How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
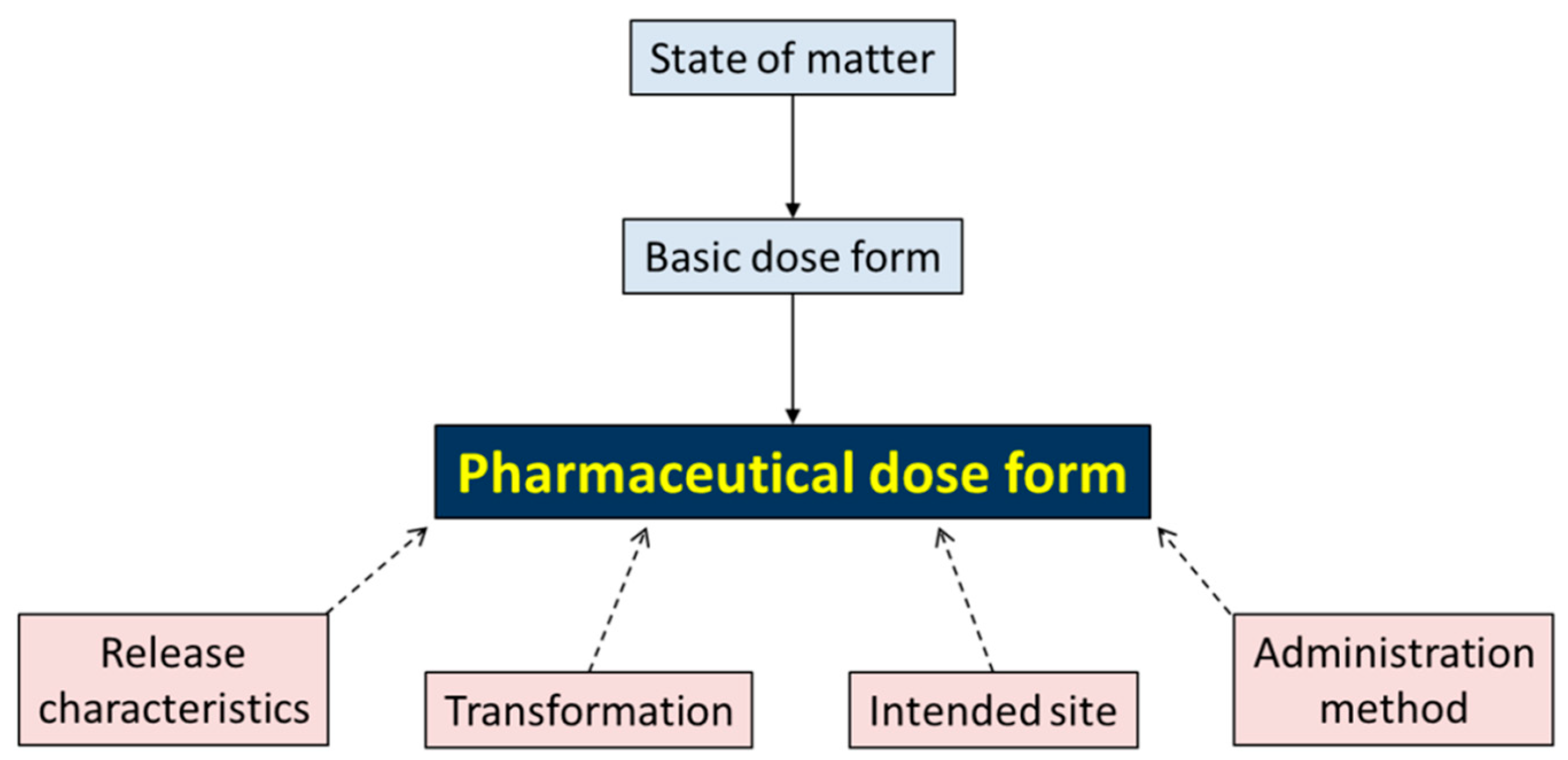
3.1. Describing EDQM as It Is
- Release characteristics (four values): conventional, prolonged, delayed, modified;
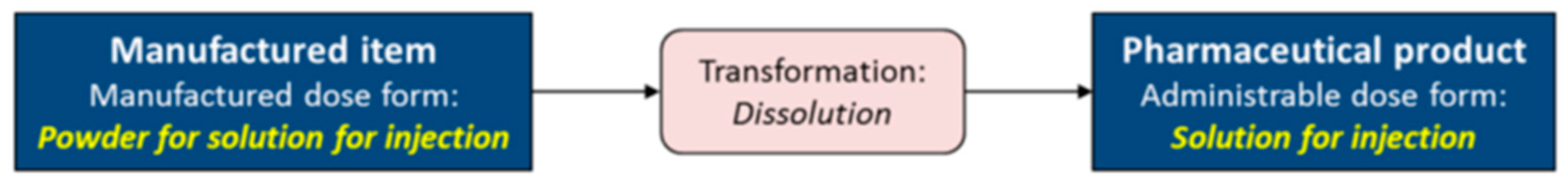
- Transformation (six values): dilution, dissolution, dispersion, mixing, no transformation, unknown;
- Intended Site (25 values): example: auricular; ocular; oral (see Supplementary File for full list);
- Administration method (19 values): example: application; inhalation; injection.
3.2. Potential Problems Identified and Approaches to Improvements
3.3. Construction of a New Basic File
3.4. Construction of the Small Ontology
3.5. Recommendations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDMP in a Capsule. Available online: https://unicom-project.eu/wp-content/uploads/2021/10/UNICOM-handboek_A4_04.pdf (accessed on 28 January 2022).
- ISO 11616:2012; Health Informatics—Identification of Medicinal Products—Data Elements and Structures for the Unique Identification and Exchange of Regulated Pharmaceutical Product Information. Available online: https://www.iso.org/standard/55035.html (accessed on 28 January 2022).
- ISO 11239:2012; Health Informatics—Identification of Medicinal Products—Data Elements and Structures for the Unique Identification and Exchange of Regulated Information on Pharmaceutical Dose Forms, Units of Presentation, Routes of Administration and Packaging. Available online: https://www.iso.org/standard/55032.html (accessed on 28 January 2022).
- EDQM Standard Terms. Introduction and Guidance for Use (Version 2.1.3—16 November 2018). Available online: https://www.edqm.eu/sites/default/files/standard_terms_introduction_and_guidance_for_use.pdf (accessed on 28 January 2022).
- Available online: https://www.fda.gov/industry/fda-resources-data-standards/identification-medicinal-products-idmp (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/wp-content/uploads/2021/11/Substance-and-Strenght_Oct.pdf (accessed on 28 January 2022).
- Available online: https://www.fda.gov/drugs/news-events-human-drugs/identification-medicinal-products-path-global-implementation-06112021-06112021 (accessed on 28 January 2022).
- Available online: https://www.ema.europa.eu/en/documents/presentation/presentation-report-who-workshop-idmp_en.pdf (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/pharmaceutical-product-identifier-phpid-is-taking-ground-under-the-leadership-of-who-umc/ (accessed on 28 January 2022).
- EMA Products Management Services—Implementation of International Organization for Standardization (ISO) Standards for the Identification of Medicinal Products (IDMP) in Europe. Introduction—EU Implementation Guide Version 2.1. Available online: https://ema.europa.eu/en/documents/regulatory-procedural-guideline/products-management-services-implementation-international-organization-standardization-iso-standards_en.pdf (accessed on 28 January 2022).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); ICH E2B(R3) Expert Working Group/Implementation Working Group. Information Paper Regarding the Use of ISO IDMP Standard in E2B(R3) Messages (Final Version, Approved on 17 June 2021). Available online: https://admin.ich.org/sites/default/files/inline-files/ICH_E2B%28R3%29_EWGIWG_Information_Paper_IDMP_Use_in_E2B%28R3%29_Messages_Final_2021_0617_0_1.pdf (accessed on 28 January 2022).
- Available online: https://www.nlm.nih.gov/research/umls/rxnorm/docs/appendix2.html (accessed on 28 January 2022).
- Available online: https://confluence.ihtsdotools.org/attachments/SNOMED_CT_Medicinal_Product_Model_Specification (accessed on 28 January 2022).
- Revised IDMP Standards to Improve Description of Medicinal Products Worldwide. Available online: https://www.iso.org/news/ref2234.html (accessed on 28 January 2022).
- Available online: https://unicom-project.eu/ (accessed on 28 January 2022).
- Standard Terms Database | EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/standard-terms-database (accessed on 28 January 2022).
- EDQM Standard Terms. Internal Controlled Vocabularies for Pharmaceutical Dose Forms (Version 1.2.0—28 January 2019). Available online: https://www.edqm.eu/sites/default/files/standard_terms_internal_vocabularies_for_pharmaceutical_dose_forms.pdf (accessed on 28 January 2022).
- Cimino, J.J. Desiderata for controlled medical vocabularies in the twenty-first century. Methods Inf. Med. 1998, 37, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Keizer, N.F.; Abu-Hanna, A.; Zwetsloot-Schonk, J.H. Understanding terminological systems. I: Terminology and typology. Methods Inf. Med. 2000, 39, 16–21. [Google Scholar] [PubMed] [Green Version]
- ISO 17117-1:2018; Health Informatics—Terminological Resources—Part 1: Characteristics. Available online: https://www.iso.org/standard/61979.html (accessed on 28 January 2022).
- ISO/TS 17117-2; Health Informatics—Terminological Resources—Part 2: Implementation Capability (TIC). Available online: https://www.iso.org/standard/76617.htm (accessed on 28 January 2022).
- ISO/TS 21526:2019; Health Informatics—Metadata Repository Requirements (MetaRep). Available online: https://www.iso.org/standard/71041.html (accessed on 28 January 2022).
- HL7 Specification: Characteristics of a Value Set Definition, Release 1. Available online: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=437 (accessed on 28 January 2022).
- Horridge, M.; Tudorache, T.; Nuylas, C.; Vendetti, J.; Noy, N.F.; Musen, M.A. WebProtégé: A collaborative Web-based platform for editing biomedical ontologies. Bioinformatics 2014, 30, 2384–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, J.; Becker, K.; Ludmann, D.; Pantazoglou, E.; Dewenter, H.; Thun, S. Intercoder Reliability of Mapping Between Pharmaceutical Dose Forms in the German Medication Plan and EDQM Standard Terms. Stud. Health Technol. Inform. 2018, 247, 845–849. [Google Scholar] [PubMed]
- Mapping Guidance for EDQM to SNOMED CT Pharmaceutical Dose Form Mapping. Available online: https://confluence.ihtsdotools.org/display/USRG/Mapping+Guidance+for+EDQM+to+SNOMED+CT+Pharmaceutical+Dose+Form+Mapping (accessed on 28 January 2022).
- Karapetian, N.; Vander Stichele, R.H.; Quintana, Y. Evaluating the Interoperability of Two Standard Terminologies for Dosage Form: RxNorm from the National Library of Medicine for the United States and EDQM from the European Directorate for the Quality in Medicines and Healthcare for Europe. SSRN. 2022. submitted. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4017191 (accessed on 12 February 2022). [CrossRef]
- Unicom Community of Expertise Webinar on 4 February 2022. Available online: https://unicom-project.eu/all-community-of-expertise-webinars-in-a-nutshell/ (accessed on 12 February 2022).
| (a) Analysis Not Taking “Systemic/Local” into Account | ||||||||||||||
| Descriptors | Characteristics | Results | Check | |||||||||||
| Number of Analysis | Basic Dose Form (PDF) | State of Matter (PDF) | Basic Dose Form (ADF) | State of Matter (ADF) | Transformation (TRAC) | Release Characteristics (RC) | Intended Site Split (ISI-s) | Administration Method (AMEC) | Systemic/Local | Total Number of Unique Combinations (UC) | Unique Combinations (UC) with 1 Occurence | Unique Combinations (UC) with 2+ Occurences | Sum of Occurences in Unique Combinations 2+ | Sum of Occurences in UC2+ and in UC1 |
| Analysis 1 | x | x | x | x | x | x | x | x | 377 | 340 | 37 | 88 | 428 | |
| Analysis 2 | x | x | x | x | x | x | 349 | 293 | 56 | 135 | 428 | |||
| Analysis 3 | x | x | x | x | 192 | 113 | 79 | 315 | 428 | |||||
| Analysis 4 | x | x | x | x | 195 | 78 | 117 | 350 | 428 | |||||
| (b) Same Analysis but Now Taking “Systemic/Local” into Account | ||||||||||||||
| Analysis 1 | x | x | x | x | x | x | x | x | x | 383 | 350 | 33 | 78 | 428 |
| Analysis 2 | x | x | x | x | x | x | x | 357 | 306 | 51 | 122 | 428 | ||
| Analysis 3 | x | x | x | x | x | 206 | 128 | 78 | 300 | 428 | ||||
| Analysis 4 | x | x | x | x | x | 274 | 197 | 77 | 231 | 428 | ||||
| LEGEND | ||||||||||||||
| Descriptors: Basic Dose Form (BDF) and State of Matter (SOM) of Pharmaceutical Dose Form (PDF) and Administrable Dose Form (ADF) | ||||||||||||||
| Characteristics: | ||||||||||||||
| Transformation (TRAc):(6 values): dilution, dissolution, dispersion, mixing, no transformation, unknown. | ||||||||||||||
| Release Characteristics (RC):(4 values): conventional, prolonged, delayed, modified. | ||||||||||||||
| Intended Site (ISI-s):(25 values): example: auricular; ocular; oral (see Supplementary file for full list). | ||||||||||||||
| Administration Method (AMEc):(19 values): example: application; inhalation; injection. (see supplementary file for full list). | ||||||||||||||
| Systemic/local: (4 values):systemic, local, local/systemic, unknown | ||||||||||||||
| Analysis 1:Taking all descriptors and all characteristics into account | ||||||||||||||
| Analysis 2:Taking the descriptors of the administrable dose form and all characteristics into account | ||||||||||||||
| Analysis 3:Taking only all characteristics into account | ||||||||||||||
| Analysis 4:Taking the Basic Dose Form of the Administrable Dose Form, RC, ISI-s, and AMEc into account (mimicing the FDA/WHO_UMC pilot approach) | ||||||||||||||
| Unique combinations (UC) with 1 occurence:a specific combination of the values of descriptors and/or characteristics, represented by one PDF | ||||||||||||||
| Can be considered as a measure of granularity of the dose form terminology and an indicator of congruence with the textual definition | ||||||||||||||
| Unique combinations with 2 or more occurences (UC2+):a specific combination of the values, represented by two or more PDFs | ||||||||||||||
| Can be considered as a measure of aggregation for ontologic class creation | ||||||||||||||
| Sum of occurences in UC2+:the number of PDFs grouped in unique combinations of values with 2 or more occurences of dose forms | ||||||||||||||
| Can be considered as an additional measure of aggregation for ontological class creation | ||||||||||||||
| Total number of unique combinations:sum of UC and UC2+ | ||||||||||||||
| Can be considered as an addtional measure of granularity of the dose form terminology | ||||||||||||||
| Check:the sum of UC and the sum of the occurences in UC2+ must always be 428 (grey cells) | ||||||||||||||
| AURICULAR | ORAL, CONVENTIONAL-RELEASE |
| Auricular dose form | Oral solid dose form |
| Auricular/nasal dose form | Oral semi-solid dose form |
| Auricular/nasal/ocular dose form | Oral drops dose form |
| Auricular/ocular dose form | Oral liquid dose form |
| CUTANEOUS | Oral effervescent or dispersible dose form |
| Cutaneous dose form | Oral/rectal dose form |
| Cutaneous/transdermal dose form | ORAL, MODIFIED-RELEASE |
| Cutaneous/nasal dose form | Oral gastro-resistant dose form |
| Cutaneous/oromucosal dose form | Oral prolonged-release dose form |
| Cutaneous/parenteral dose form | Other oral modified-release dose form |
| DENTAL | OROMUCOSAL |
| Dental dose form | Oromucosal spray dose form |
| ENDOCERVICAL | Oromucosal solid dose form |
| Endocervical dose form | Oromucosal prolonged-release dose form |
| EXTRACORPOREAL | Oromucosal liquid dose form |
| Extracorporeal dose form | Oromucosal gargling/mouthwash dose form |
| EXTRACORPOREAL/PARENTERAL | Sublingual dose form |
| Dialysis dose form | PARENTERAL |
| GASTRIC | Implantation prolonged-release dose form |
| Gastric dose form | Injection prolonged-release dose form |
| GASTROENTERAL | Injection dose form |
| Gastroenteral dose form | Infusion dose form |
| INTRAPERITONEAL | Infusion/injection dose form |
| Intraperitoneal dose form | PULMONARY |
| INTRAUTERINE | Vapour dose form |
| Intrauterine dose form | Nebuliser dose form |
| Intrauterine device | Pressurised inhalation dose form |
| INTRAVESICAL | Inhalation dose form |
| Intravesical/intraurethral dose form | Medicinal gas dose form |
| OCULAR | Endotracheopulmonary instillation dose form |
| Ocular semi-solid dose form | RECTAL |
| Ocular drops dose form | Rectal systemic dose form |
| Ocular rinsing dose form | Rectal local dose form |
| Ocular intraocular dose form | TRANSDERMAL |
| Ocular prolonged-release dose form | Transdermal prolonged-release dose form |
| NASAL | Transdermal dose form |
| Nasal spray dose form | VAGINAL |
| Nasal solid or semi-solid dose form | Vaginal prolonged-release dose form |
| Nasal drops dose form | Vaginal dose form |
| Endosinusial dose form | Vaginal device |
| Nasal/ocular/pulmonary dose form | MISCELLANEOUS |
| Radiopharmaceutical dose form | |
| Wound dressings prolonged-release dose form | |
| Ungrouped dose form |
| 1. The relationship between transformable Pharmaceutical dose forms (hence, the manufactured dose forms) and their administrable dose forms (and their state of matter and basic dose form) needs to be made more explicit in the EDQM dose form Terminology 2. The role of the characteristics in the EDQM dose form terminology, albeit not definitional, can be more then informational and may become the basis of systematic revision and ontology creation. 3. The value sets of the characteristics need to be slightly revised, taking into account multiplicity, systemic effect, and sublingual use. The coding system of the values needs to be corroborated and strengthened by adopting ISO/CEN norms for coding systems. 4. A new characteristic could be added, based on systemic and/or local effect. This would greatly facilitate the automated identification of polypharmacy as the concomitant use of 5 or more chronic drugs with systemic effect. 5. A simple ontology for dose forms may enable more rigorous standardization of dose forms in national regulatory agencies and facilitate semantic interoperability the alignment with other dose form terminologies, used in clinical care and research. 6. Standardization of national dose form identification to EDQM will require special training of regulatory experts in industry and agencies, and validation procedures. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Stichele, R.H.; Roumier, J.; van Nimwegen, D. How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology. Appl. Sci. 2022, 12, 4337. https://doi.org/10.3390/app12094337
Vander Stichele RH, Roumier J, van Nimwegen D. How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology. Applied Sciences. 2022; 12(9):4337. https://doi.org/10.3390/app12094337
Chicago/Turabian StyleVander Stichele, Robert H., Joseph Roumier, and Dirk van Nimwegen. 2022. "How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology" Applied Sciences 12, no. 9: 4337. https://doi.org/10.3390/app12094337
APA StyleVander Stichele, R. H., Roumier, J., & van Nimwegen, D. (2022). How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology. Applied Sciences, 12(9), 4337. https://doi.org/10.3390/app12094337






