Insight into the Preparation of MgAl-Layered Double Hydroxide (LDH) Intercalated with Nitrates and Chloride Adsorption Ability Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Agents
2.2. Preparation of LDH
2.3. Characterization of LDH
2.4. Chloride Adsorption Behavior of the Synthesized LDH Powders
3. Results and Discussion
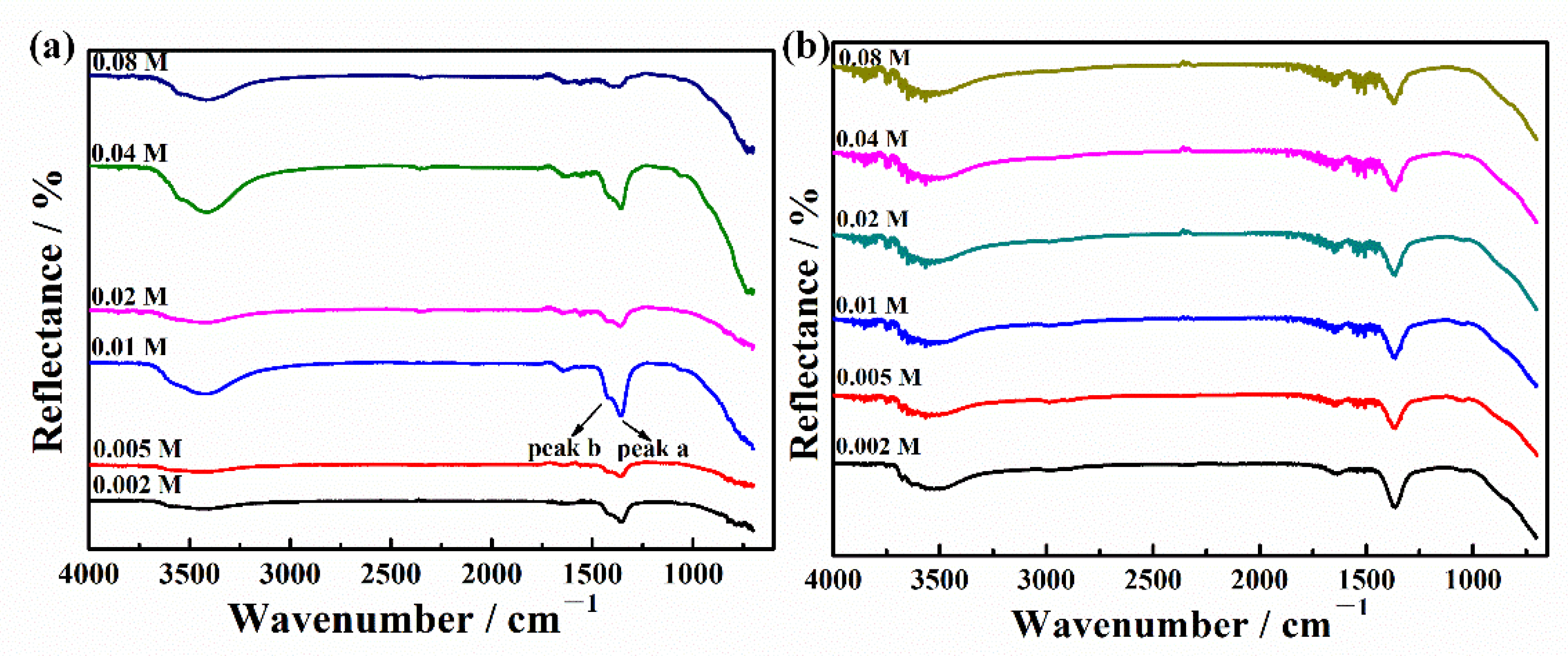
3.1. Characterization of LDH Powder
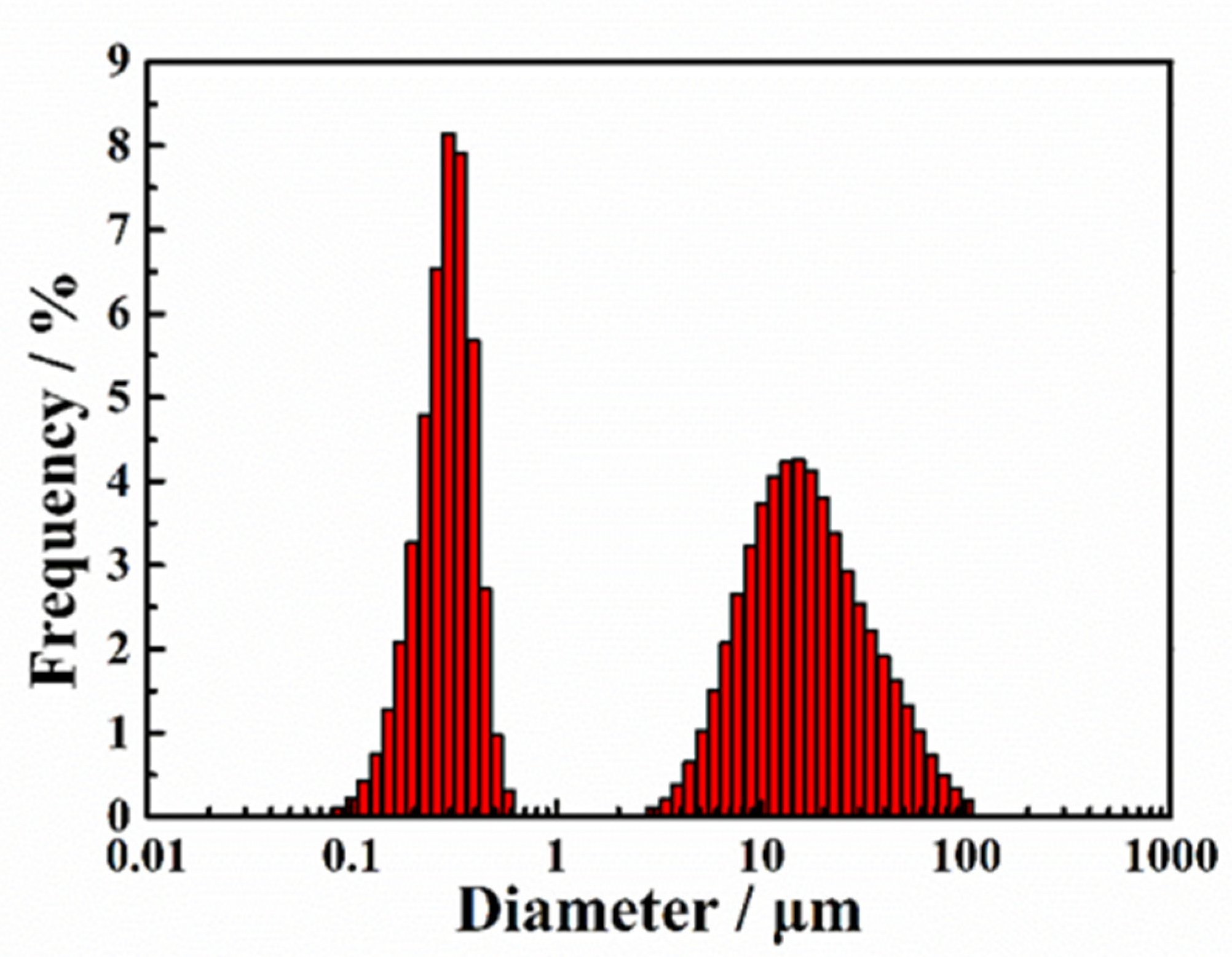
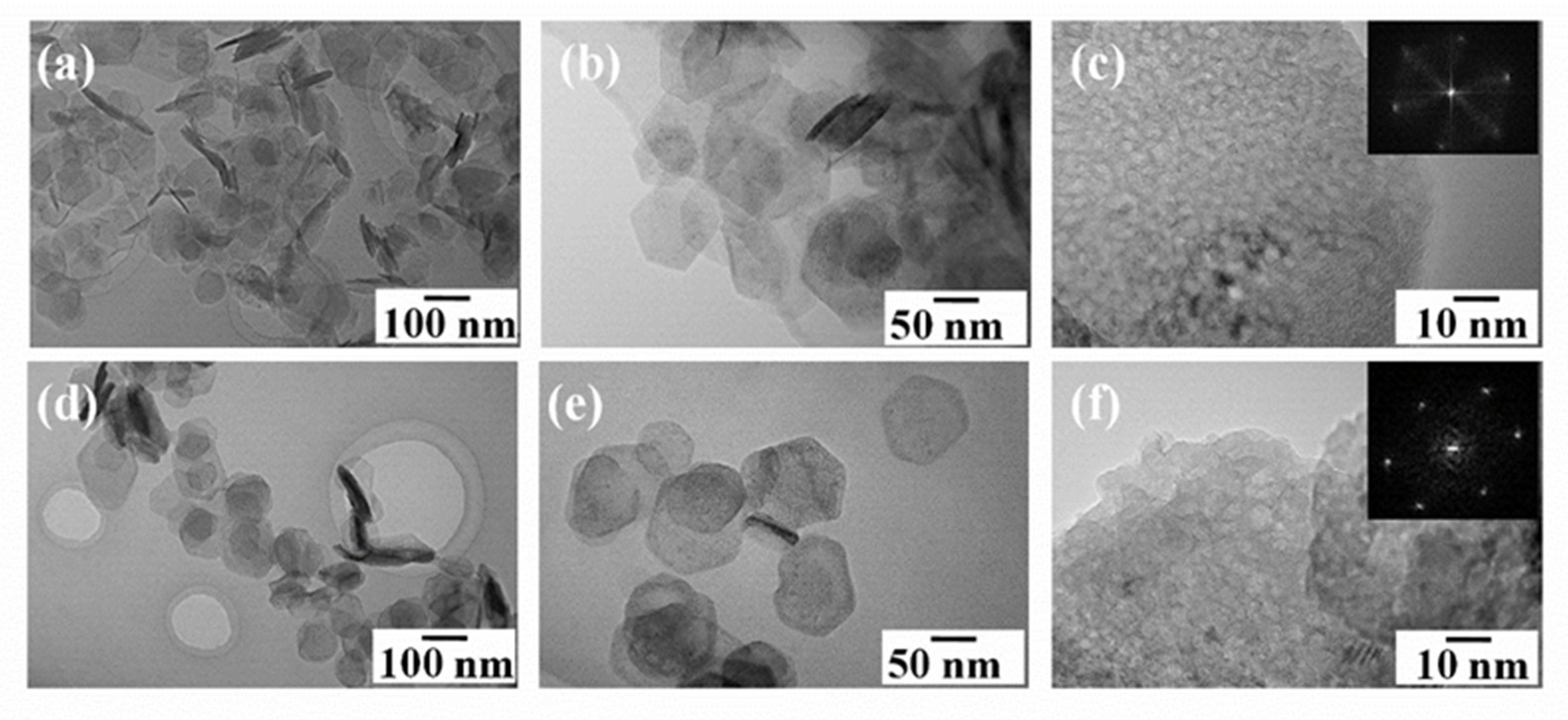
3.1.1. Morphology
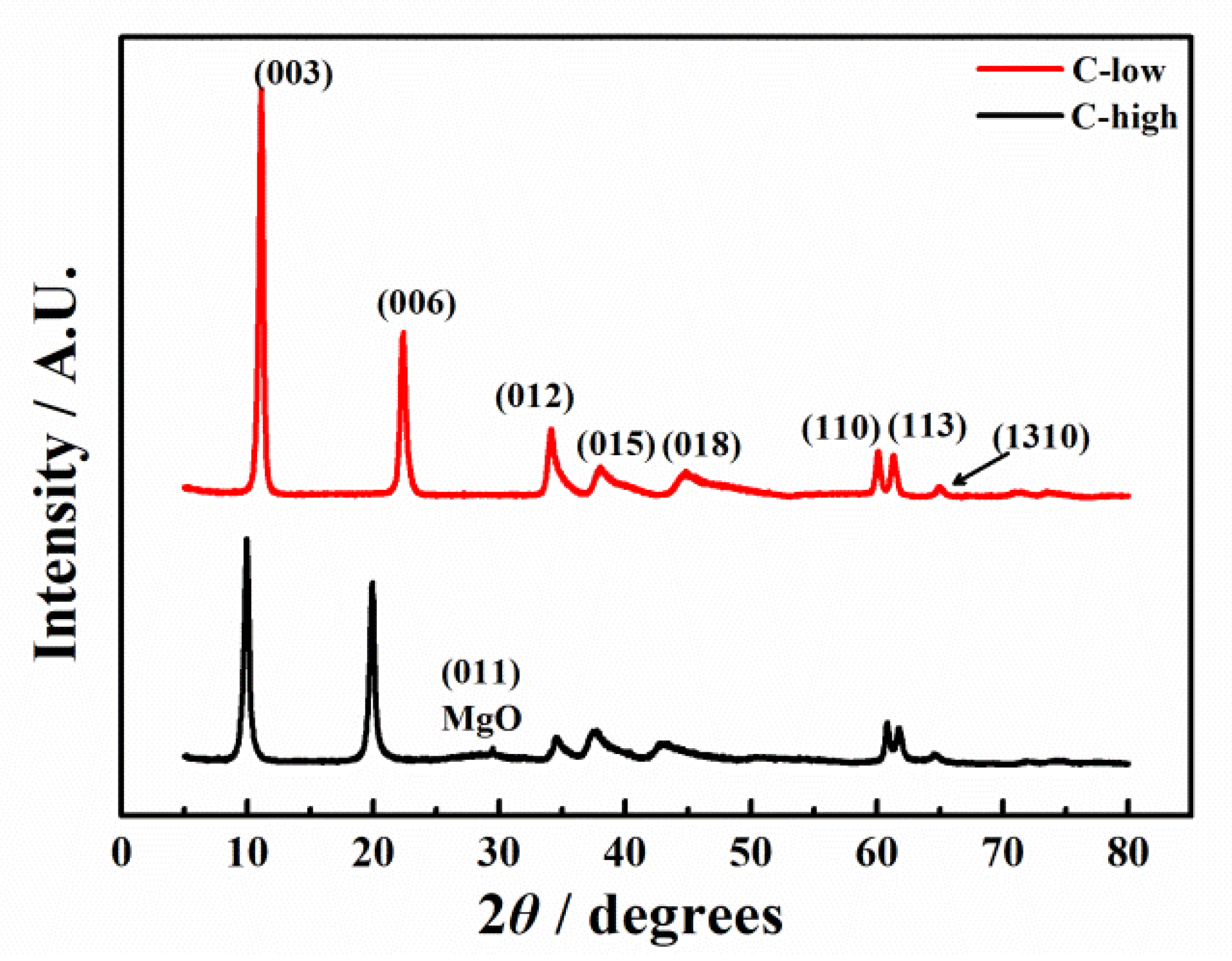
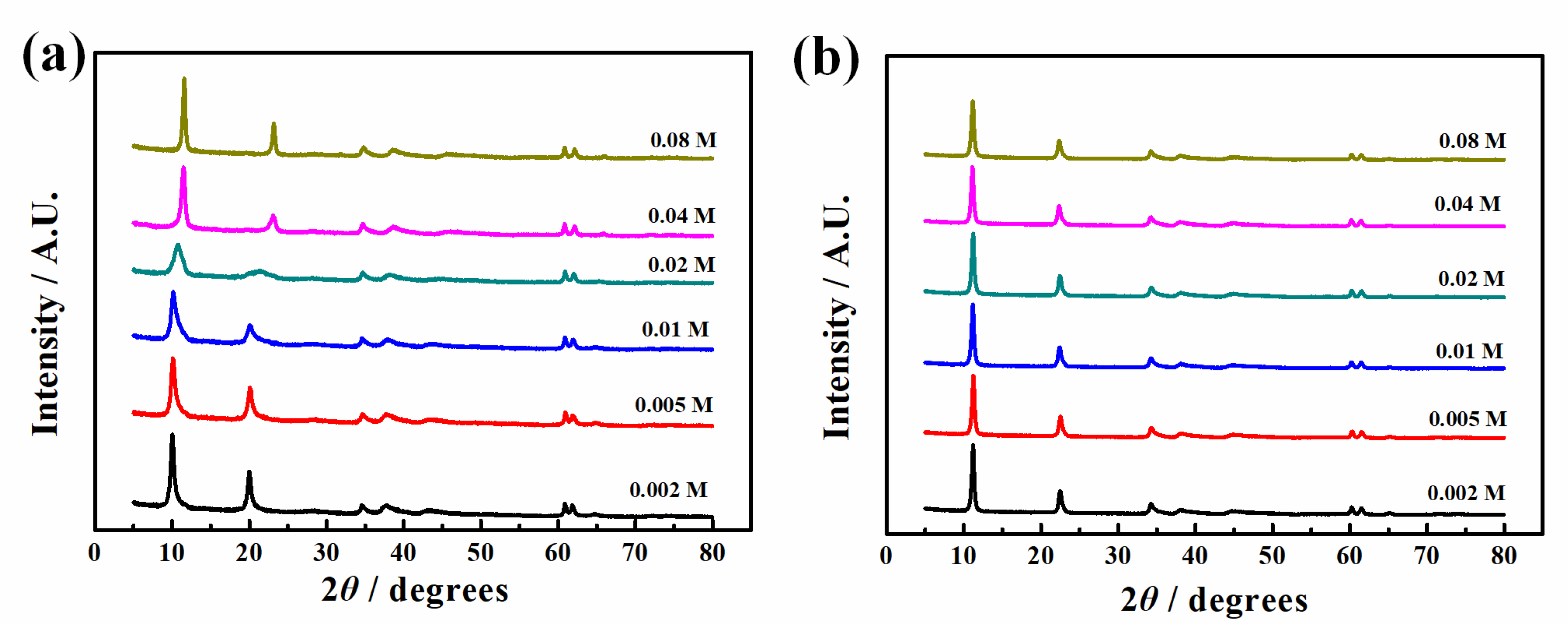
3.1.2. Structure
3.1.3. Composition
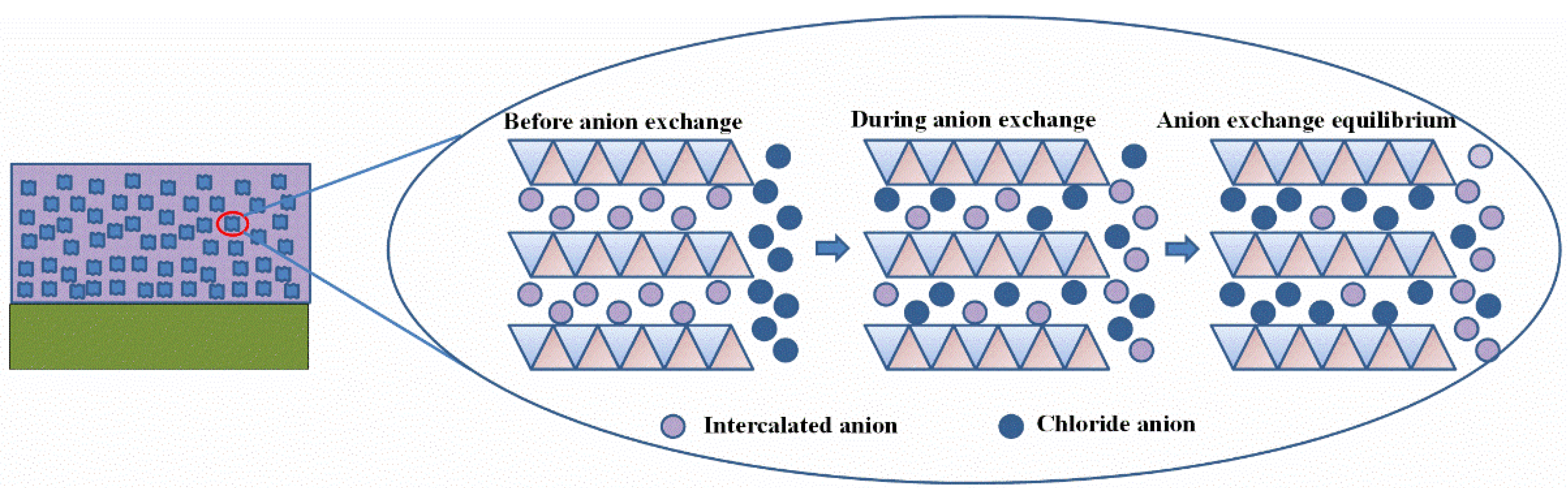
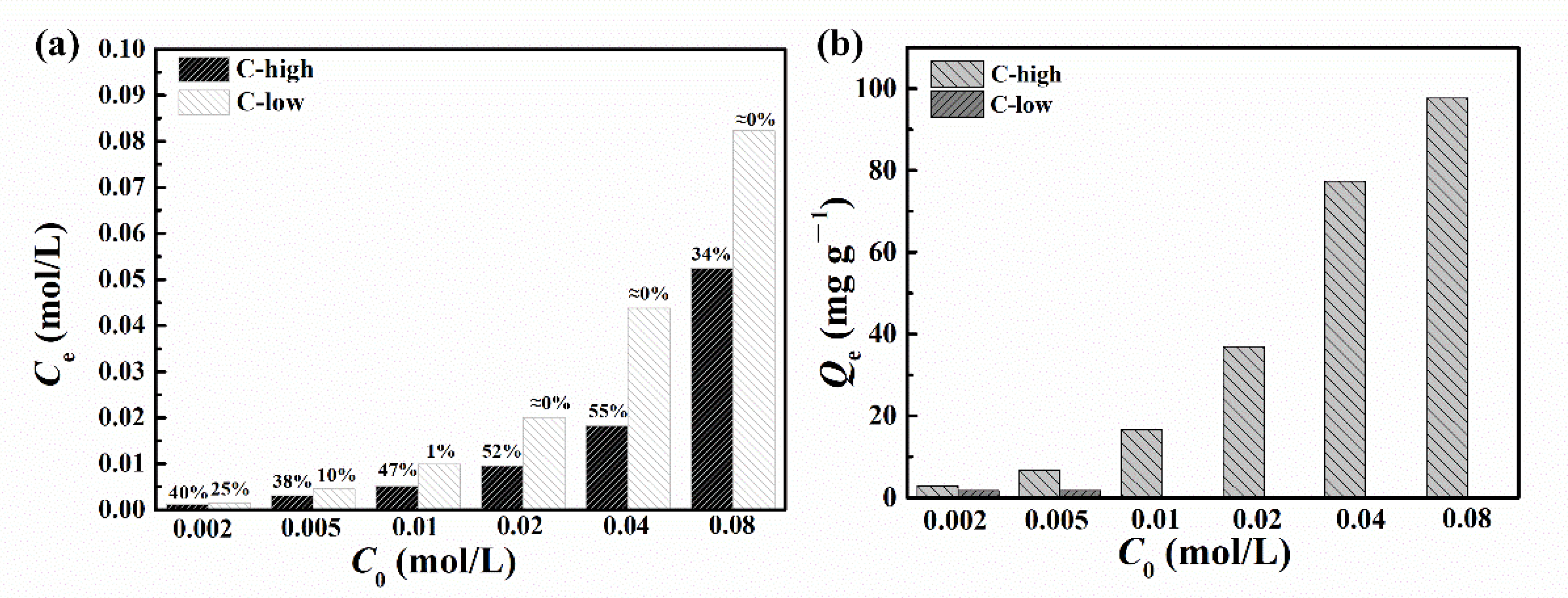
3.2. Chloride Adsorption Behavior of LDH
4. Conclusions
- (1)
- The change of reactant concentration hardly had an influence on the morphology and size of the LDH platelets, while the pH difference caused by different metal ion concentrations may have a remarkable effect on the intercalated anions. The difference in reactant concentration resulted in the change of pH value of the reactant solution. An obviously higher pH with a value of 12.7 in the case of C-low leads to the intercalation of a larger number of carbonates, resulting in a lower chloride adsorption capability due to the difficulty of anion exchange reaction. This finding emphasizes the importance of pH value in LDH synthesis, which can be easily ignored when other conditions were changed. pH should be controlled well during the LDH synthesis process to guarantee the quality of the synthesized LDH. This result could provide meaningful guidance into further preparation of MgAl-LDH.
- (2)
- The sample of C-high presents superior chloride adsorption ability with a Qm value of 155.88 mg g−1, which was much better than that reported in the literature. This work is able to provide a solid foundation for the production of LDH with a strong chloride adsorbing property and further industrial application in the field of corrosion protection in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C. Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: A review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Li, S.; Xue, B. Fabrication of inhibitor anion-intercalated layered double hydroxide host films on aluminum alloy 2024 and their anticorrosion properties. J. Coat. Technol. Res. 2015, 12, 293–302. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, S.; Yang, D.; Liu, S.; Zhao, H.; Wang, L.; Xue, Q. Active anti-corrosion of epoxy coating by nitrite ions intercalated MgAl LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Farashi, S. Active corrosion protection of Mg-Al-PO43− LDH nanoparticle in silane primer coated with epoxy on mild steel. J. Taiwan Inst. Chem. Eng. 2017, 75, 248–262. [Google Scholar] [CrossRef]
- Li, W.H.; Tian, H.W.; Wang, D.P. Controlled Release of Nitrate and Molybdate Intercalated in Zn-Al-Layered Double Hydroxide Nanocontainers towards Marine Anticorrosion Applications. Colloid Interfac. Sci. 2018, 24, 18–23. [Google Scholar] [CrossRef]
- Chen, J.; Fang, L.; Wu, F.; Xie, J.; Hu, J.; Jiang, B.; Luo, H. Corrosion resistance of a self-healing rose-like MgAl-LDH coating intercalated with aspartic acid on AZ31 Mg alloy. Prog. Org. Coat. 2019, 136, 105234. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.; Zhao, W. Designing novel organic inhibitor loaded MgAl-LDHs nanocontainer for enhanced corrosion resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Xu, S.; Evans, D.G.; Duan, X. Preparation of layered double hydroxide films with different orientations on the opposite sides of a glass substrate by in situ hydrothermal crystallization. Chem. Commun. 2009, 6836–6838. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Qiu, S.; Wei, J.; Zhu, X.; Zhao, H.; Xue, Q. Sulfonated polyaniline assisted hierarchical assembly of graphene-LDH nanohybrid for enhanced anticorrosion performance of waterborne epoxy coatings. Chem. Eng. J. 2021, 426, 131269. [Google Scholar] [CrossRef]
- Kosari, A.; Visser, P.; Tichelaar, F.; Eswara, S.; Audinot, J.N.; Wirtz, T.; Zandbergen, H.; Terryn, H.; Mol, J.M.C. Cross-sectional characterization of the conversion layer formed on AA2024-T3 by a lithium-leaching coating. Appl. Surf. Sci. 2020, 512, 145665. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Chen, Y.; Zhong, Z.; Ci, W.; Wu, J.; Xie, Z.; Yuan, Y.; Pan, F.A. self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 2022, 194, 109941. [Google Scholar] [CrossRef]
- Hu, T.; Ouyang, Y.; Xie, Z.H.; Wu, L. One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT composite coating on magnesium alloy under mild conditions. J. Mater. Sci. Technol. 2021, 92, 225–235. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, C.; Liu, A.; Li, W. The effect of layered double hydroxides intercalated with vitamin B3 on the mechanical properties, hydration and pore structure of cement-based materials. Mater. Lett. 2021, 300, 130228. [Google Scholar] [CrossRef]
- Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Vieira, J.M.; Brett, C.M.A. Ce-substituted Mg-Al layered double hydroxides to prolong the corrosion protection lifetime of aluminium alloys. Appl. Surf. Sci. 2022, 573, 151527. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Mana, Y. The effect of interlayer spacing on the inhibitor release capability of layered double hydroxide based nanocontainers. J. Clean. Prod. 2020, 251, 119676. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.D.; Nguyen, A.S.; Paint, Y.; Gonon, M.; To, T.X.H.; Olivier, M.G. A comparative study of the structure and corrosion resistance of ZnAl hydrotalcite conversion layers at different Al3+/Zn2+ ratios on electrogalvanized steel. Surf. Coat. Technol. 2022, 429, 127948. [Google Scholar] [CrossRef]
- Wu, W.; Sun, X.; Zhu, C.L.; Zhang, F.; Zeng, R.C.; Zou, Y.H.; Li, S.Q. Biocorrosion resistance and biocompatibility of Mg–Al layered double hydroxide/poly-L-glutamic acid hybrid coating on magnesium alloy AZ31. Prog. Org. Coat. 2020, 147, 105746. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.; Tang, A.; Zhang, G.; Atrens, A.; Pan, F. Doublely-doped Mg-Al-Ce-V2O74− LDH composite film on magnesium alloy AZ31 for anticorrosion. J. Mater. Sci. Technol. 2021, 64, 66–72. [Google Scholar] [CrossRef]
- Wei, J.; Xu, J.; Mei, Y.; Tan, Q. Chloride adsorption on aminobenzoate intercalated layered double hydroxides: Kinetic, thermodynamic and equilibrium studies. Appl. Clay Sci. 2020, 187, 105495. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Zhao, Y.; Jiang, L.; Mei, Y.; Chen, P. Chloride removal and corrosion inhibitions of nitrate, nitrite-intercalated Mg Al layered double hydroxides on steel in saturated calcium hydroxide solution. Appl. Clay Sci. 2018, 163, 129–136. [Google Scholar] [CrossRef]
- Chen, M.; Wu, F.; Yu, L.; Cai, Y.; Chen, H.; Zhang, M. Chloride binding capacity of LDHs with various divalent cations and divalent to trivalent cation ratios in different solutions. CrystEngComm 2019, 21, 6790–6800. [Google Scholar] [CrossRef]


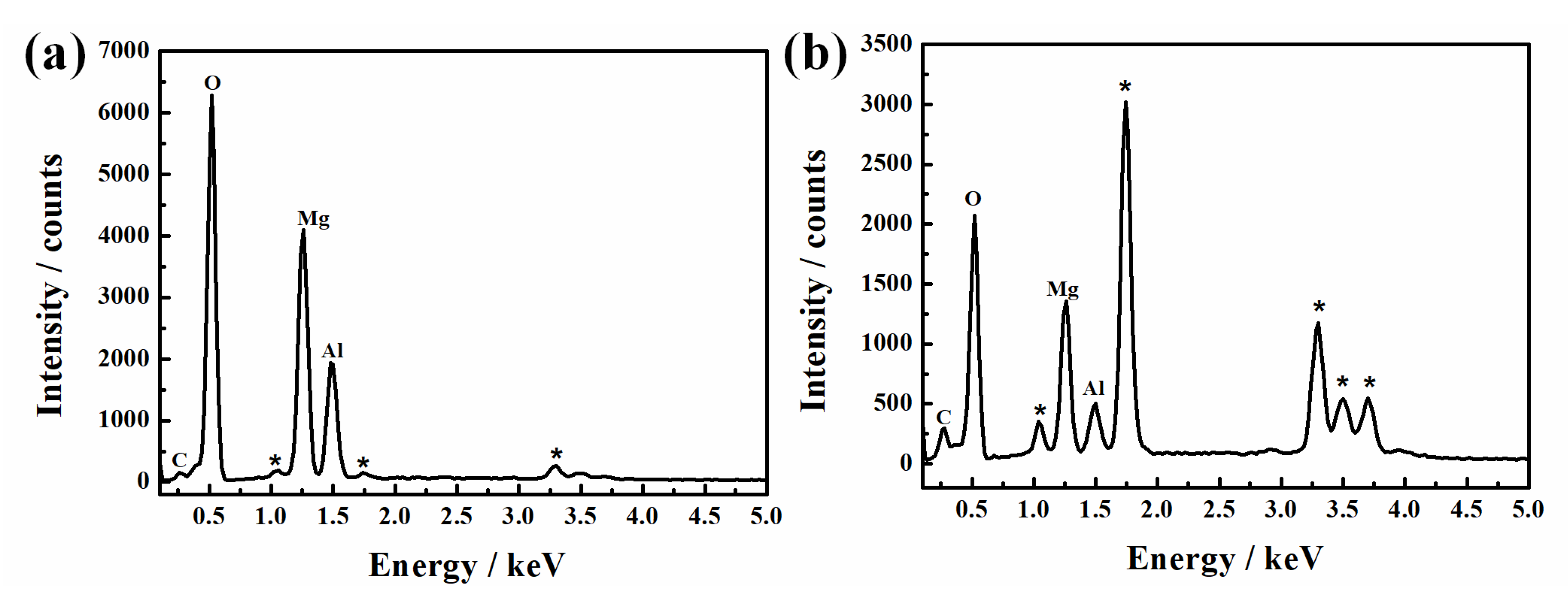
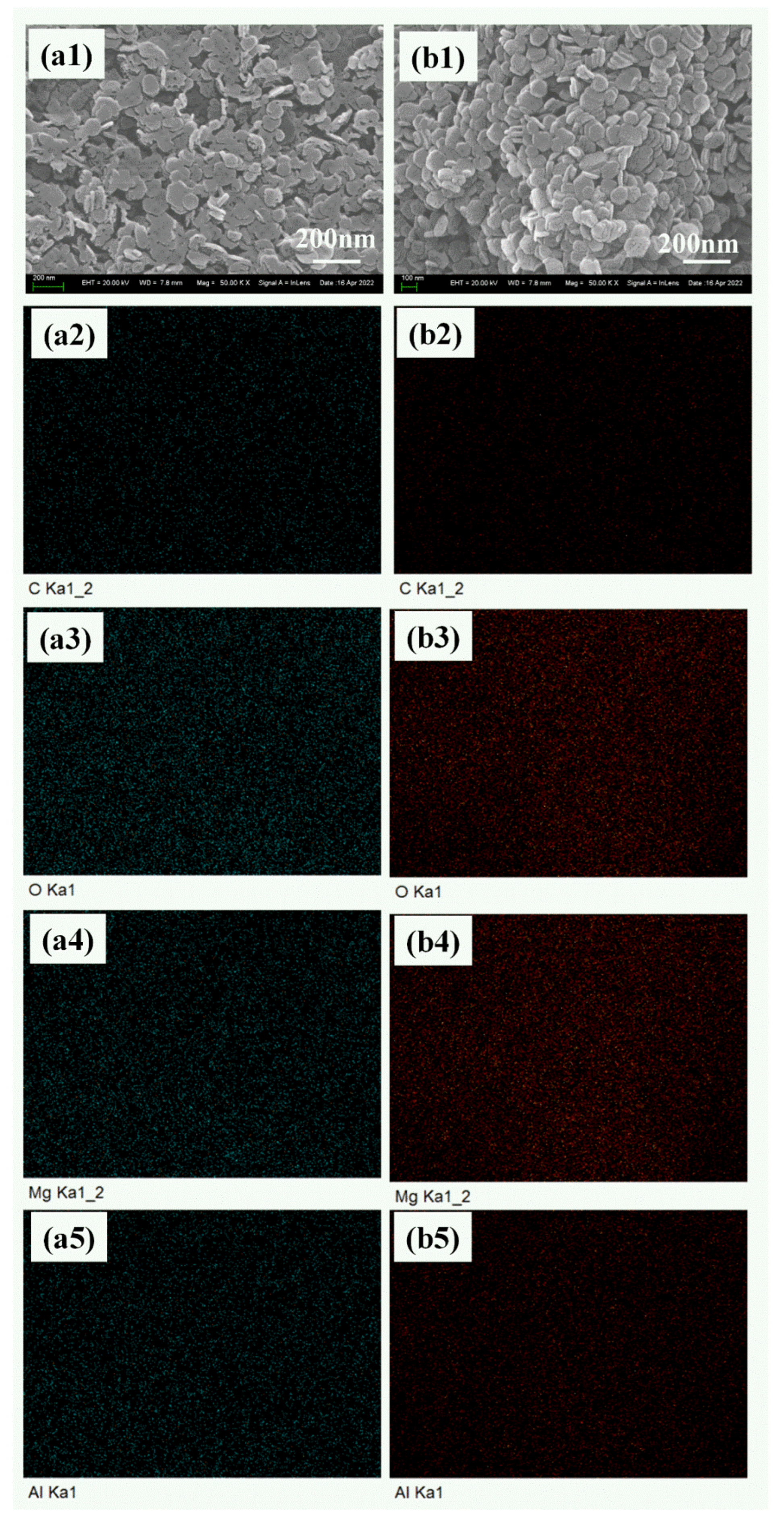



| Element (At.%) | C | O | Mg | Al | Mg/Al Ratio |
|---|---|---|---|---|---|
| C-high | 13.97 ± 1.36 | 68.40 ± 0.70 | 11.49 ± 0.43 | 6.13 ± 0.32 | 1.88 ± 0.07 |
| C-low | 23.64 ± 1.63 | 62.59 ± 0.84 | 10.11 ± 0.69 | 3.66 ± 0.10 | 2.79 ± 0.16 |
| LDH | Qm (mg g−1) | Literature |
|---|---|---|
| MgAl-LDH-NO3− | 155.88 | Our work |
| MgAl-LDH-pAB | 33.54 | 22 |
| MgAl-LDH-NO3− | 107.00 | 23 |
| MgAl-LDH-NO2− | 88.75 | 23 |
| MgAl-LDH-NO3− | 111.29 | 24 |
| CaAl-LDH-NO3− | 115.55 | 24 |
| ZnAl-LDH-NO3− | 148.28 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Fang, S.; Chen, K.; Qi, H.; Zhang, X.; Huang, C.; Wang, J.; Liu, J. Insight into the Preparation of MgAl-Layered Double Hydroxide (LDH) Intercalated with Nitrates and Chloride Adsorption Ability Study. Appl. Sci. 2022, 12, 4492. https://doi.org/10.3390/app12094492
Cao Y, Fang S, Chen K, Qi H, Zhang X, Huang C, Wang J, Liu J. Insight into the Preparation of MgAl-Layered Double Hydroxide (LDH) Intercalated with Nitrates and Chloride Adsorption Ability Study. Applied Sciences. 2022; 12(9):4492. https://doi.org/10.3390/app12094492
Chicago/Turabian StyleCao, Yanhui, Shuo Fang, Kaifeng Chen, Haixia Qi, Xinyue Zhang, Congshu Huang, Jingjing Wang, and Jianchun Liu. 2022. "Insight into the Preparation of MgAl-Layered Double Hydroxide (LDH) Intercalated with Nitrates and Chloride Adsorption Ability Study" Applied Sciences 12, no. 9: 4492. https://doi.org/10.3390/app12094492
APA StyleCao, Y., Fang, S., Chen, K., Qi, H., Zhang, X., Huang, C., Wang, J., & Liu, J. (2022). Insight into the Preparation of MgAl-Layered Double Hydroxide (LDH) Intercalated with Nitrates and Chloride Adsorption Ability Study. Applied Sciences, 12(9), 4492. https://doi.org/10.3390/app12094492







