Abstract
As the development and use of living modified organisms (LMOs) steadily increase, new risk assessment methods that reflect domestic natural ecosystems are being developed. Although LM plants are fundamentally necessary for environmental risk assessment, the introduced gene products and LMO proteins can replace transgenic plants. However, their use is problematic because of instability and indirect assessment data issues. This study proposes a risk assessment tool and scheme for introducing LMO proteins into genetically modified crops. The agroinfiltration method for transient LMO gene expression in plants is a practical tool which can be used to rapidly verify the putative risks of LMO proteins against insects using an LM crop mimic plant with a stably expressed LMO protein. This study used Nicotiana tabacum leaves, which transiently but stably expressed the insecticidal LMO protein Vip3Aa, for LMO risk assessments against Spodoptera litura. The Vip3Aa protein was stably expressed for 5 d in the agroinfiltrated plants, and the protein was active against target insects for environmental LMO risk assessments. In the toxicity evaluation of Vip3Aa-expressing plants against S. litura, the number of deaths was higher in the Vip3Aa-infiltrated N. tabacum-fed group than that in the recombinant Vip3Aa-fed group. In addition, the cumulative number of deaths in the infiltration leaf-fed group was approximately 12-fold higher than that in the protein-fed group under low dosage conditions. This study aimed to develop a transient expression model which can be used to evaluate whether the overall risk of LMO protein is acceptable for use. These results support the usefulness of the transient expression model using an agroinfiltration method as a rapid risk validation tool for LMO proteins against herbivorous insects before producing transgenic plants.
1. Introduction
Various living modified organisms (LMOs) have been developed owing to advances in modern biotechnology, but there is concern about their potential risks to human health and the environment [1]. As a result, the Cartagena Protocol on Biosafety (CPB) to the Convention on Biological Diversity has developed an international standard for the use of LMOs [2,3,4,5,6]. Therefore, the Transboundary Movement, Etc. of LMOs Act (hereafter, the LMOs Act) was enacted in South Korea [7]. In particular, the Ministry of Environment (MOE) is in charge of the overall development and use of LMOs for environmental remediation [8,9,10,11]. The MOE is also responsible for the risk review and assessment of LMOs for other uses in natural ecosystems and LMOs for environmental remediation. The National Institute of Ecology (NIE), an LMO risk review agency and institute for LMO risk assessment under the MOE, has been handed responsibility for LMO safety management. The NIE establishes standards and methods for reviewing and assessing the risk of LMOs for environmental remediation. Furthermore, it consults on the effects of new LMOs on the natural ecosystem [10,11,12].
The environmental risk assessment includes environmental exposure experiment results obtained from laboratory and field tests using submitted LMOs and their genetic products [13,14]. Moreover, the risk review must be accompanied by a risk assessment of the environment of each participating country [8,9,10,15]. However, the risk assessment of LMOs is not easy because of the closed technical trade secrets of manufacturers and practical limitations in time, methods, lack of human resources, and places to conduct an assessment within the review period in each participating country [6,7,8,16,17,18]. Additionally, environmental risk assessments should consider the food chain in the ecosystem [19,20]. Therefore, new domestically applicable ecological risk assessment tools are required to resolve these issues and complement risk assessment reviews.
Traditional environmental risk assessment methods are performed in two distinct steps: a high-dose toxicity evaluation of recombinant LM proteins (laboratory trial) and an exposure assessment of LM plants in an isolated field (field trial) [19,20,21,22]. However, all studies must confirm the equivalence between the recombinant and expressed proteins in the LM plant [22].
The aim of this study was to propose a risk assessment tool for LMO proteins using an agroinfiltration method for the putative risks associated with LMO proteins in LM crop mimic plants. In particular, the focus was placed on developing evaluation techniques which can determine whether the valued general risk of an LMO protein is manageable in potentially diverse environments. The NIE dedicated this effort to examining the applicability of risk assessment in the case of the insecticidal Vip3Aa LMO protein, with the process comprising a comprehensive laboratory and semi-field trial, as in the tiered system for risk assessment [19,20,21,22,23]. The Vip3Aa-infiltrated Nicotiana tabacum plant, as a new risk assessment tool, provided the advantage of stable LMO protein production and rapid risk validation against herbivorous insects before producing transgenic plants. In addition, this approach provides the opportunity to consider the food chain relationship between target and non-target insects and the benefits in terms of the environmental risk assessment of LM crop mimic plants, despite the absence of LMO plants.
This study supports the usefulness of agroinfiltrated plants as a rapid risk validation tool for LMO proteins in the context of quick LMO risk assessment before import approval during the risk review process.
2. Materials and Methods
2.1. Construction for Transient Expression of the Vip3Aa Gene
The pCAMBIA1300 vector derived from the pPZP vector was used for the agroinfiltration of Vip3Aa into tobacco plants. The Vip3Aa gene was excised with BamHI and XbaI fragments and ligated into the pCAMBIA1300 vector. The resulting construct was named pCAMBIA1300-Vip3Aa.
2.2. Growing Plant Material for Agroinfiltration
Tobacco (N. tabacum) plants were grown in a growth chamber at 24 °C under a 16-h light/8-h dark cycle. Each construct was inserted into Agrobacterium tumefaciens strain GV3101 (Intact Genomics, Inc., St. Louis, MO, USA) by freeze-thawing, followed by selection on yeast extract peptone (YEP) plates containing the appropriate antibiotics. Transformed leaves were harvested daily for 0–6 d. Agrobacterium-mediated transient expression in tobacco leaf epidermal cells was performed as described previously [24,25,26].
2.3. Confirmation of the Vip3Aa Gene’s Introduction
Total RNA was extracted from agroinfiltrated tobacco leaves using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol and quantified using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). First-strand cDNA was synthesized using ReverTra Ace -α-® (Toyobo, Osaka, Japan). RT-PCR was performed using the SlogTM 2× EF-Tag PCR premix (Slogent, Daejeon, Korea). The primers for RT-PCR and qRT-PCR were as follows: forward primer (Vip3Aa F BamH I) 5′-GGA TCC ATG AAC AAG AAT AAT ACT AAA TTA AGC AC-3′ and reverse primer (Vip3Aa XhoI R) 5′-CTC GAG TCC ATT TTA CTT TTT AAA ATT AAA TAC-3′ for RT-PCR and forward primer (Vip3Aa 536aa F BamHI) 5′- GGA TCC AGC AAT ATT GTA GAG AAC GG-3′ and reverse primer (Vip3Aa 654aa R XhoI) 5′- CTC GAG TCC ATT TTG ACT TTT TAA AAT TAA A-3′ for qRT-PCR. qRT-PCR was performed using Maxima SYBR Green/Rox qPCR Master Mix (Thermo Fisher Scientific) with TC-EF-1a as an internal control [27]. The cDNA samples were analyzed using qRT-PCR to determine the expression levels of Vip3Aa using an ABI PRISM1 7300 unit (Applied Biosystems, Foster City, CA, USA).
2.4. Expression of Vip3Aa Protein
Negative control plants and Vip3Aa-agroinfiltrated plant leaves were ground in liquid N2, resuspended in lysis buffer (100 mM Tris [pH 7.5], 300 mM NaCl, 5 mM EDTA, 5% glycerol, 0.1% SDS, protease cocktail, and 1 mM PMSF) at 4 °C for 30 min, and then centrifuged at 12,000 rpm for 15 min. Vip3Aa protein expression was visually confirmed according to the method provided with the Vip3Aa ELISA Kit (Agdia, Elkhart, IN, USA) to determine the protein using the collected supernatant. Next, to confirm the expression level of the Vip3Aa protein, TMB solution was added, and the absorbance at 650 nm was measured.
Protein bands were detected using an enhanced chemiluminescence kit (Elpis Biotech, Daejeon, Korea) and membrane exposure to the ChemiDoc Gel Imaging System (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked for 30 min with 5% bovine serum albumin (Merck, Darmstadt, Germany) in PBS containing 0.1% Tween 20 (PBST), washed with PBST, and incubated for 2 h with primary antibodies against Vip3Aa (AbClon, Seoul, Korea) diluted in PBST buffer (1:2000) at room temperature. After washing, membranes were incubated with anti-rabbit IgG-conjugated horseradish peroxidase (1:5000; Cell Signaling Technology, Danvers, MA, USA) for 1 h.
2.5. Insect Bioassays of the Vip3Aa Protein
Fresh recombinant Vip3A protein was prepared shortly before the insect bioassay and stored at −70 °C for the test duration [28]. Spodoptera litura eggs were provided by the National Institute of Crop Science (Wanju, Korea). For the insecticidal activity assay of Vip3Aa protein, freshly hatched neonate S. litura larvae were kept under controlled conditions in an insect-rearing room at 25 ± 1 °C and 60% relative humidity for 16 and 8 h (light/dark).
For the artificial diet-overlay bioassay, the first instar larvae were fed with two concentrations (40 and 100 ng/mg) of recombinant Vip3Aa protein and buffer 1 (1× PBS) in an artificial diet. An artificial diet without anything else was also maintained as a control (mock). Fresh recombinant Vip3A protein dilutions (200 µL) and control solution were dispensed uniformly onto the surface of 1 g of artificial diet and allowed to dry on a clean bench. Thereafter, the larvae were placed on each test plate using a soft brush, and the plates were covered with sterilized gauze and a perforated plastic cap. Subsequently, an artificial diet-overlay bioassay was performed in our insect-rearing room for 12 d. During the bioassay period, 200 µL of each recombinant Vip3Aa protein dilution (or control buffer) was added every 24 h, and the number of dead larvae was scored daily.
The insecticidal activity of Vip3Aa was evaluated using the second- or third-day leaves after agroinfiltration. The experiment was set up on test plates, with leaves placed on moist filter paper. First, agroinfiltration transient leaves and control leaves (mock, buffer 2: infiltration buffer, and buffer 3: containing vector) were placed in a test plate. Afterward, first-instar larvae were added to each test plate, and the plates were covered with sterilized gauze and a perforated plastic cap. The plates were then kept in an insect-rearing room for 12 d under the same conditions as the artificial diet-overlay bioassay. During the bioassay period, fresh leaves were changed every 48 h, and the number of dead larvae was recorded daily. For the artificial diet-overlay bioassay and insecticidal activity of Vip3Aa, 10 S. litura larvae were used. The treatments were performed in duplicate and repeated five times. Larvae that were motionless when gently touched with a soft brush were considered dead. Furthermore, in the bioassay, the mortality trend was assessed in terms of time in days until 50% lethality (LT50).
2.6. Statistical Analysis
One-way analysis of variance (ANOVA) was used for multiple comparisons, followed by Dunnett’s test in GraphPad Prism (version 9.0; GraphPad Inc., San Diego, CA, USA). The experimental values are expressed as mean ± standard error of the mean. We determined statistical significance at p < 0.05.
3. Results and Discussion
3.1. Domestic and International Regulatory Frameworks for LMOs
Since 2008, South Korea has had a national management system for research on the testing, production, import, export, and use of genetically modified organisms (GMOs) based on the LMO law, an act under the biosafety protocol [8,13]. The system designates the Ministry of Trade, Industry, and Energy as the national institution responsible for LMO safety management in South Korea, the Ministry of Foreign Affairs as the national liaison, and the relevant administrative agencies as accountable for each use. The Korean government is in charge of the risk review and assessment of all LMOs for import or export and the safe management of LMOs. In particular, the MOE, Ministry of Agriculture, Food, and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), and Ministry of Health and Welfare (MOHW) undergo consultation reviews for the environmental risk of LMOs that are released or are likely to be released into the environment [8,9,10,11]. Furthermore, each relevant institution convenes a risk review expert committee to evaluate risks by examining the submitted documents. Since 2015, the NIE, an LMO risk review agency of the MOE, has also been responsible for the effects of LMOs on natural ecosystems. In addition, the NIE was designated as an institute of LMO risk assessment by the MOE in December 2018 and is equipped with isolation field facilities to conduct LMO risk assessment [10,11]. The LMO risk review agency can request an LMO risk assessment from the institute of LMO risk assessment during the risk review process for a new LMO. The NIE has also been drawing up guidelines on risk review and assessment methods to establish a safety management system for LMOs for the purpose of environmental remediation and the general safe management of LMOs in natural ecosystems [10,11]. The LMO safety management strategies of the MOE and NIE are shown in Figure 1.
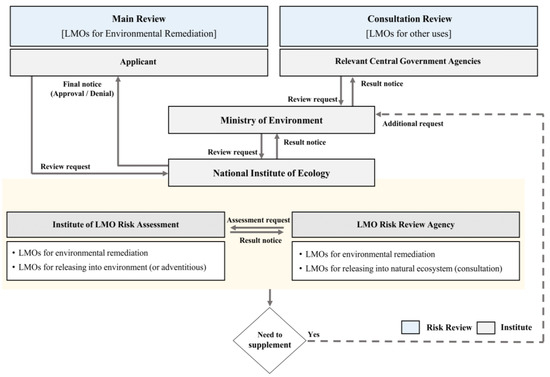
Figure 1.
The safety-management framework of the Ministry of Environment and the National Institute of Ecology for LMO risk review and assessment.
3.2. Approach to New Tools
The approach proposed by the EPA for conducting LMO risk assessment for target organisms is based on the traditional steps, namely laboratory assessment (toxicity assessment) and field trials (exposure assessment) (Figure 2a) [23]. Among laboratory-based risk assessment methods, the artificial feeding bioassay is one of the most common approaches used for toxicity assessment; it uses a recombinant LMO protein diluted to the correct concentration and pipetted onto the surface of a solidified artificial diet [29,30,31]. Its advantages are that it provides rapid and easy application and uses significantly less LMO protein [29]. However, with artificial diets, there are disadvantages in that there can be an uneven distribution of LMO protein on the artificial diet surface, leading to non-uniform treatment and the inconsistent exposure of larvae [30]. Another major drawback of artificial diets is the instability of LMO proteins in artificial diets [30]. On the other hand, field trials have the advantage of using LMOs directly to evaluate putative ecological risks in the food chain. Furthermore, field trials have been used to collect a vast amount of otherwise anonymous information on how LMOs affect environmental changes in the field and natural ecosystems [31]. Nevertheless, there are three reasons for this lack of consideration in domestic field trials. (1) The disadvantage of field testing is that it is time-consuming and costly [8,18]. (2) Human resources and legal forces are inadequate to strictly manage all the confined fields for LMO risk assessment [7,18,32]. (3) There is a lack of long-term field trial data for LMOs that modify the domestic state; therefore, it is difficult to set the isolation distance [33]. Eventually, an approved LMO cannot be guaranteed upon adventitious release into the domestic environment.
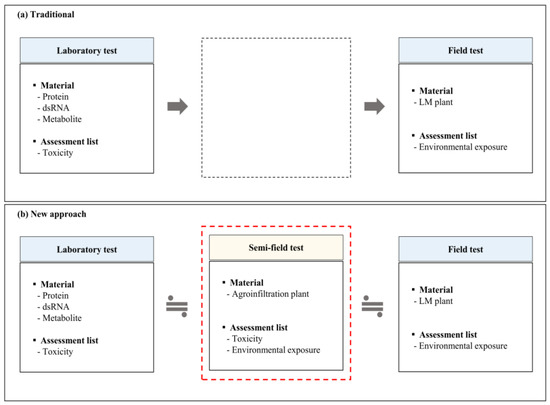
Figure 2.
Scheme of the new risk assessment strategy. (a) Traditional risk assessment methods include laboratory and field trials. (b) A new risk assessment approach based on transient expression models of plants using an agroinfiltration method.
We propose a new LMO risk assessment tool that compensates for limitations associated with laboratory assessments and field trials. Agroinfiltrated plants with transiently expressed LMO proteins were used in new trials that indirectly reflect the domestic natural ecosystem. The risk assessment of the scenario of the target species inhabiting South Korea using a mimic plant can overcome the disadvantages of present environmental risk assessment methods. The use of Vip3Aa-agroinfiltrated plants can be regarded as a semi-field trial at an intermediate stage between laboratory and field trials (Figure 2b). The application of this tool in LMO risk assessment and review may have several advantages: (1) self-production would be possible without security disputes with LMO developers; (2) transient expression plants have a short production period; (3) a semi-field trial would be possible as an evaluation method using plants; (4) domestic target insects could be used; and (5) the risk to the natural ecosystem would be low, even if the agroinfiltrated plants were unintentionally released from the isolated field.
3.3. Vip3Aa Protein Expression and Stability
In transient protein expression using agroinfiltration, the introduced gene directly expresses the desired protein without fusion into the plant genome. This strategy provides the advantage of a significantly improved protein accumulation level and faster protein production timeline [34]. Thus, the production level of the LMOs protein can be easily amplified by increasing the number of host plants. In order to evaluate the risk of Vip3Aa protein, the Vip3Aa-agroinfiltration transient plants were extensively engineered to achieve high protein accumulation levels in tobacco leaves. To take advantage of the risk assessment tool based on agroinfiltration, it is essential to confirm the concentration of Vip3Aa protein expressed in real LMOs and infiltrated plants as LM mimic organisms. The expression level of Vip3Aa protein in infiltrated leaves was investigated using ELISA assay and compared with those of LM crops such as Bt corn (MIR162) and cotton (COT102) (Table S1, Figure 3) [35,36]. The expression level of Vip3Aa in the agroinfiltrated plants (170–220 ng/mg, fresh weight) was similar to that of LM corn MIR162 (87.5~339 ng/mg, dry weight). Moreover, Vip3Aa in the agroinfiltrated plants was stably expressed until 5 d (Figure 3). The standard deviation of the Vip3Aa protein expression in the agroinfiltrated plant was smaller than that of the two LM plants.
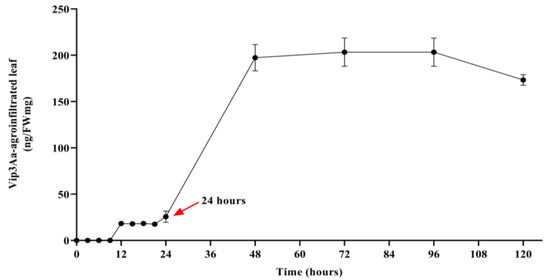
Figure 3.
The expression level of Vip3Aa protein in the agroinfiltrated tobacco leaves. The red arrow indicates the starting point of Vip3Aa expression.
To investigate the stability of the Vip3Aa protein, we analyzed its expression levels in the recombinant Vip3Aa-treated artificial diet (100 ng/mg) once and in the infiltrated plant leaves (the 3rd-day sample after infiltration) using western blot analysis for 60 h (Figure 4). While the Vip3Aa protein in the agroinfiltrated plant leaves slightly decreased until 60 h, the recombinant Vip3Aa in the artificial diet significantly reduced after 15 h and was barely detectable after 21 h.
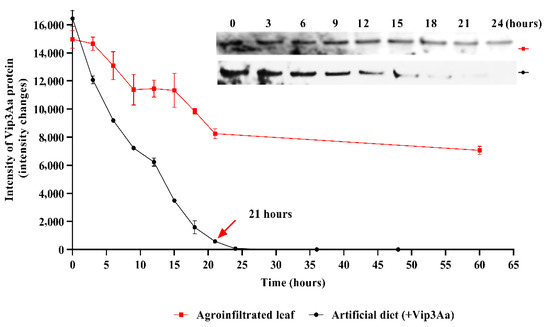
Figure 4.
The stability of Vip3Aa in the recombinant protein-treated artificial diet and infiltrated plant leaves. The stability of the Vip3Aa protein in the recombinant Vip3Aa-treated artificial diet (100 ng/mg) and third-day plant leaves after Vip3Aa-agroinfiltration. Each sample was collected per 3 h, and the protein expression level was quantified by immunoblot analysis with anti-Vip3Aa Ab. Protein intensity was measured using the ImageJ (version 1.53n) program. The red arrow indicates the last indication time point of Vip3Aa protein, and inset data shows the western blot assay data until 24 h. All values represent the mean ± standard deviation (SD) of triplicate experiments.
3.4. Risk Assessment Using an Agroinfiltrated Plant
To verify the applicability of the infiltrated plant as a replacement for the LM crop, a bioassay for S. litura was performed using an artificial diet and an agroinfiltrated plant. The Vip3A protein concentration used in the diet-overlay bioassay was determined using the methods of Bergamasco et al. and Gupta et al. [37,38]. A measurement endpoint is generally demonstrated as a significant increase in lethal toxin concentration, killing 50% of the insects (LC50) [39]. The response criteria used to assess mortality included severe growth inhibition and death. Therefore, the time in days until 50% lethality (LT50) is necessary to perform a risk assessment of the new approach. First, the LT50 was investigated by insect bioassays using an artificial diet and an agroinfiltrated plant (Table 1). As shown in Table 1, we confirmed that the LT50 value of transient agroinfiltrated plants was more efficient than the artificial diet. In addition, the LT50 value of agroinfiltrated plants was lower than that of LM crops or LM proteins [38,40]. This result suggests that agroinfiltrated plants can be used as an effective material for LMO risk assessment using insect bioassays.

Table 1.
The survival time of S. litura larvae using the insect bioassay.
We performed a bioassay for S. litura using an artificial diet and agroinfiltrated plant (Figure 4). The duration of the bioassay can drastically affect the final toxicity outcome of the LM protein [41]. Therefore, we fed the protein-treated artificial diet and the agroinfiltrated leaves to the first instar larvae of S. litura (n = 10, duplicate) for 12 d (refer to Table 1) (approximately twice that of the LT50 value). The recombinant protein was added to the artificial diet every 24 h, and the leaves on the third day after infiltration were replaced every 48 h (Figure 5).
Figure 5.
Insecticidal activity for S. litura using the recombinant Vip3Aa-treated-artificial diet and the Vip3Aa-agroinfiltrated plant leaf. (a) The recombinant Vip3Aa protein immersion artificial diet and (b) agroinfiltrated plant leaf were used for insect bioassay as feed for S. litura. The experiment was repeated five times. (c,d) Impact of Vip3Aa on the survival of S. litura on the artificial diet (c) and agroinfiltrated leaf-feeding (d) for 6 and 12 d. Number, feeding time (day); dead, dead insect; Con, mock; Buffer 1, PBS buffer; LC, Vip3Aa protein (40 ng/mg); HC, Vip3Aa protein (100 ng/mg); Buffer 2, infiltration buffer; Buffer 3, vector; leaf: Vip3Aa-agroinfiltrated leaf. Values are expressed as the means ± SEM. ****, p < 0.0001compared with the control.
In the insect bioassay using the recombinant Vip3Aa-treated artificial diet, dead individuals were detected from the fifth day (LC, 40 ng/mg) and the third day (HC, 100 ng/mg) after feeding (Figure 5a,c). The total number of deaths in the low- and high-concentration groups was 6 and 70, respectively. Bioassays against S. litura larvae showed that both the 100 ng/mg Vip3Aa-treated artificial diet and agroinfiltrated leaves had a similar toxicity after 12 d (Figure 5c,d). However, in the agroinfiltration leaf-feeding experiment, dead individuals were found on the first day after feeding and 72 of 100 larvae were overlaid with Vip3Aa-expressed plant leaves (Figure 5b,d). Based on these results, we confirmed that the agroinfiltration method as a risk assessment tool was more efficient than using a recombinant protein-based artificial diet.
Although the agrobacterium penetration method is used in various fields to identify protein functions in plants, studies on the risk assessment of species have not been tried. The main objective of this study was to show the usefulness of a new risk assessment tool for evaluating the putative risks of the natural ecosystem on LM proteins. The agrobacterium infiltration model was used as a substitute for LM crops that take a long time to construct LM plants and elucidate the insect risk assessment of LM proteins quickly. Our results suggest that the new approach may be used more efficiently in terms of time and cost than artificial feed bioassays in laboratory trials with recombinant protein for LMO risk assessment. Transiently expressed LM protein in plants can satisfy laboratory trials, including toxicity tests with high-dose recombinant protein and direct environmental exposure assay using the LM crop.
Future work based on this study will establish guidelines for LMO risk assessment for Lepidoptera in the potential receiving environments of South Korea. Therefore, we are improving this tool to assess the valued overall risk of Lepidoptera. Our findings could serve as a valuable reference for future studies seeking to develop appropriate assessment techniques to determine risk factors affecting the environmental ecosystem in South Korea.
4. Conclusions
In this study, we verified the applicability of agroinfiltration transient plants to suggest a new risk assessment tool that can substitute traditional risk assessment. In particular, this effort was dedicated to examining the applicability of risk assessment tools for insecticide-resistant LMOs considering domestic natural ecosystems. The Vip3Aa protein concentrations expressed in MIR162 (R1) and agroinfiltrated plants were similar. In addition, the standard deviation of the expression level of the Vip3Aa protein expressed in agroinfiltration transient expression plants (193.3 ± 18.7) was smaller than that of MIR162 (228 ± 102). Agroinfiltration transient expression of Vip3Aa protein was expressed in a much more uniform distribution pattern than in the case of MIR162-expressed Vip3Aa protein. We confirmed that the Vip3Aa protein in agroinfiltration transient plant tissues was stably expressed for approximately 5 d at room temperature. Moreover, the group fed Vip3Aa-transient-expressed leaves showed more effective insecticidal action than the recombinant protein group. In summary, agroinfiltrated plants expressing LMO genes can mimic LMOs to rapidly assess risks before LMO plant production.
As an alternative way to address traditional risk assessment limitations, developing effective risk assessment tools based on scientific evidence can act as a means of strict safety management review for the import approval of LMOs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12094397/s1, Table S1. Comparison of Vip3Aa protein expression levels in LM cotton, maize, and agroinfiltration transient model plants. ELISA was used to quantify the levels of Vip3Aa proteins in LM events. Protein levels were determined in leaves (V4–V6 and R1 stages). Figure S1. Gene expression analysis in Vip3Aa agroinfiltration transient plant leaves. qRT-PCR was performed using Vip3Aa gene-specific primers, and TC-EF-1a genes were used as internal controls. Days indicate expression times after agroinfiltration.
Author Contributions
Conceptualization, S.-H.Y. and J.R.L.; methodology, S.-H.Y., Y.J.J. and J.R.L.; validation, S.-H.Y., Y.J.J. and J.R.L.; formal analysis, S.-H.Y., Y.J.J. and J.R.L.; investigation, S.-H.Y., Y.J.J. and J.R.L.; resources, Y.J.J. and J.R.L.; data curation, S.-H.Y. and J.R.L.; writing—original draft preparation, S.-H.Y. and J.R.L.; writing—review and editing, J.R.L.; visualization, S.-H.Y. and J.R.L.; supervision, J.R.L.; project administration, J.R.L.; funding acquisition, J.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Institute of Ecology (NIE), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIE-A-2022-7 and NIE-A-2022-10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prakash, D.; Verma, S.; Bhatia, R.; Tiwary, B.N. Risks and precautions of genetically modified organisms. Int. Sch. Res. Not. 2011, 2011, 369573. [Google Scholar] [CrossRef]
- Keiper, F.; Atanassova, A. Regulation of synthetic biology: Developments under the convention on biological diversity and its protocols. Front. Bioeng. Biotechnol. 2020, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Falkner, R. The influence of the Cartagena Protocol on biosafety: Comparing Mexico, China and South Africa. Glob. Environ. Politics 2006, 6, 23–55. [Google Scholar] [CrossRef]
- Kimani, V.; Gruère, G. Implications of import regulations and information requirements under the Cartagena Protocol on biosafety for GM commodities in Kenya. AgBioForum 2010, 13, 222–241. [Google Scholar]
- Entine, J.; Felipe, M.S.S.; Groenewald, J.H.; Kershen, D.L.; Lema, M.; McHughen, A.; Nepomuceno, A.L.; Ohsawa, R.; Ordonio, R.L.; Parrott, W.A.; et al. Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res. 2021, 30, 551–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, T. The Regulatory Framework for Genetically Modified Agricultural Products in Korea; Food and Fertilizer Technology Center: Taipei, Taiwan, 2003; Volume 527, pp. 1–8. [Google Scholar]
- Jang, H.M. Status of policies relating biosafety. J. Plant Biotechnol. 2003, 5, 13–17. [Google Scholar]
- Kim, E.S. Technocratic precautionary principle: Korean risk governance of genetically modified organisms. New Genet. Soc. 2014, 33, 204–224. [Google Scholar] [CrossRef]
- Choi, W.K.; Jo, B.H.; Seol, M.A.; Eum, S.J.; Park, J.H.; Song, H.R. Presence of environmental risk assessments for LMOs in nature and future considerations based on new biotechnologies. Korean J. Int. Agric. 2014, 26, 297–302. [Google Scholar] [CrossRef]
- Nam, K.H.; Han, S.M. Seed germination of sunflower as a case study for the risk assessment and management of transgenic plants used for environmental remediation in South Korea. Sustainability 2020, 12, 10110. [Google Scholar] [CrossRef]
- Kim, I.R.; Lim, H.S.; Choi, W.K.; Kang, D.I.; Lee, S.Y.; Lee, J.R. Monitoring living modified canola using an efficient multiplex PCR assay in natural environments in South Korea. Appl. Sci. 2020, 10, 7721. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, I.R.; Lee, S.H.; Choi, W.K.; Yoon, Y.M.; Lee, J.R. Establishment and application of a monitoring strategy for living modified cotton in natural environments in South Korea. Appl. Sci. 2021, 11, 10259. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, H.M. Risk assessment of genetically modified organisms. J. Toxicol. Pub. Health 2003, 19, 1–12. [Google Scholar]
- Oh, S.W.; Kim, E.H.; Lee, S.Y.; Baek, D.Y.; Lee, S.G.; Kang, H.J.; Chung, Y.S.; Park, S.K.; Ryu, T.H. Compositional equivalence assessment of insectresistant genetically modified rice using multiple statistical analyses. GM Crops Food 2021, 12, 303–314. [Google Scholar] [CrossRef] [PubMed]
- R&D LMO Safety Management. Statute Book II, Cheongju, Korea. 2021. Available online: https://www.ip-korea.org/lib/pdf_view.php (accessed on 28 December 2021).
- Kim, S.B.; Kim, Y.H. Status and prospect of safety evaluation of genetically modified microorganism (GMM) for domestic and foreign food application. Food Sci. Ind. 2019, 52, 150–170. [Google Scholar] [CrossRef]
- Nakai, S.; Hoshikawa, K.; Shimono, A.; Ohsawa, R. Transportability of confined field trial data from cultivation to import countries for environmental risk assessment of genetically modified crops. Transgenic Res. 2015, 24, 929–944. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Hendley, P.; Bigler, F.; Mayeregger, E.; Parker, R.; Rubinstein, C.; Satorre, E.; Solari, F.; McLean, M.A. Transportability of confined field trial data for environmental risk assessment of genetically engineered plants: A conceptual framework. Transgenic Res. 2014, 23, 1025–1041. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010, 8, 1879. [Google Scholar] [CrossRef]
- Gray, A. Problem formulation in environmental risk assessment for genetically modified crops: A practitioner’s approach. Collect. Biosaf. Rev. 2012, 6, 10–65. [Google Scholar]
- Bratlie, S.; Halvorsen, K.; Myskja, B.K.; Mellegård, H.; Bjorvatn, C.; Frost, P.; Heiene, G.; Hofmann, B.; Jensen, A.H.; Larsen, T.H.; et al. A novel governance framework for GMO. EMBO Rep. 2019, 20, e47812. [Google Scholar] [CrossRef]
- Hilbeck, A.; Meier, M.; Römbke, J.; Jänsch, S.; Teichmann, H.; Tappeser, B. Environmental risk assessment of genetically modified plants-concepts and controversies. Environ. Sci. Eur. 2011, 23, 13. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Jacobs, E.; Raybould, A.; Nickson, T.E.; Sowig, P.; Willekens, H.; Van Der Kouwe, P.V.; Layton, R.; Amijee, F.; Fuentes, A.M.; et al. A tiered system for assessing the risk of genetically modified plants to non-target organisms. Environ. Biosaf. Res. 2006, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Goodin, M.M.; Zaitlin, D.; Naidu, R.A.; Lommel, S.A. Nicotiana benthamiana: Its history and future as a model for plant-pathogen interactions. MPMI 2008, 8, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A highly efficient Agrobacterium-mediated method for transient gene expression and functional studies in multiple plant species. Plant Commun. 2020, 1, 100028. [Google Scholar] [CrossRef] [PubMed]
- Lisong, M.; Lukasik, E.; Gawehns, F.; Takken, F.L.W. The use of agroinfiltration for transient expression of plant resistance and fungal effector proteins in Nicotiana benthamiana leaves. Methods Mol. Biol. 2012, 835, 61–74. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, M.; Song, B.; Hou, C.; Hu, D.; Li, X.; Wang, Z.; Fan, H.; Bi, L.; Liu, J.; et al. Dufulin activates HrBP1 to produce antiviral responses in tobacco. PLoS ONE 2012, 7, e37944. [Google Scholar] [CrossRef]
- Jung, Y.J.; Yoo, S.H.; Lee, J.R. Purification and risk assessment of Bacillus thuringiensis Vip3Aa protein against Apis mellifera. Korean J. Environ. Biol. 2019, 37, 585–591. [Google Scholar] [CrossRef]
- Hervet, V.A.D.; Laird, R.A.; Floate, K.D.A. Review of the McMorran diet for rearing Lepidoptera Species with addition of a further 39 species. J. Insect Sci. 2016, 15, 19. [Google Scholar] [CrossRef]
- Sivasupramaniam, S.; Head, G.P.; English, L.; Li, Y.J.; Vaughn, T.T. A global approach to resistance monitoring. J. Invertebr. Path. 2007, 95, 224–226. [Google Scholar] [CrossRef]
- Faure, J.D.; Napier, J.A. Europe’s first and last field trial of gene-edited plants? Elife 2018, 7, e42379. [Google Scholar] [CrossRef]
- Jung, H.J.; Han, T.H. Prospect of GMO monitering of LMO facilities with NGOs. Trends Agric. Life Sci. 2017, 54, 23–28. [Google Scholar] [CrossRef]
- Lee, B.K. A study on the establishment of isolation distances for environmental release of biotech crops. Korean J. Agric. Sci. 2017, 44, 188–195. [Google Scholar] [CrossRef]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, H.; Birch, A.N.; Casacuberta, J.; Schrijver, A.D.; Gralak, M.A.; Guerche, P.; Jones, H.; Manachini, B.; Messean, A.; Nielsen, E.E.; et al. Risk assessment of information on the subcombination Bt11 3 MIR162, related to the application of Syngenta (EFSA-GMO-DE-2009-66) for authorization of food and feed containing, consisting and produced from genetically modified maize Bt11 3 MIR162 3 MIR604 3 GA21. EFSA J. 2017, 15, 4745. [Google Scholar] [CrossRef][Green Version]
- Syngenta Australia Pty Ltd. Risk Assessment and Risk Management Plan for DIR 157. Commercial Release of Cotton Genetically Modified for Insect Resistance (COT102); Australian Government Department of Health Office of the Gene Technology Regulator: Canberra, Australia, 2018.
- Bergamasco, V.B.; Mendes, D.R.P.; Fernandes, O.A.; Desidério, J.A.; Lemos, M.V.F. Bacillus thuringiensis Cry1Ia10 and Vip3Aa protein interactions and their toxicity in Spodoptera spp. (Lepidoptera). J. Invertebr. Pathol. 2013, 112, 152–158. [Google Scholar] [CrossRef]
- Khan, M.H.; Jander, G.; Mukhtar, Z.; Arshad, M.; Sarwar, M.; Asad, S. Comparison of in vitro and in planta toxicity of Vip3A for lepidopteran herbivores. J. Econ. Entomol. 2020, 113, 2959–2971. [Google Scholar] [CrossRef]
- Siegfried, B.D.; Spencer, T.; Crespo, A.L.; Storer, N.P.; Head, G.P.; Owens, E.D.; Guyer, D. Ten years of Bt resistance monitoring in the European corn borer: What we know, what we don’t know, and what we can do better. Am. Entomol. 2007, 53, 208–214. [Google Scholar] [CrossRef]
- Rangeshwaran, R.; Velavan, V.; Lewis, F.M.; Kumari, S.; Shylesha, A.N.; Mohan, M.; Kumar, S.; Sivakumar, G. Cloning, expression and bioassay of Vip3A protein from an indigenous Bacillus thuringiensis Isolate. J. Pure Appl. Microbiol. 2016, 10, 1533–1539. [Google Scholar]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).