Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases Selection and Search Strategy
2.2. Study Selection
- -
- Interventional studies (either randomized or non-randomized controlled clinical trials);
- -
- Observational studies (either analytical or descriptive);
- -
- Case series or Case reports regarding the effects of MFs on osseointegration of dental implants were selected.
3. Results
3.1. In Vitro Studies
3.1.1. Static Magnetic Fields from Permanent Magnets
3.1.2. Pulsed ElectroMagnetic Fields
3.2. In Vivo Studies
3.2.1. Static Magnetic Fields from Permanent Magnets
3.2.2. Pulsed ElectroMagnetic Fields
3.3. Clinical Studies
3.3.1. Static Magnetic Fields from Permanent Magnets
3.3.2. Pulsed ElectroMagnetic Fields
4. Discussion
4.1. Limitations of Available Scientific Research
4.2. Indications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar] [PubMed]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.Y.; Anderson, D.E. Bone and cartilage interfaces with orthopedic implants: A literature review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Goiato, M.C.; Dos Santos, D.M.; Santiago, J.F.; Moreno, A.; Pellizzer, E.P. Longevity of dental implants in type IV bone: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing three major determinants for late implant failures in the Brånemark system. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Huynh-Ba, G.; Friedberg, J.R.; Vogiatzi, D.; Ioannidou, E. Implant failure predictors in the posterior maxilla: A retrospective study of 273 consecutive implants. J. Periodontol. 2008, 79, 2256–2261. [Google Scholar] [CrossRef]
- Sverzut, A.T.; Stabile, G.A.V.; de Moraes, M.; Mazzonetto, R.; Moreira, R.W.F. The influence of tobacco on early dental implant failure. J. Oral Maxillofac. Surg. 2008, 66, 1004–1009. [Google Scholar] [CrossRef]
- Urban, T.; Kostopoulos, L.; Wenzel, A. Immediate implant placement in molar regions: Risk factors for early failure. Clin. Oral Implants Res. 2012, 23, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F. Endosseous implants. J. Am. Dent. Assoc. 2001, 132, 1452. [Google Scholar]
- Lupi, S.M.; Torchia, M.; Rizzo, S. Biochemical modification of titanium oral implants: Evidence from in vivo studies. Materials 2021, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar]
- Annunziata, M.; Guida, L. The effect of titanium surface modifications on dental implant osseointegration. Front. Oral Biol. 2015, 17, 62–77. [Google Scholar]
- Lew, W.Z.; Feng, S.W.; Lee, S.Y.; Huang, H.M. The review of bioeffects of static magnetic fields on the oral tissue-derived cells and its application in regenerative medicine. Cells 2021, 10, 2662. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; D’Angelo, C.; Murmura, G.; Reale, M.; Caputi, S. Human gingival fibroblasts exposed to extremely low-frequency electromagnetic fields: In vitro model of wound-healing improvement. Int. J. Mol. Sci. 2019, 20, 2108. [Google Scholar] [CrossRef]
- Androjna, C.; Fort, B.; Zborowski, M.; Midura, R.J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Jiang, L.; Li, H. Therapeutic effect of pulsed electromagnetic field on bone wound healing in rats. Electromagn. Biol. Med. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Cai, J.; Li, W.; Sun, T.; Li, X.; Luo, E.; Jing, D. Pulsed electromagnetic fields preserve bone architecture and mechanical properties and stimulate porous implant osseointegration by promoting bone anabolism in type 1 diabetic rabbits. Osteoporos. Int. 2018, 29, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Grace, K.L.R.; Revell, W.J.; Brookes, M. The effects of pulsed electromagnetism on fresh fracture healing: Osteochondral repair in the rat femoral groove. Orthopedics 1998, 21, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Glazer, P.A.; Heilmann, M.R.; Lotz, J.C.; Bradford, D.S. Use of electromagnetic fields in a spinal fusion: A rabbit model. Spine 1997, 22, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Takano-Yamamoto, T.; Kawakami, M.; Sakuda, M. Effect of a pulsing electromagnetic field on demineralized bone-matrix-induced bone formation in a bony defect in the premaxilla of rats. J. Dent. Res. 1992, 71, 1920–1925. [Google Scholar] [CrossRef]
- Bruce, G.K.; Howlett, C.R.; Huckstep, R.L. Effect of a static magnetic field on fracture healing in a rabbit radius. Preliminary results. Clin. Orthop. Relat. Res. 1987, 222, 300–306. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, S.; Zhang, M.; Zhou, Z.; Zhang, X.; Li, W.; Cai, H.; Zhao, B.C.; Lee, E.S.; Jiang, H.B. Effects of physical stimulation in the field of oral health. Scanning 2021, 2021, 5517567. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef]
- He, Y.; Yu, L.; Liu, J.; Li, Y.; Wu, Y.; Huang, Z.; Wu, D.; Wang, H.; Wu, Z.; Qiu, G. Enhanced osteogenic differentiation of human bone-derived mesenchymal stem cells in 3-dimensional printed porous titanium scaffolds by static magnetic field through up-regulating Smad4. FASEB J. 2019, 33, 6069–6081. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Memè, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of supercharged cover screw as static magnetic field generator for bone healing, 1st part: In vitro enhancement of osteoblast-like cell differentiation. J. Biol. Regul. Homeost. Agents 2017, 31, 215–220. [Google Scholar] [PubMed]
- Kim, H.J.; Chang, I.T.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Jang, J.H. Effect of magnetic field on the fibronectin adsorption, cell attachment and proliferation on titanium surface. Clin. Oral Implants Res. 2005, 16, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, W.; Yan, L.; Cheng, S.; Li, X.; Qiao, S. 3D-printed Ti6Al4V scaffolds combined with pulse electromagnetic fields enhance osseointegration in osteoporosis. Mol. Med. Rep. 2021, 23, 410. [Google Scholar] [CrossRef]
- Bloise, N.; Petecchia, L.; Ceccarelli, G.; Fassina, L.; Usai, C.; Bertoglio, F.; Balli, M.; Vassalli, M.; De Angelis, M.G.C.; Gavazzo, P.; et al. The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces. PLoS ONE 2018, 13, e0199046. [Google Scholar] [CrossRef]
- Jing, D.; Zhai, M.; Tong, S.; Xu, F.; Cai, J.; Shen, G.; Wu, Y.; Li, X.; Xie, K.; Liu, J.; et al. Pulsed electromagnetic fields promote osteogenesis and osseointegration of porous titanium implants in bone defect repair through a Wnt/β-catenin signaling-associated mechanism. Sci. Rep. 2016, 6, 32045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, Y.; Li, F.; Li, D.; Jing, D.; Guo, T.; Luo, E.; Ma, C. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014, 10, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Aybar, B.; Ergüven, M.; Emes, Y.; Bultan, Ö.; Akça, K.; Yalçin, S.; Baysal, U.; Işsever, H.; Çehreli, M.C.; et al. The effects of pulsed electromagnetic field (PEMF) on osteoblast-like cells cultured on titanium and titanium-zirconium surfaces. J. Craniofac. Surg. 2013, 24, 2127–2134. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Sbarra, M.S.; Visai, L.; De Angelis, M.G.C.; Mazzini, G.; Benazzo, F.; Magenes, G. Ultrasonic and electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto a titanium plasma-spray surface. Tissue Eng. Part C Methods 2009, 15, 233–242. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Magenes, G. Electromagnetically enhanced coating of a sintered titanium grid with human SAOS-2 osteoblasts and extracellular matrix. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 3582–3585. [Google Scholar]
- Fassina, L.; Saino, E.; Visai, L.; Silvani, G.; De Angelis, M.G.C.; Mazzini, G.; Benazzo, F.; Magenes, G. Electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffolds. J. Biomed. Mater. Res. Part A 2008, 87, 750–759. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Magenes, G. Physically enhanced coating of a titanium plasma-spray surface with human SAOS-2 osteoblasts and extracellular matrix. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6415–6418. [Google Scholar]
- Li, X.; Wu, J.; Li, D.; Zou, Q.; Man, Y.; Zou, L.; Li, W. Pro-osteogenesis and in vivo tracking investigation of a dental implantation system comprising novel mTi implant and HYH-Fe particles. Bioact. Mater. 2021, 6, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamada, S.; Jinno, Y.; Arai, K.; Galli, S.; Ichikawa, T.; Jimbo, R. Bone-forming effect of a static magnetic field in rabbit femurs. Int. J. Periodontics Restor. Dent. 2019, 39, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Diiorio, D.; Meme, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of supercharged cover screw as static magnetic field generator for bone healing, 2nd part: In vivo enhancement of bone regeneration in rabbits. J. Biol. Regul. Homeost. Agents 2017, 31, 481–485. [Google Scholar] [PubMed]
- Kim, E.C.; Leesungbok, R.; Lee, S.W.; Hong, J.Y.; Ko, E.J.; Ahn, S.J. Effects of static magnetic fields on bone regeneration of implants in the rabbit: Micro-CT, histologic, microarray, and real-time PCR analyses. Clin. Oral Implants Res. 2017, 28, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Leesungbok, R.; Ahn, S.J.; Lee, S.W.; Park, G.H.; Kang, J.S.; Choi, J.J. The effects of a static magnetic field on bone formation around a sandblasted, large-grit, acid-etched-treated titanium implant. J. Oral Implantol. 2013, 39, 248–255. [Google Scholar] [CrossRef]
- Nunes, C.M.M.; Ferreira, C.L.; Bernardo, D.V.; Lopes, C.C.R.; Collino, L.; da Silva Lima, V.C.; de Camargo Reis Mello, D.; de Vasconcellos, L.M.R.; Jardini, M.A.N. Evaluation of pulsed electromagnetic field protocols in implant osseointegration: In vivo and in vitro study. Clin. Oral Investig. 2021, 25, 2925–2937. [Google Scholar] [CrossRef]
- Cai, J.; Shao, X.; Yang, Q.; Yang, Y.; Yan, Z.; Luo, E.; Feng, X.; Jing, D. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone 2020, 133, 115266. [Google Scholar] [CrossRef]
- Barak, S.; Neuman, M.; Iezzi, G.; Piattelli, A.; Perrotti, V.; Gabet, Y. A new device for improving dental implants anchorage: A histological and micro-computed tomography study in the rabbit. Clin. Oral Implants Res. 2016, 27, 935–942. [Google Scholar] [CrossRef]
- Grana, D.R.; Marcos, H.J.A.; Kokubu, G.A. Pulsed electromagnetic fields as adjuvant therapy in bone healing and peri-implant bone formation: An experimental study in rats. Acta Odontol. Latinoam. 2008, 21, 77–83. [Google Scholar]
- Akca, K.; Sarac, E.; Baysal, U.; Fanuscu, M.; Chang, T.L.; Cehreli, M. Micro-morphologic changes around biophysically-stimulated titanium implants in ovariectomized rats. Head Face Med. 2007, 3, 28. [Google Scholar] [CrossRef][Green Version]
- Özen, J.; Atay, A.; Orucß, S.; Dalkiz, M.; Beydemir, B.; Develi, S. Evaluation of pulsed electromagnetic fields on bone healing after implant placement in the rabbit mandibular model. Turk. J. Med. Sci. 2004, 34, 91–95. [Google Scholar]
- Buzzá, E.P.; Shibli, J.A.; Barbeiro, R.H.; de Albergaria Barbosa, J.R. Effects of electromagnetic field on bone healing around commercially pure titanium surface: Histologic and mechanical study in rabbits. Implant Dent. 2003, 12, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ochi, M.; Abiko, Y.; Hirose, Y.; Kaku, T.; Sakaguchi, K. Pulsed electromagnetic fields promote bone formation around dental implants inserted into the femur of rabbits. Clin. Oral Implants Res. 2000, 11, 354–360. [Google Scholar] [CrossRef]
- Ijiri, K.; Matsunaga, S.; Fukuyama, K.; Maeda, S.; Sakou, T.; Kitano, M.; Senba, I. The effect of pulsing electromagnetic field on bone ingrowth into a porous coated implant. Anticancer Res. 1996, 16, 2853–2856. [Google Scholar] [PubMed]
- Gujjalapudi, M.; Anam, C.; Mamidi, P.; Chiluka, R.; Gautamkumar, A.; Bibinagar, R. Effect of magnetic field on bone healing around endosseous implants—An in-vivo study. J. Clin. Diagn. Res. 2016, 10, ZF01–ZF04. [Google Scholar] [CrossRef] [PubMed]
- Siadat, H.; Bassir, S.H.; Alikhasi, M.; Shayesteh, Y.S.; Khojasteh, A.; Monzavi, A. Effect of static magnetic fields on the osseointegration of immediately placed implants: A randomized controlled clinical trial. Implant Dent. 2012, 21, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bud, E.S.; Bud, A.; Păcurar, M.; Vlasa, A.; Lazăr, A.P.; Lazăr, L. Clinical studies regarding electromagnetic stimulation in proximity of dental implants on patients with/without orthodontic treatment. J. Clin. Med. 2020, 9, 3983. [Google Scholar] [CrossRef]
- Nayak, B.P.; Dolkart, O.; Satwalekar, P.; Kumar, Y.P.; Chandrasekar, A.; Fromovich, O.; Yakobson, E.; Barak, S.; Dayube, U.; Shibli, J.A. Effect of the pulsed electromagnetic field (PEMF) on dental implants stability: A randomized controlled clinical trial. Materials 2020, 13, 1667. [Google Scholar] [CrossRef]
- Barak, S.; Matalon, S.; Dolkart, O.; Zavan, B.; Mortellaro, C.; Piattelli, A. Miniaturized electromagnetic device abutment improves stability of the dental implants. J. Craniofac. Surg. 2019, 30, 1055–1057. [Google Scholar] [CrossRef]
- EI Fadly, M.A.; Selim, H.A.; Katamish, M.A.; Metwally, S.A. Evaluation of the effect of pulsed electromagnetic fields on osseointegration of immediate dental implants. Egypt. J. Oral Maxillofac. Surg. 2014, 5, 84–91. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.; Hall, B.J.; Doyle, J.; Waters, E. “Scoping the scope” of a cochrane review. J. Public Health 2011, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Speranza, M.C.; Scianguetta, S.; Tramontano, A.; Bencivenga, D.; Stampone, E.; Negri, A.; Nobili, B.; Locatelli, F.; et al. Iron overload enhances human mesenchymal stromal cell growth and hampers matrix calcification. Biochim. Biophys. Acta 2016, 1860, 1211–1223. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Bencivenga, D.; Stampone, E.; Perrotta, S.; Oliva, A.; Della Ragione, F. Tyrosine kinase inhibitors and mesenchymal stromal cells: Effects on self-renewal, commitment and functions. Oncotarget 2017, 8, 5540–5565. [Google Scholar] [CrossRef]
- Galli, C.; Pedrazzi, G.; Guizzardi, S. The cellular effects of pulsed electromagnetic fields on osteoblasts: A review. Bioelectromagnetics 2019, 40, 211–233. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and mechanisms of exogenous electromagnetic field on bone cells: A review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus—A systematic review. Int. J. Implant Dent. 2016, 2, 5. [Google Scholar] [CrossRef]
- De Medeiros, F.C.F.L.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar] [PubMed]
- Gaetani, R.; Ledda, M.; Barile, L.; Chimenti, I.; De Carlo, F.; Forte, E.; Ionta, V.; Giuliani, L.; D’Emilia, E.; Frati, G.; et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc. Res. 2009, 82, 411–420. [Google Scholar] [CrossRef] [PubMed]
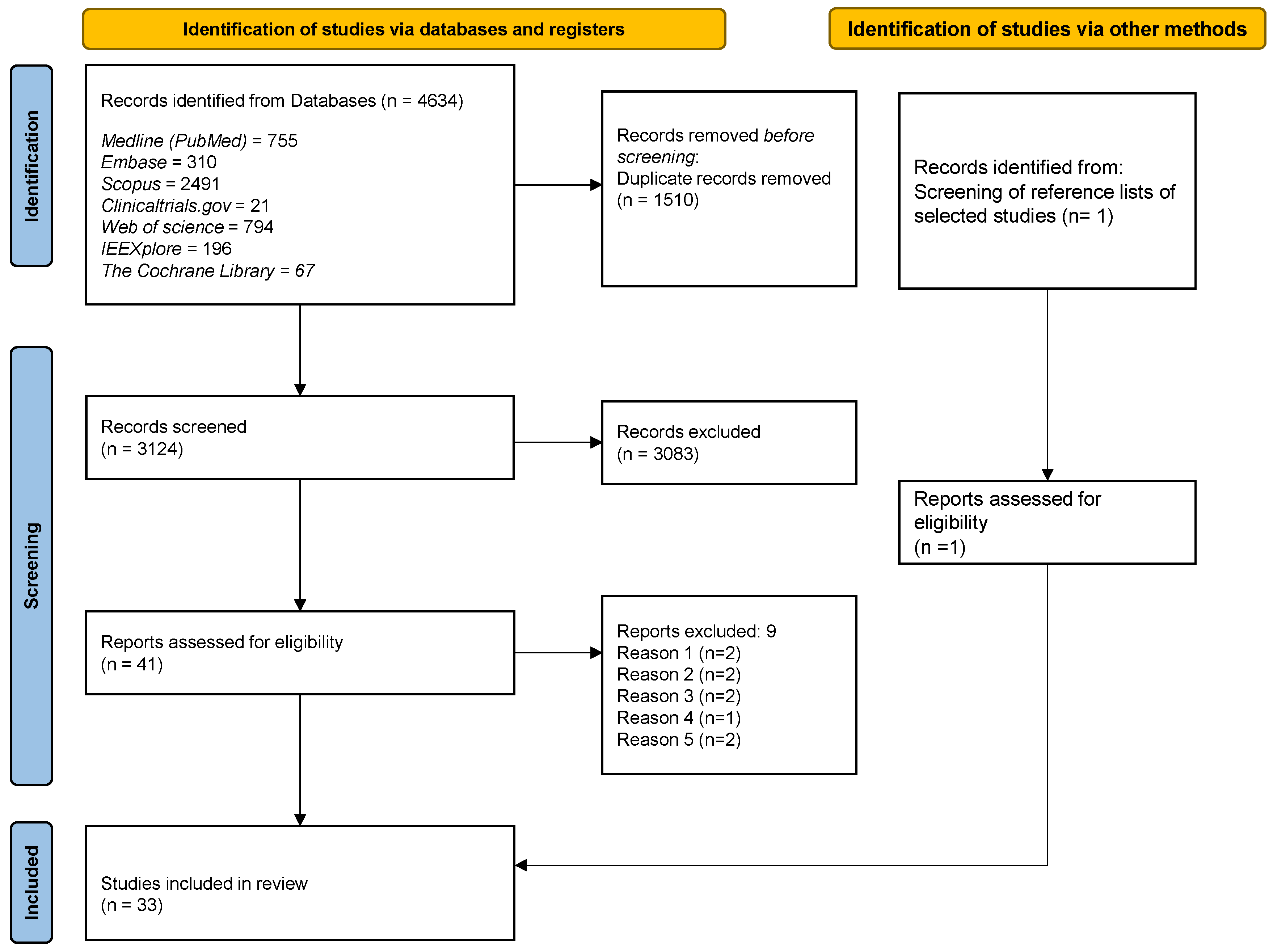
Electronic databases:
|
| Filters: English language |
Inclusion criteria:
|
Exclusion criteria:
|
Additional sources:
|
| Authors, Year | Stimulation Details | Study Groups | Follow-Up | Main Findings |
|---|---|---|---|---|
| SFM Stimulation | ||||
| He et al., 2019 [31] | Amplitude: 50, 100, 150 mT | hBMSC cultured on Ti scaffolds with SFM at 50 mT, 100 mT and 150 mT and without SFM. | 7, 14 days | Positive effects of SFM on osteoblast-related factors and ECM production, but not on cell proliferation and viability. |
| Bambini et al., 2017a [32] | Further details are not reported | MG63 cells were cultured with: Ti implant with magnetic cover screw; implant without magnetic cover screw; cells in direct contact with the magnetic cover screw and magnets free in the culture medium (only cells). | 24, 48, 72 h | Negative effect of SFM on cell proliferation. Positive effects of SFM on transcription of osteogenesis genes and matrix mineralization. |
| Kim et al., 2005 [33] | Amplitude: 1, 2, 3, 5, 7, 10 mT | TE-85 cells seeded onto Ti disks at different distances from Nd–Fe–B magnet (1, 2, 3, 5, 7, 10 mT) and control group. | 2 h | Positive effects of SFM on cell attachment, but not on cell proliferation. |
| PEMF Stimulation | ||||
| Ye et al., 2021 [34] | Amplitude: 1 mT; Frequency: 50 Hz | BMSCs from osteoporotic rabbits cultured on porous Ti implants with PEMF stimulation 2 h/day and control cells. | 1, 4, 7, 14, 21 days | Positive effects of PEMF on cell proliferation spreading and lamellipodia extension, expression of osteoblast-related factors and ECM mineralization. |
| Bloise et al., 2018 [35] | Amplitude: 2 ± 0.2 mT; Frequency: 75 ± 2 Hz; Duty cycle 1/10 | hBM-MSCs were grown in osteogenic or proliferation medium on TiO2 substrate with/without PEMF stimulation to evaluate the effect of surface nano-topography in combination with PEMF exposure in cell differentiation. | 3, 28 days | Positive effects of PEMF on osteoblast-related factors and intracellular Ca concentration. |
| Jing et al., 2016 [36] | Amplitude: 2.0 mT Frequency: 15 Hz Pulse shaping: pulsed bursts (burst width, 5 ms; pulse width, 0.2 ms; pulse wait, 0.02 ms; burst wait, 60 ms; pulse rise, 0.3 μs; pulse fall, 2.0 μs), | Osteoblast-like MC3T3-E1 cells exposed to PEMF and unexposed control cells on porous Ti implants | 3 days | Positive effects of PEMF on cell proliferation and attachment. |
| Wang et al., 2014 [37] | Amplitude: 48 mT Frequency: 15 Hz Pulse shaping: pulsed bursts | Rat calvarial osteoblasts plated on 3 different implant surfaces, with PEMF and without (control): polished flat; Micro-topographical (sand-blasted with large grit and acid etched); Nano-topographical (anodized nanotubular-structured surface). | 0.5; 1; 1.5 h –1, 4, 7 days | Positive effects of PEMF on cell adhesion and proliferation, on osteoblast-related factors expression and ECM mineralization, expecially on nano-structured surfaces. |
| Atalay et al., 2013 [38] | Amplitude: 0.2 mT; Frequency: not reported | Rat calvarial osteoblasts plated on 3 different Ti discs, with PEMF and without (control): TiZr discs with hydrophilic sandblasted acid-etched surfaces; cpTi discs with hydrophilic sandblasted acid-etched surfaces; machine surface cpTi discs. | 24, 72 h | Positive effects of PEMF on cell proliferation on cpTi surfaces, but not on TiZr surfaces. |
| Fassina et al., 2009 [39] | US: Average power 149 mW; Frequency: 1.5 MHz PEMF: Amplitude: 2 ± 0.2 mT; Frequency 75 ± 2 Hz; Duty cycle 1/10 | SAOS-2 cultured on Ti plasma spray disks divided into 3 groups: cells receiving US waves; cells receiving electromagnetic waves; cells not treated (control). | 22 days | Positive effects of PEMF and US on cell proliferation and ECM production. |
| Fassina et al., 2008a [40] | Amplitude: 2 ± 0.2 mT; Frequency 75 ± 2 Hz; Duty Cycle: 1/10 | SAOS-2 cultured on Ti sintered grids exposed or not (control) to PEMF. | 22 days | Positive effects of PEMF on cell proliferation and ECM production. |
| Fassina et al., 2008b [41] | PEMF: intensity 2 ± 0.2 mT; Frequency: 75 ± 2 Hz; Duty cycle: 1/10 | SAOS-2 cultured on Ti fiber-mesh sheets exposed or not (control) at PEMF. | 22 days | Positive effects of PEMF on cell proliferation, osteoblast-related factors expression and ECM production. |
| Fassina et al. 2007 [42] | US: Average power 149 mW; Frequency: 1.5 MHz PEMF: Amplitude: 2 ± 0.2 mT; Frequency 75 ± 2 Hz; Duty cycle 1/10 | SAOS-2 cultured on Ti plasma spray disks exposed to PEMF, to ultrasonic stimulus, or not exposed (control). | 22 days | Positive effects of PEMF and US on cell proliferation and ECM production. |
| Authors, Year | Type of Stimulation | Implant Site, Animal Model | Study Groups | Follow-Up | Main Findings |
|---|---|---|---|---|---|
| SFM Stimulation | |||||
| Li et al., 2021 [43] | Amplitude: 0.3–9.4 mT in the middle position, 0.2–1.4 mT in the upper or lower position of implant | Alveolar bone, dog | 2 dogs receiving Ti + HA, mTi + HA and mTi + HYH-Fe implants; samples harvested at 8 weeks (dog1) and 12 weeks (dog2). 12 weeks used for in situ fluorescence evaluation. | 8, 10, 12 weeks | Positive effects of SFM on trabecular bone formation. |
| He et al., 2019 [31] | Amplitude: 100 mT | Mandibular ramus, rat | 12 rats: 6 stimulated by permanent magnets 12 h/day and 6 controls. | 6, 12 weeks | Positive effects of SFM on bone ingrowth and osseointegration of Ti scaffolds. |
| Naito et al., 2019 [44] | Amplitude: 43–162 mT | Femur, rabbit | 6 rabbits (12 implants): 6 containing neodymium magnets and 6 controls. | 12 weeks | Positive effects of SFM on BIC. |
| Bambini et al., 2017b [45] | Characteristics not reported | Tibia, rabbit | 12 rabbits (24 implants): 1 implant receiving magnetic cover screw, 1 control implant in each animal. | 15, 30 days | Positive results of SFM on BIC. |
| Kim et al., 2017 [46] | Amplitude: 15 mT | Tibia, rabbit | 27 rabbits (54 implants), each animal received 1 implant exposed to magnet and 1 control. | 1, 4, 8 weeks | Positive effects of SMF on bone formation and BIC. |
| Leesungbok et al., 2013 [47] | Amplitude: 15.34 mT | Tibia, rabbit | 10 rabbits (40 sandblasted, large-grit, acid-etched implants): test rabbits treated with neodymium magnets and controls. | 3, 6 weeks | Positive effects of SFM on BIC at 3 weeks. |
| PEMF Stimulation | |||||
| Ye et al., 2021 [34] | Amplitude: 1 mT; Frequency 50 Hz | Femur, rabbit | 12 osteoporotic rabbits receiving porous Ti implants and exposed to PEMF 2 h/day and 12 osteoporotic rabbits receiving porous Ti implants only. | 6, 12 weeks | Positive effects of PEMF on bone formation on porous Ti implants. |
| Nunes et al., 2020 [48] | Amplitude: 1 ± 1 mT in 200 μs Frequency: 15 Hz Pulse shaping: 25 cycles at each period | Tibia, rat | 60 rats (180 implants); 20 control group, 20 with 3 h/day exposure to PEMF, 20 with 1 h/day exposure to PEMF. | 3, 7, 21, 45 days | Positive effects of PEMF on bone parameters, implant removal torque and BIC. |
| Cai et al., 2020 [49] | Amplitude: 2.0 mT. Frequency: 15 Hz Pulse shaping: pulsed bursts (burst width, 5 ms; pulse width, 0.2 ms; pulse wait, 0.02 ms; burst wait, 60 ms; pulse rise, 0.3 μs; pulse fall, 2.0 μs) | Femur, rabbit | 24 rabbits (24 implants): control group; osteoporotic rabbits group; osteoporotic rabbits with PEMF exposure group. | 4 weeks | Positive effects of PEMF on peri-implant bone and osteoblast-related factors. |
| Cai et al., 2018 [21] | Amplitude: 2.0 mT. Frequency: 15 Hz Pulse shaping: burst width, 5 ms; pulse width, 0.2 ms; pulse wait, 0.02 ms; burst wait, 60 ms; pulse rise, 0.3 μs; pulse fall, 2.0 μs | Femur, rabbit | 24 rabbits: 8 diabetic rabbits with 2 h/day PEMF exposure for 8 weeks; 8 diabetic rabbits; 8 non-diabetic rabbits (control). | 8 weeks | Positive effects of PEMF on peri-implant bone. |
| Jing et al., 2016 [36] | Amplitude: 2.0 mT. Frequency: 15 Hz Pulse shaping: pulsed bursts (burst width, 5 ms; pulse width, 0.2 ms; pulse wait, 0.02 ms; burst wait, 60 ms; pulse rise, 0.3 μs; pulse fall, 2.0 μs) | Femur, rabbit | 24 rabbits (24 implants): control group and test group (with PEMF exposure). | 6, 12 weeks | Positive effects of PEMF on peri-implant bone growth and on expression of osteoblast-related factors. |
| Barak et al., 2016 [50] | Amplitude 0.4–0.2 mT (source at 1 and 2 mm away from the implant surface, respectively); Frequency: 10 Hz | Tibia, rabbit | 22 rabbits. (22 implants): 11 implants with a healing cup emitting PEMF; 11 implants with a control healing cup. | 2, 4 weeks | Positive effects of PEMF on BIC. |
| Grana et al., 2008 [51] | Amplitude: 72 mT; Pulse shaping: sinusoidal bursts at 50 Hz for 60 ms, then a dead time of 450 ms | Tibia, rat | 60 rats: 30 rats in the test group treated with PEMF twice/day for 30 min each session; 30 rats in the control group. | 5, 10, 20 days | Positive effects of PEMF on BIC and peri-implant ossification. |
| Akca et al., 2007 [52] | MECHVIB: frequency 50 Hz PEMF: intensity 0.2 mT; frequency not reported | Tibia, rat | 15 osteoporotic rats (30 implants): 5 rats in the control group, 5 rats treated with PEMF 4 h/day, 5 rats treated with MECHVIB 14 min/day. | 2 weeks | Positive effects of MECHVIB-stimulated on peri-implant bone volume. No positive effects of PEMF on peri-implant bone volume. |
| Ozen et al., 2004 [53] | Amplitude: 0.2 mT; Frequency 100 Hz; Duty Cycle: 1/400 | Mandible, rabbit | 28 rabbits (28 implants): 14 in the control group; 14 exposed to PEMF 4 h/day for 2 weeks. | 2, 8 weeks | Positive effects of PEMF on osteoblast number and peri-implant bone formation at 8 weeks. |
| Buzzá et al., 2003 [54] | Amplitude: not reported; Frequency: 20 Mc pulse width 85 μs; intensity not reported | Tibia, rabbit | 12 rabbits: 6 rabbits in the PEMF stimulated group; 6 rabbits in the control group. | 21, 42 days | No positive effects of PEMF on peri-implant bone or removal torque. |
| Matsumoto et al., 2000 [55] | Amplitude: 0.2 mT, 0.3 mT, 0.8 mT; Frequency 100 Hz, Duty cycle 1/400 | Femur, rabbit | 45 rabbits: rabbits receiving PEMF at 0.2 mT or 0.3 mT or 0.8 mT for 8 h/day for 2 weeks; rabbits receiving PEMF at 0.2 mT for 4 h/day or 8 h/day for 2 weeks; rabbits receiving PEMF at 0.2 mT for 1 or 2 or 4 weeks; control rabbits. | 1, 2, 4 weeks | Positive effects of PEMF on BIC. |
| Ijiri et al., 1996 [56] | Amplitude: 0.2 mT; Frequency 10 Hz; Duty cycle 1/4000 | Humerus, rabbit | 20 rabbits: 5 receiving PEMF 5 h/day; 5 receiving PEMF 10 h/day; 5 receiving immobilization 5 h/day; 5 receiving immobilization 10 h/day. | 2 weeks | Positive effects of PEMF on peri-implant bone. |
| Authors, Year | Design | Type and Time of Stimulation | Patients Carachteristics | Number of Implants | Implant Carachteristics | Implant Location | Placement Protocol | Loading Protocol | Follow-Up | Quality Assessment (Judgment; Tool) | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SFM Stimulation | |||||||||||
| Gujjalapudi et al., 2016 [57] | CCT | Amplitude: 50–245 mT; 12–15 h/day for 90 days | 10 patients (age between 50–75 years) | 20 implants, 2 per patient (one exposed to SFM, one as control) | NR | Anterior mandible | 3–6 months after extraction | NR | RFA at 0, 1, 2, 3 months | Moderate risk; ROBINS-I | Positive effects of SFM on implant stability at 1, 2 and 3 months. |
| Siadat et al., 2012 [58] | RCT | Amplitude: 186 mT; 24 h/day for 90 days | 20 patients (11 F, 9 M; age between 23–60 years) | 20 implants, 1 per patient (10 exposed to SMF, 10 controls) | Rough (blasted/acid etched) surface; 4.1 mm in diameter; 10–12 mm in lenght | Anterior maxilla | Immediate placement | Conventional loading | RFA and radiographs at 0, 1, 2, 3 months | Some concerns; RoB 2 | Positive effects of SFM on implant stability at 1 month and on peri-implant marginal bone loss at 2 months. |
| PEMF Stimulation | |||||||||||
| Bud et al., 2020 [59] | CCT | Characteristics not reported; 24 h/day for 60 days | 29 patients (14 F, 15 M; age between 30–60 years) | 53 implants (25 exposed to PEMF, 28 controls) | Rough surface; diameter and length NR | NR | NR | NR | Cone Beam Tomography at 0 and 60 days | Moderate risk; ROBINS-I | No positive effects of PEMF on bone radiodensity around implants. |
| Nayak et al., 2020 [60] | RCT | Amplitude: 0.05–0.5 mT; Frequency 10–50 kHz; 24 h/day for 30 days | 19 patients (10 F, 9 M; average age 37+/−9.7) | 40 implants (20 exposed to PEMF, 20 controls) | Rough (blasted/acid etched) surface; 3.75 in diameter; 10–11.5 mm in lenght | Maxilla and mandible | 3–6 months after extraction | NR | RFA at 0, 2, 4, 8, 12 weeks; radiographs at 0, 6 and 12 weeks | Some conerns; RoB 2 | Positive effects of PEMF on implant stability and peri-implant bone loss. |
| Barak et al., 2019 [61] | Retrospective study | Characteristics not reported; 24 h/day for 8 weeks | 12 patients (7 F, 5 M; age between 34–69 years) | 28 implants (12 exposed to PEMF, 16 controls) | Rough surface; diameter and length NR | Maxilla and mandible | NR | NR | RFA at 0, 2, 4 and 8 weeks | Moderate risk; ROBINS-I | Positive effects of PEMF on implant stability. |
| EI Fadly et al., 2014 [62] | RCT | Amplitude: not reported; Frequency 2–4 Hz; 2 h/day for 12 days | 8 patients (7 F, 1 M; age between 25–45 years) | 12 implants (6 exposed to PEMF, 6 controls) | Surface carachteristics NR; diameter: 3.4–3.8 mm; length 12–14 mm. | Maxillary anterior or premolar region | Immediate placement | NR | RFA at 0, 3, 6 months; radiographa at 0, 1, 3, 6 and 12 months | Some concerns; RoB 2 | Positive effects of PEMF on peri-implant radiodensity and peri-implant bone loss, but not on implant stability of immediate post-exctravite implants. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecoro, G.; Bencivenga, D.; Annunziata, M.; Cennamo, N.; Della Ragione, F.; Formisano, A.; Piccirillo, A.; Stampone, E.; Volpe, P.A.; Zeni, L.; et al. Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review. Appl. Sci. 2022, 12, 4496. https://doi.org/10.3390/app12094496
Cecoro G, Bencivenga D, Annunziata M, Cennamo N, Della Ragione F, Formisano A, Piccirillo A, Stampone E, Volpe PA, Zeni L, et al. Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review. Applied Sciences. 2022; 12(9):4496. https://doi.org/10.3390/app12094496
Chicago/Turabian StyleCecoro, Gennaro, Debora Bencivenga, Marco Annunziata, Nunzio Cennamo, Fulvio Della Ragione, Alessandro Formisano, Angelantonio Piccirillo, Emanuela Stampone, Pio Antonio Volpe, Luigi Zeni, and et al. 2022. "Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review" Applied Sciences 12, no. 9: 4496. https://doi.org/10.3390/app12094496
APA StyleCecoro, G., Bencivenga, D., Annunziata, M., Cennamo, N., Della Ragione, F., Formisano, A., Piccirillo, A., Stampone, E., Volpe, P. A., Zeni, L., Borriello, A., & Guida, L. (2022). Effects of Magnetic Stimulation on Dental Implant Osseointegration: A Scoping Review. Applied Sciences, 12(9), 4496. https://doi.org/10.3390/app12094496












