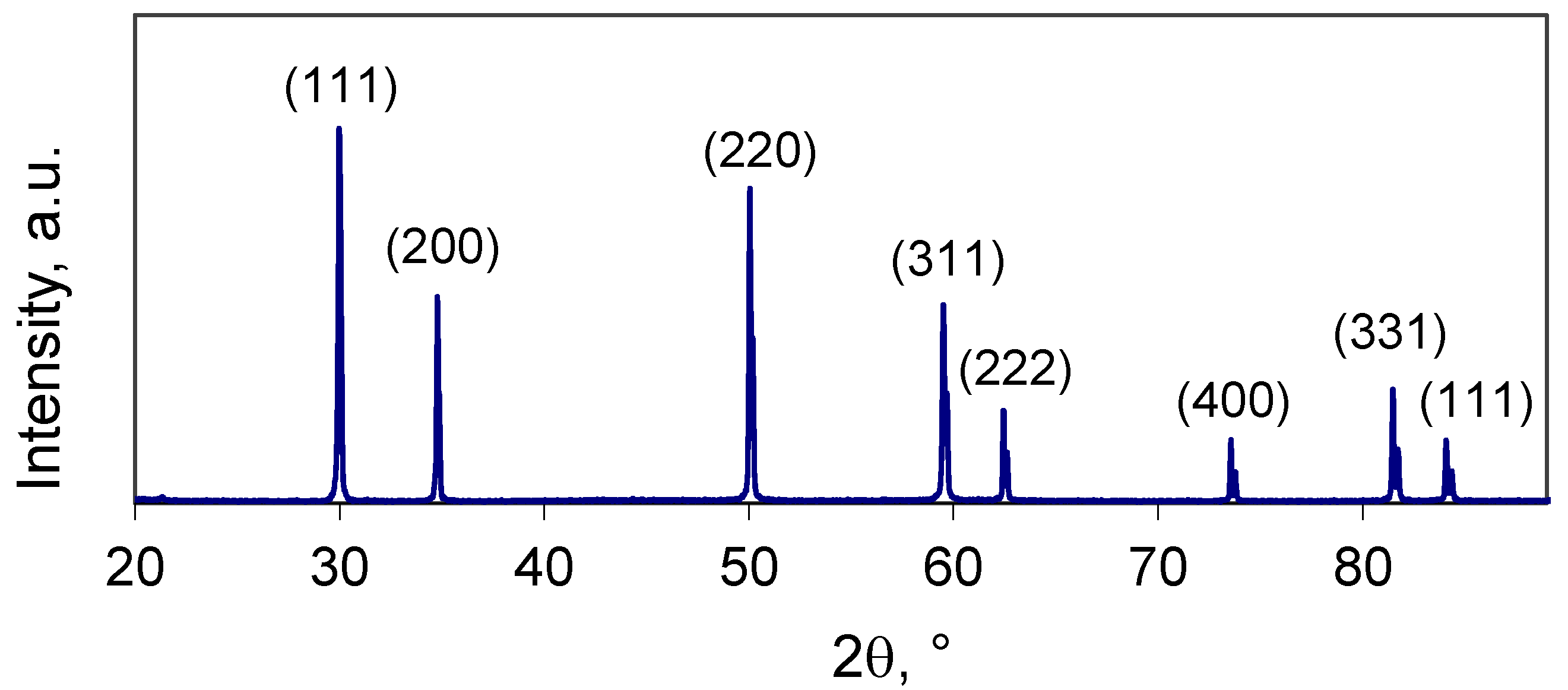
Figure 3 shows the XRD pattern of the sintered YSZ sample. The observed diffraction peaks are related to the YSZ phase, which is confirmed by the ICDD file (030–1468) for the 8 mol.% Y
2O
3-doped ZrO
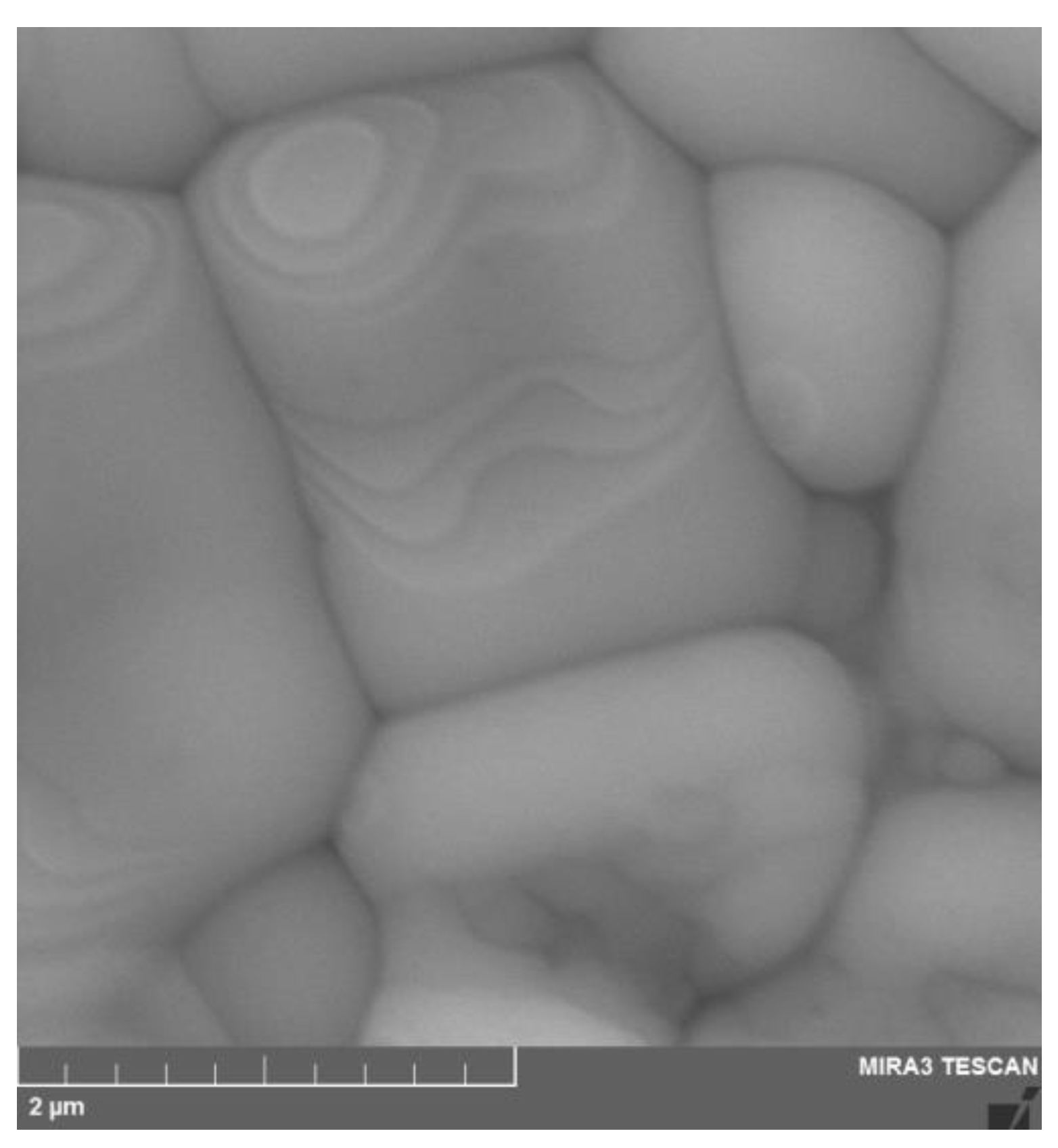
2 powder with a cubic fluorite structure. SEM image of the surface of the YSZ ceramic sample is shown in
Figure 4. The micrograph reveals a dense grained microstructure of the sintered ceramics, which is needed to avoid gas leakage through the electrolyte membrane.
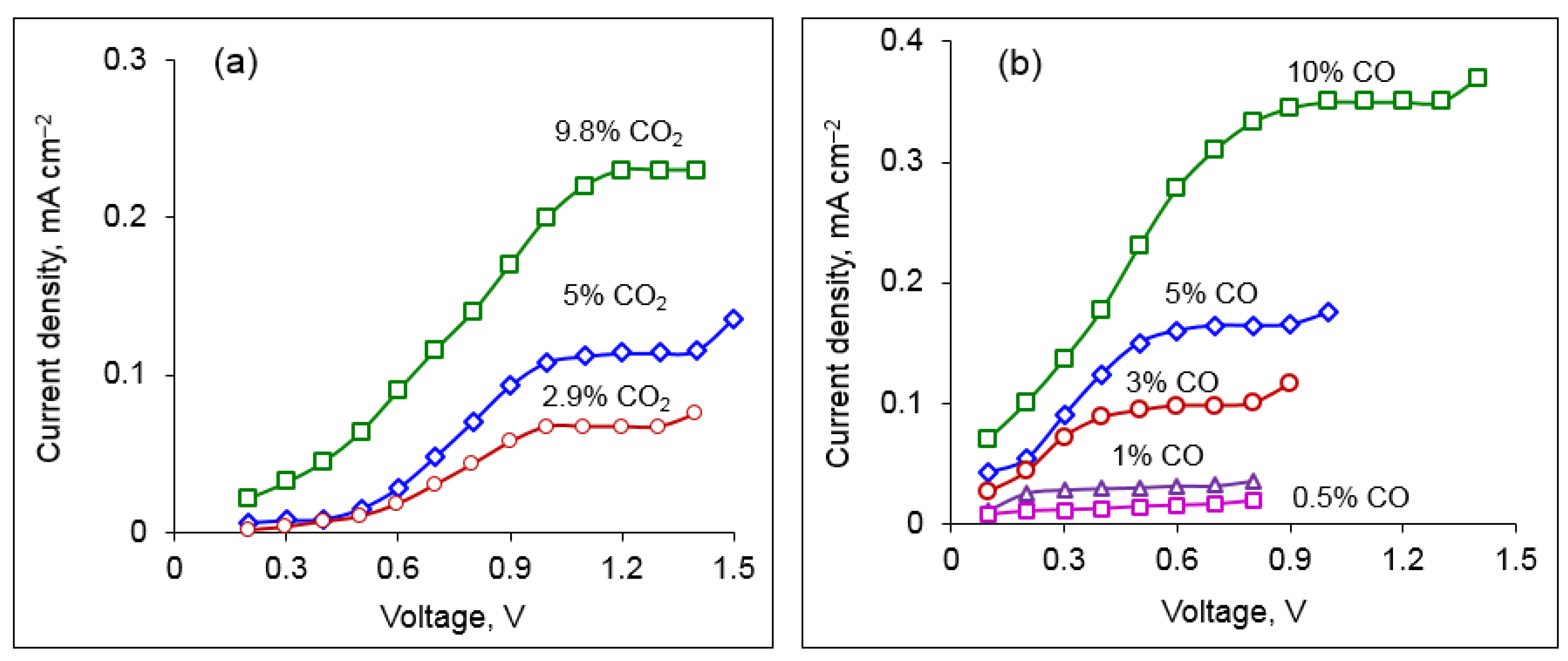
At the first stage of testing, the sensor response in binary mixtures, N
2 + CO and N
2 + CO
2 was studied. To avoid the interference with water, the gaseous mixtures were dried with phosphorus pentoxide. The current–voltage dependences measured on the CO
2 and CO cells (cell 1 and cell 2, respectively) at 700 °C are presented in
Figure 5. It can be seen that the obtained curves look similar: the current increases with the applied voltage at low potential differences, then a plateau is observed, which corresponds to the stationary state described in
Section 2.2. The difference in the voltage values at which the limiting current is attained in the CO
2 and CO cells can be caused by different catalytic activities of Pt electrodes to reactions 1 and 2.
With further increasing voltage, the limiting current plateau is followed by the next step of rising the sensor current (
Figure 5). We assume that this can be explained by the partial reduction of YSZ in harsh conditions of a large DC voltage applied across the electrolyte under a low oxygen activity in the surrounding atmosphere. The reduction is accompanied by the generation of electrons, according to the following reaction [
19]:
Concentration of electrons n increases with decreasing the oxygen partial pressure in line with the mass action law for the reaction 5 as follows:
That means that with decreasing the concentration of COx, the concentration of electrons rises, and consequently, the electronic current increases.
The above assumption is supported by previously reported data on the blackening of YSZ from the cathode side related to electron association with point defects under a current load, and even the metallization of YSZ from the cathodic side [
20,
21,
22]. However, the reoxidation of the electrolyte occurs after removal of the DC load so that the traces of metallicity, including the black color, disappear [
21]. Appearance of the electronic charge carriers in YSZ should result (i) in a decrease in the oxide-ion flux across the electrolyte at a given applied DC voltage because of short circuiting, and (ii) in an extension of the effective area of the electrode reactions. As a consequence, the sensor current rises upon increasing the voltage at high DC loads.
As can be seen in
Figure 5, the limiting currents in the CO
2 and CO cells increase with a rise in the carbon oxides content.
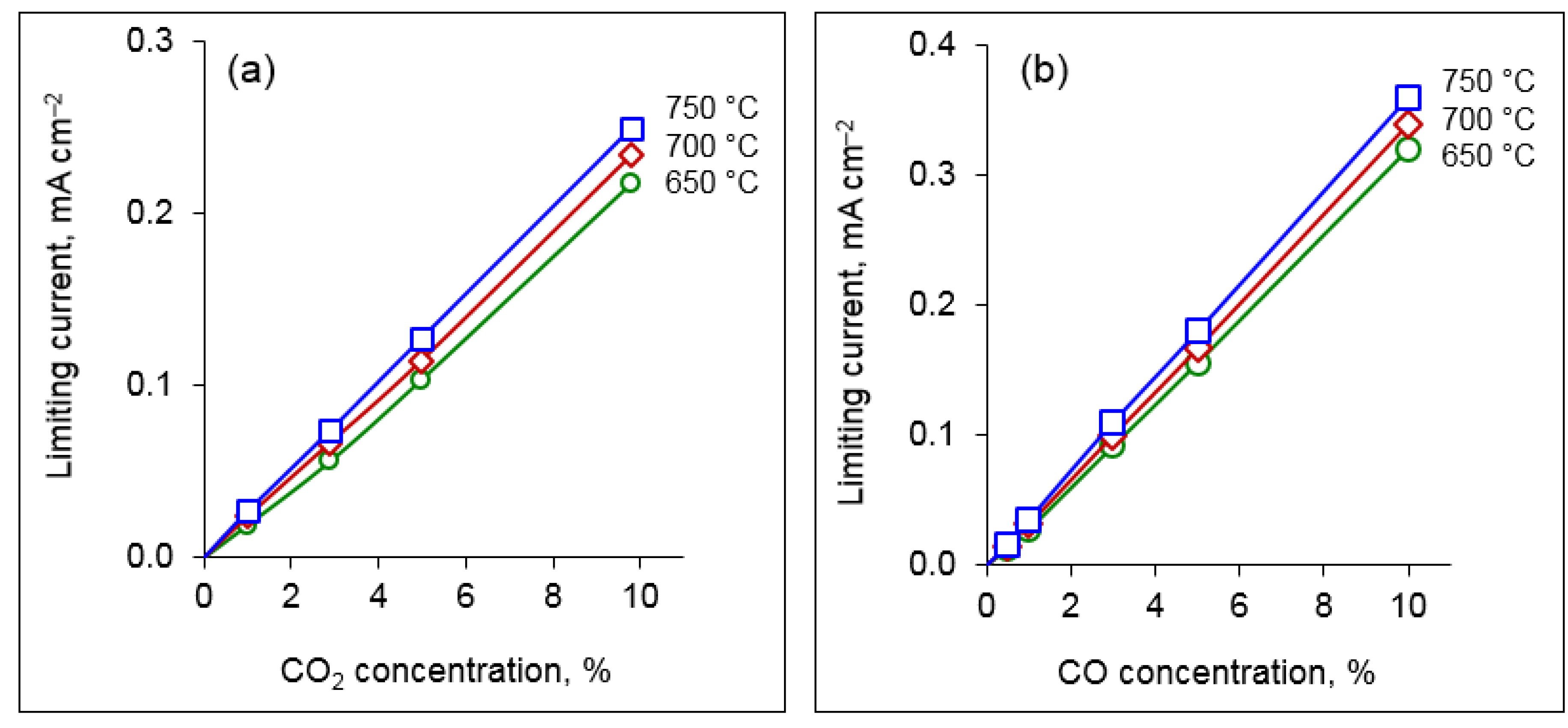
Figure 6 displays the sensor signal vs. CO
x concentration. The dependences are linear in agreement with Equation (4). The limiting current increases with temperature, which can be explained by faster diffusion of the corresponding gaseous species. The slope of the limiting current–concentration lines determines the sensitivity of the sensor; as can be seen in
Figure 6, the sensitivity increases with temperature and reaches the values of 25 mA cm
−2 per 1 vol.% CO
2 and 36 mA cm
−2 per 1 vol.% CO at 750 °C.
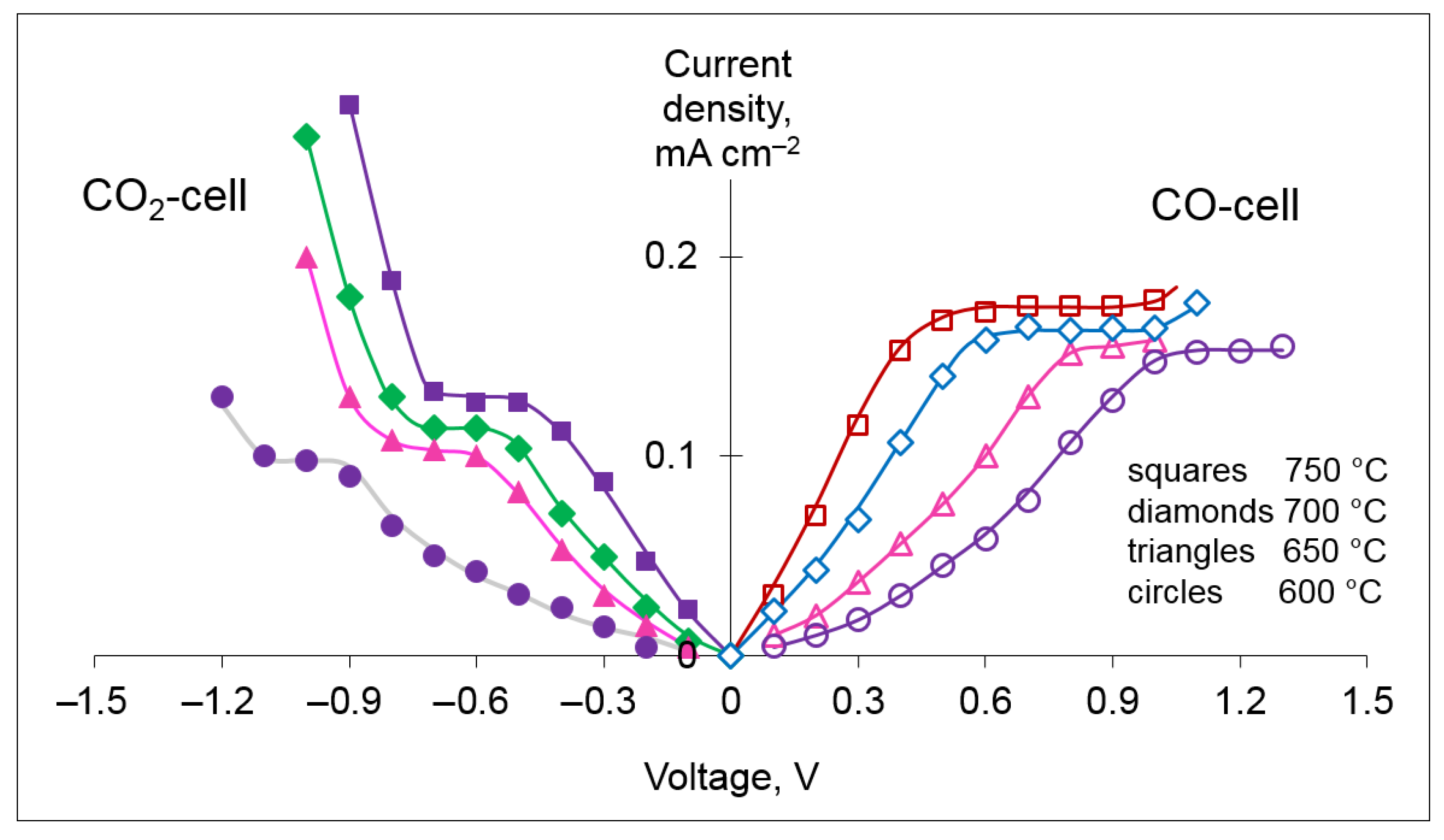
The next stage of testing was aimed at the study of the sensing properties in dry ternary mixtures N
2 + CO + CO
2. The current–voltage curves of the CO
2 and CO cells measured in the N
2 + 4.9 vol.% CO + 4.9 vol.% CO
2 gas at the temperatures of 600–750 °C are shown in
Figure 7. For more comfortable visual perception, it was taken into account that the DC voltage was applied to the CO
2 and CO cells in the opposite directions. For both cells, the slope of the initial step of the current–voltage dependences decreases with decreasing temperature, which is definitely related to lowering the ionic conductivity of YSZ and the slowing down of the electrochemical reactions on the electrodes. The values of limiting the current of the CO
2 and CO cells in the ternary gaseous mixtures N
2 + CO + CO
2 are similar to those measured in the binary mixtures N
2 + CO and N
2 + CO
2 at the corresponding concentration of CO or CO
2 (see
Figure 5), indicating that there is no perceptible cross-sensing effect toward the counterpart. The fact that the designed amperometric sensor is capable of simultaneous measuring of the concentrations of the two oxides of carbon is of high importance, as these gases coexist in many practical applications.
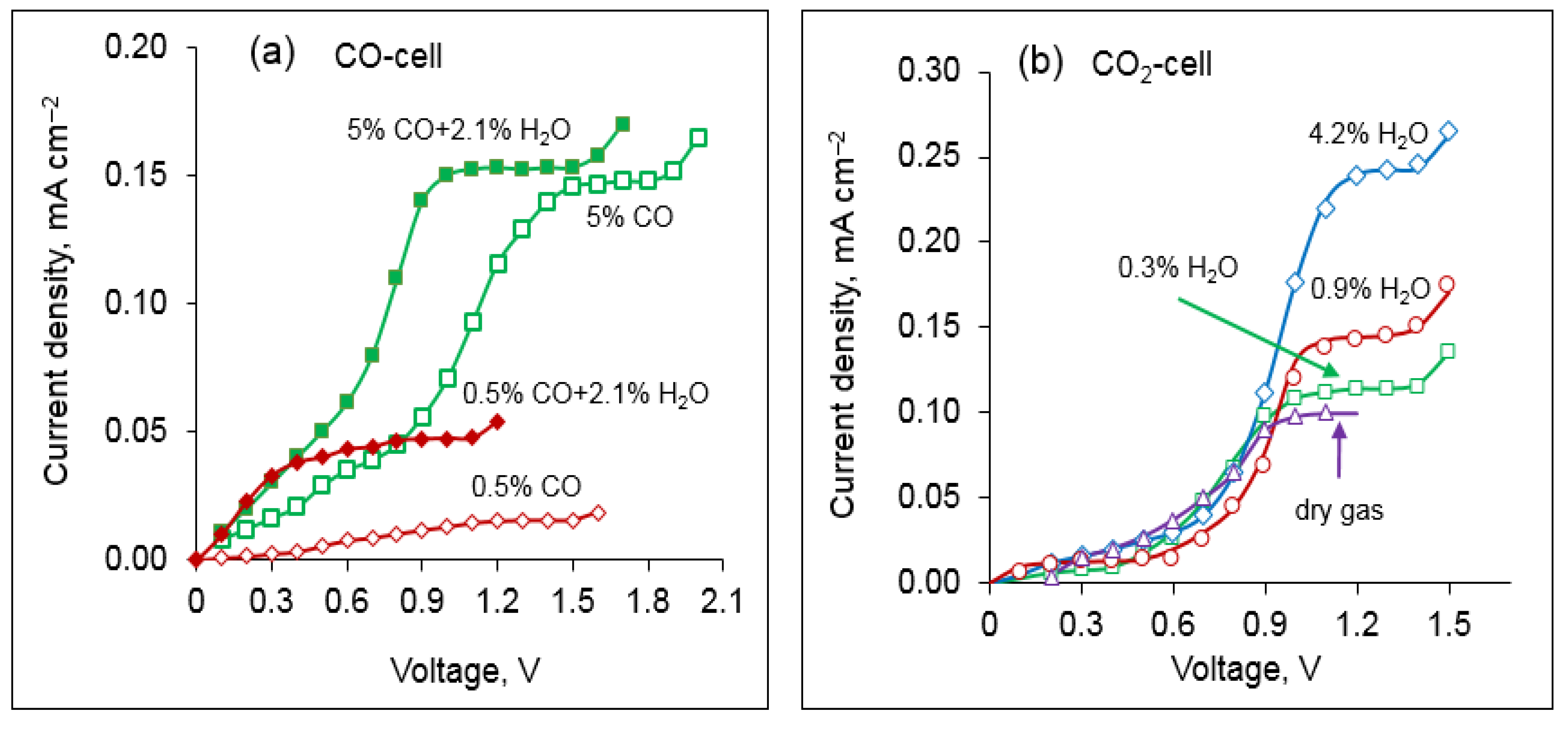
It is important to evaluate the role of water vapor in the gas-sensing properties as some amount of water vapor may exist in the analyzed gas. For this purpose, the binary mixtures N
2 + CO and N
2 + CO
2 were bubbled through a water bath kept in a thermostat. The measured dependences of the sensor current on the applied voltage in dry and wet atmospheres are shown in
Figure 8. As can be seen, water vapor strongly affects the CO-sensing properties at low concentrations of CO (
Figure 8a). This can be explained by the interaction of CO and H
2O at the temperatures above 500 °C according to the following reaction:
As a result, the hot gaseous mixture contains not only the carbon oxides and water vapor, but also hydrogen, which is oxidized together with CO on electrode 7. As far as the diffusion coefficient for H2 being almost four times higher than that for CO, the limiting current of the CO cell significantly increases in the presence of water vapor. When the CO concentration is high compared to that of H2O, the cross-sensing effect toward water is reduced due to a small concentration of the produced hydrogen.
The limiting current in the CO
2 cell also changes in the presence of water vapor in the analyzed gas (
Figure 8b). The effect of H
2O on sensing CO
2 can be explained by the electrolysis of water on electrode 4, which occurs along with the electrolysis of carbon dioxide (reaction 1) as follows:
The Gibbs energy for carbon dioxide splitting is close to that of water splitting in the considered temperature range (∆G is ~180 kJ/mol and ~200 kJ/mol for CO
2 and H
2O splitting, respectively, at 600 °C [
23]); therefore, the decomposition of H
2O and CO
2 should occur under similar values of the applied voltage. The presence of water vapor in the gas provides a larger number of oxide ions to be pumped out into the inner chamber of the CO
2 cell across the electrolyte; consequently, the limiting current increases as the H
2O concentration rises.
Thus, to avoid the cross-sensitivity effect toward water and ensure the proper detection of CO and CO2 concentrations by the designed sensor, the analyzed gas is to be preliminarily dried.
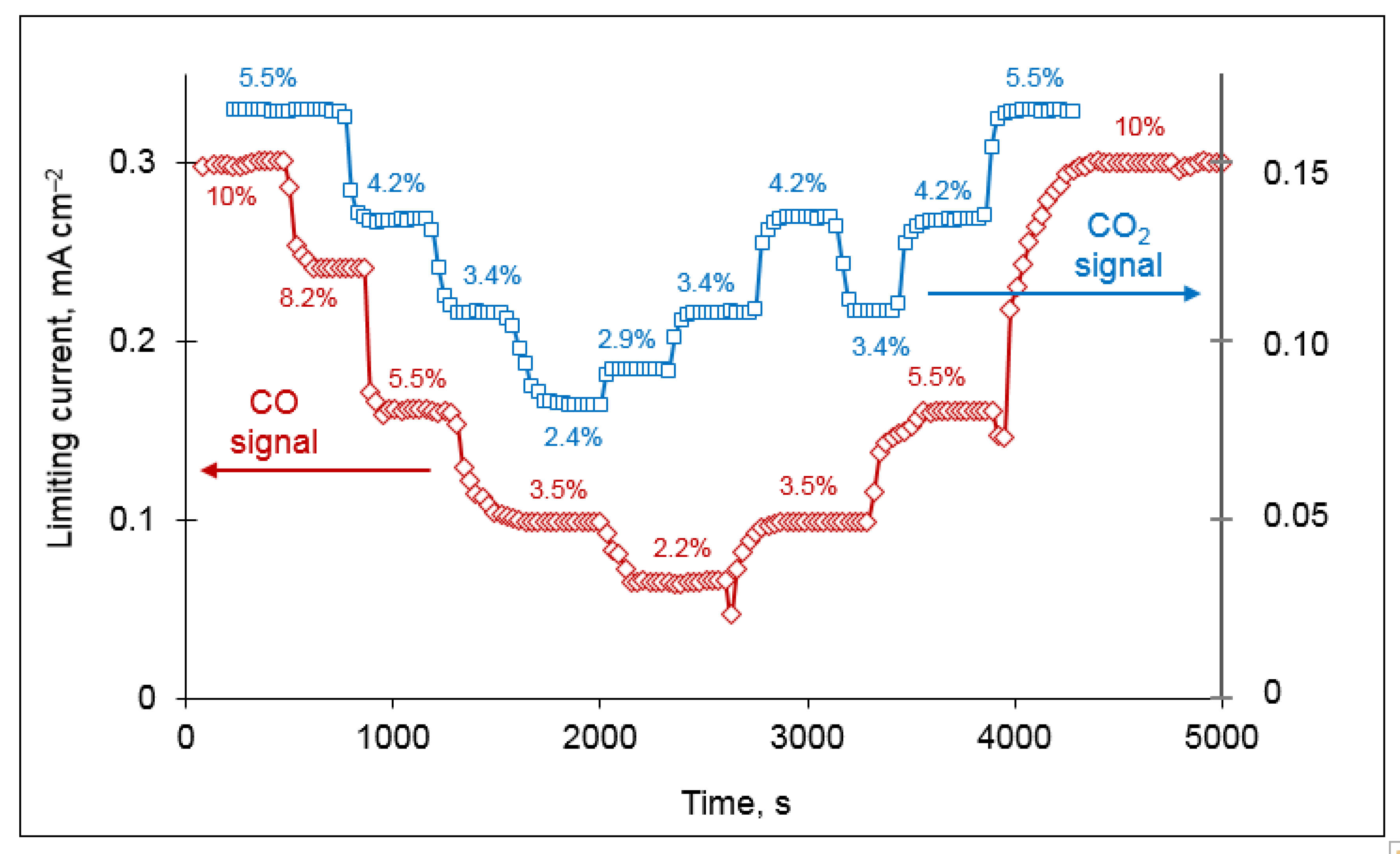
A reliable sensor has to satisfy such requirements as good reproducibility of the sensor signal and a sufficiently short transient time (the time required for 90% change of the sensor signal after a change in gas composition). The dynamic response of the sensor limiting currents to stepwise changes in the concentrations of CO and CO
2 in the gaseous mixtures N
2 + CO and N
2 + CO
2 at 600 °C is presented in
Figure 9. It can be seen that the sensor signal is well reproduced, with a scatter below 1%. The longest transient time for the CO and CO
2 sensing reaches ~6 min, which is in accordance with the requirements of practical applications.
Long-term stability testing of the sensor is currently being conducted; the sensor readings currently remain stable for nearly 2000 h, with a scatter not exceeding 1%. This demonstrates good long-term stability and reliability of the sensor. It is worth noting that the calibration of each sensor of the proposed design is required as well as of all amperometric-type sensors because deviations in geometric parameters of a diffusion barrier may lead to dispersion of the sensor signal. It should be noted that calibration of the amperometric-type sensors is simple due to the linear relationship between the limiting current and the concentration of a target gaseous species (Equation (4)).