Predictive Model of Lyme Disease Epidemic Process Using Machine Learning Approach
Abstract
:1. Introduction
- Models and methods of epidemic processes simulation should be analyzed.
- Models and methods of the Lyme disease epidemic process simulation should be analyzed.
- Data on Lyme disease morbidity in the Kharkiv region (Ukraine) should be analyzed.
- The Lyme disease epidemic process machine learning model based on neural networks should be developed.
- The experimental study of the developed model with actual statistical data on Lyme disease morbidity should be provided.
- The results of the developed model should be compared with other Lyme disease epidemic process models.
- The development of a simulation machine learning model of the Lyme disease epidemic process and the comparison of the results with other approaches will allow for the estimating of the accuracy of the neural network approach applied to the simulation of the epidemic process simulation, specifically vector-borne diseases.
- The development of a machine learning model based on neural networks of the Lyme disease epidemic process will allow for the predicting of the epidemic situation without an additional measurement of climate parameters, using indicators of the number of ticks and their infection with borrelia in any selected territory to build the model.
- The application of the machine learning model based on neural networks to the Lyme disease epidemic process in the Kharkiv region (Ukraine) will allow for the estimating of its dynamics, contributing to closing a severe gap in understanding the spread of Lyme borreliosis in specific spatial and temporal settings, assessing the risks of Lyme disease in humans, and mitigating the impact of tick-borne infections in the future.
2. Background
2.1. Lyme Disease Epidemiology
2.2. Current Research Analysis
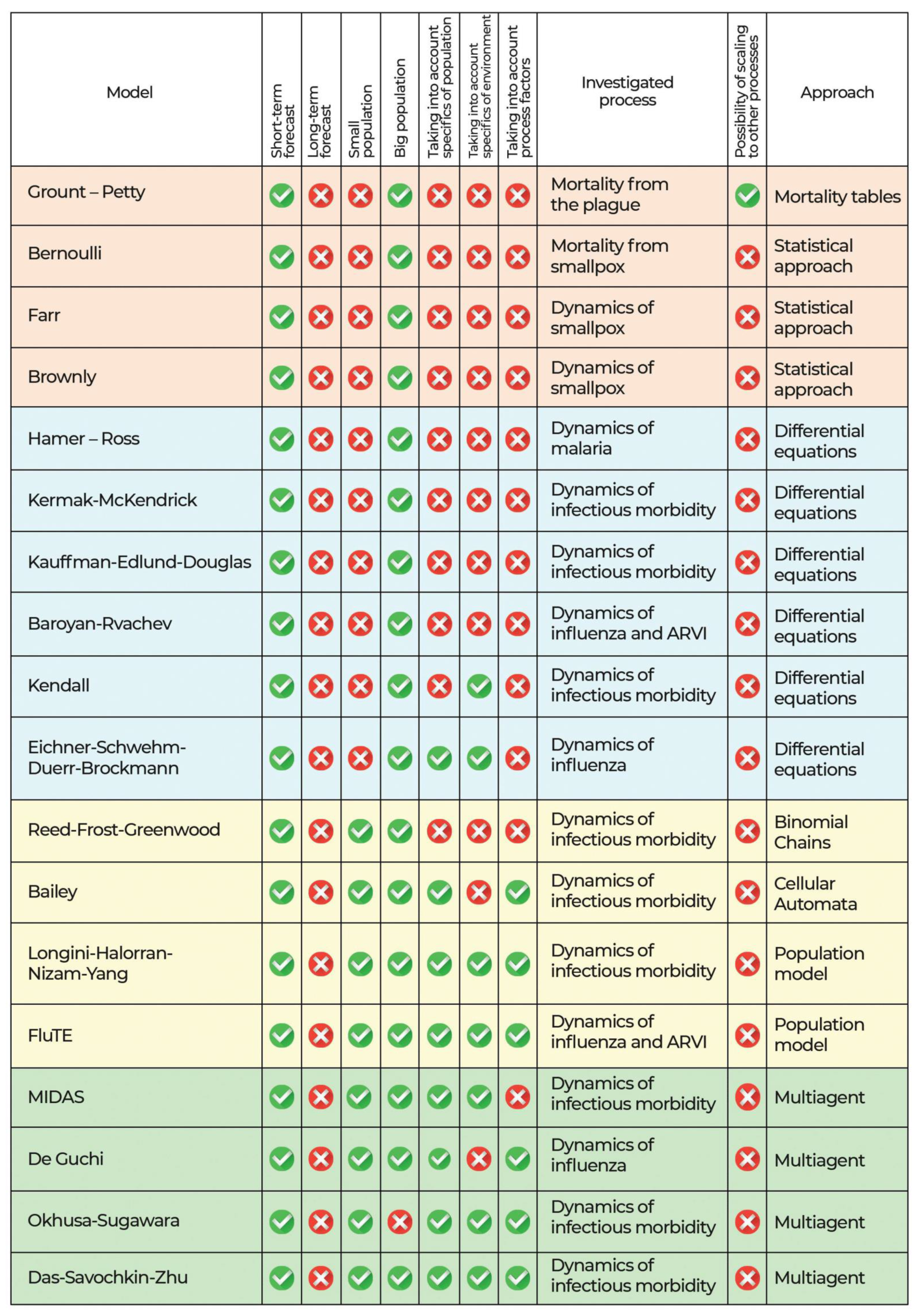
- Models aimed at the theoretical study of the epidemic process of Lyme disease;
- Simulation models aimed at modeling certain aspects of the epidemic process of Lyme disease.
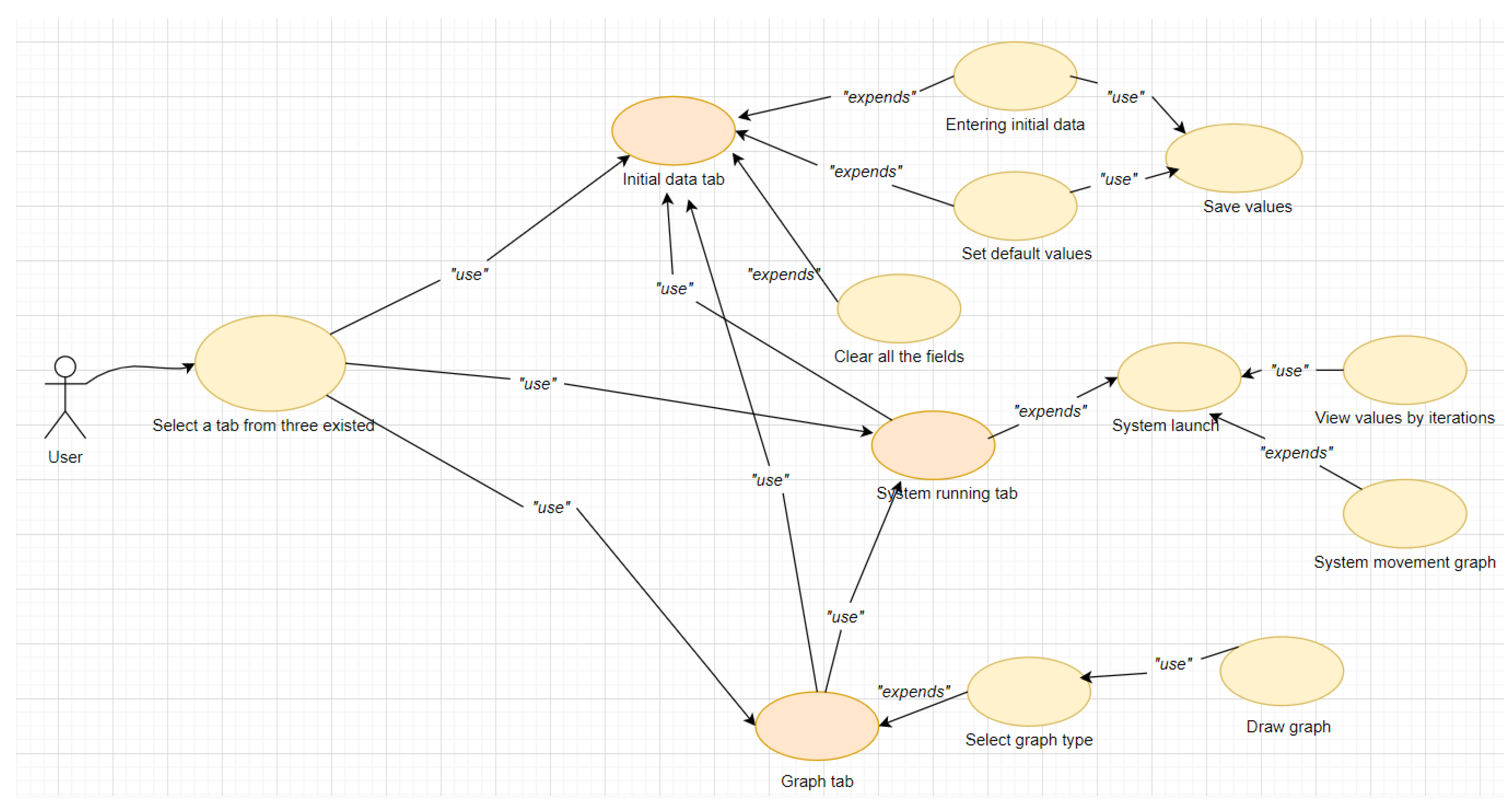
3. Materials and Methods
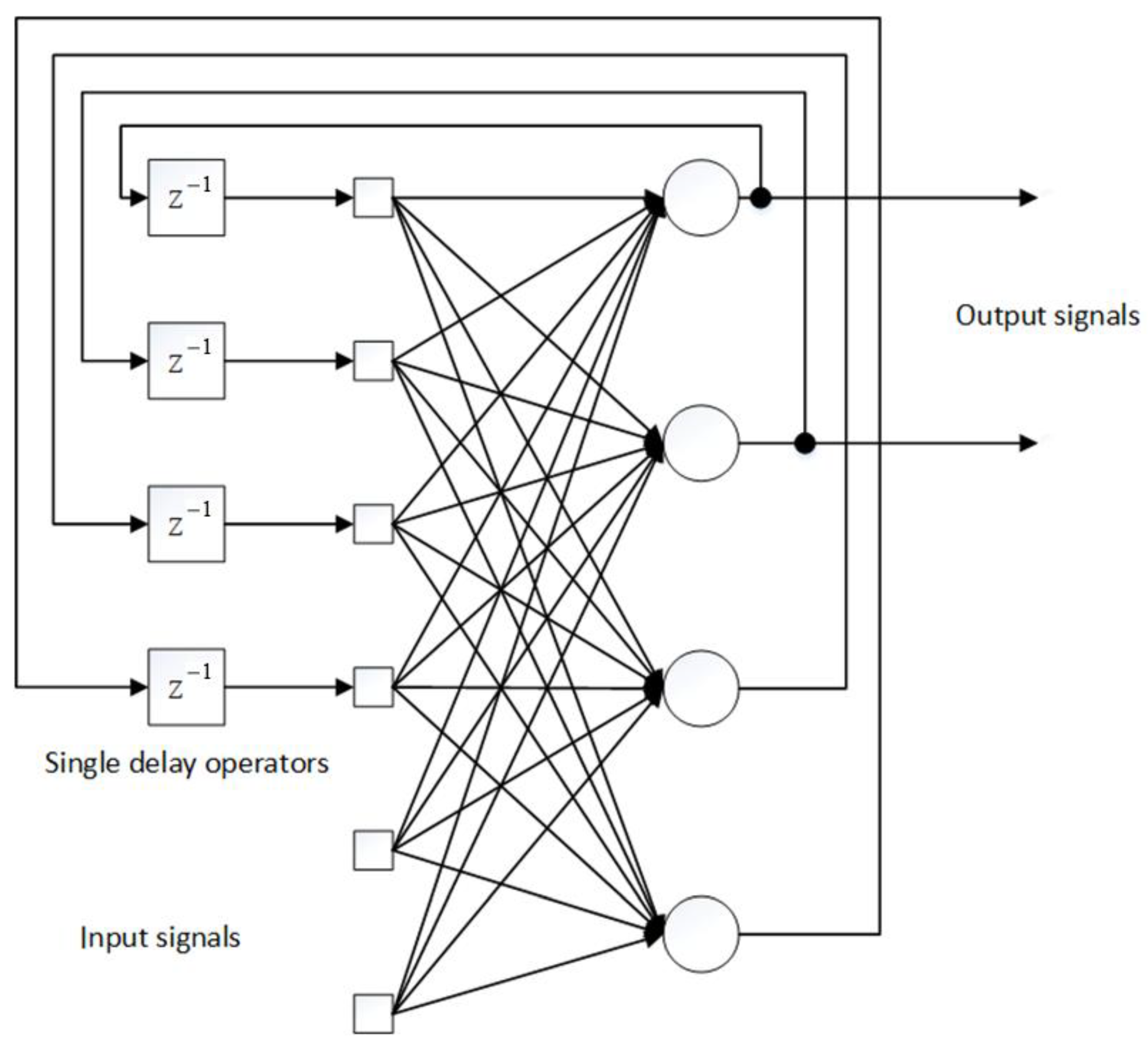
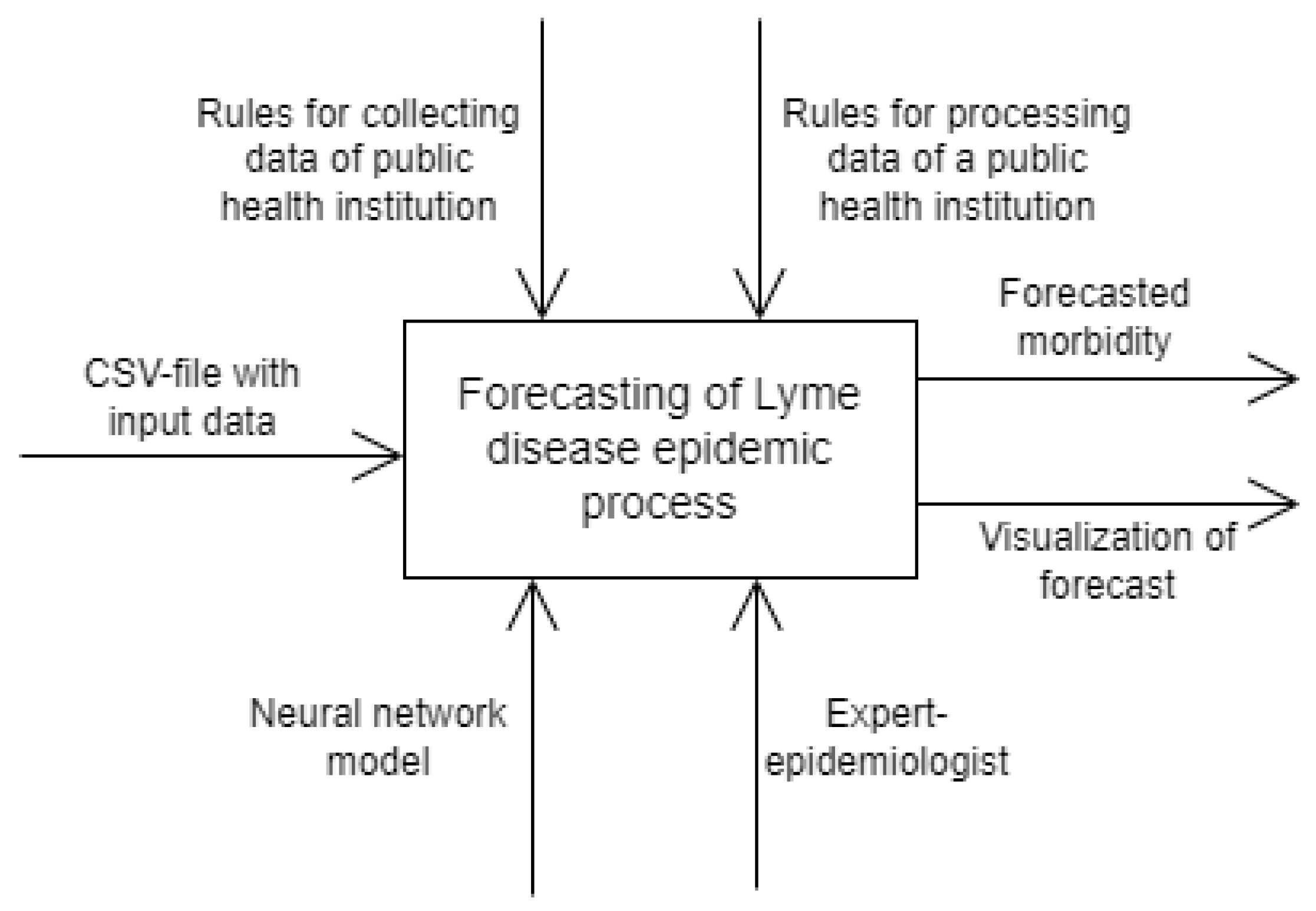
3.1. Model of Lyme Disease Epidemic Process
- setting the initial conditions for all synaptic weights of the network in the form of sufficiently small random numbers so that the activation functions of neurons do not enter saturation mode at the initial stages of learning (protection from network “paralysis”);
- feed to the input of the network of method x and calculate the outputs of all neurons for given values ;
- for a given training vector d and the calculated intermediate outputs , the calculation of local errors for all layers;
- clarification of all synaptic scales by the formula
- input to the network input of the following method x, etc.
- Initialization of synaptic weights with small random values.
- Selecting the next training pair from the training set.
- Feeding the input vector to the input of the network.
- Network output calculation.
- Calculate the difference between the network output and the required output.
- Neural network weight adjustment to minimize error.
- Iteration of the algorithm until the error is minimized.
- The number of input neurons is 48.
- The number of hidden neurons is 72.
- The number of output neurons is 24.
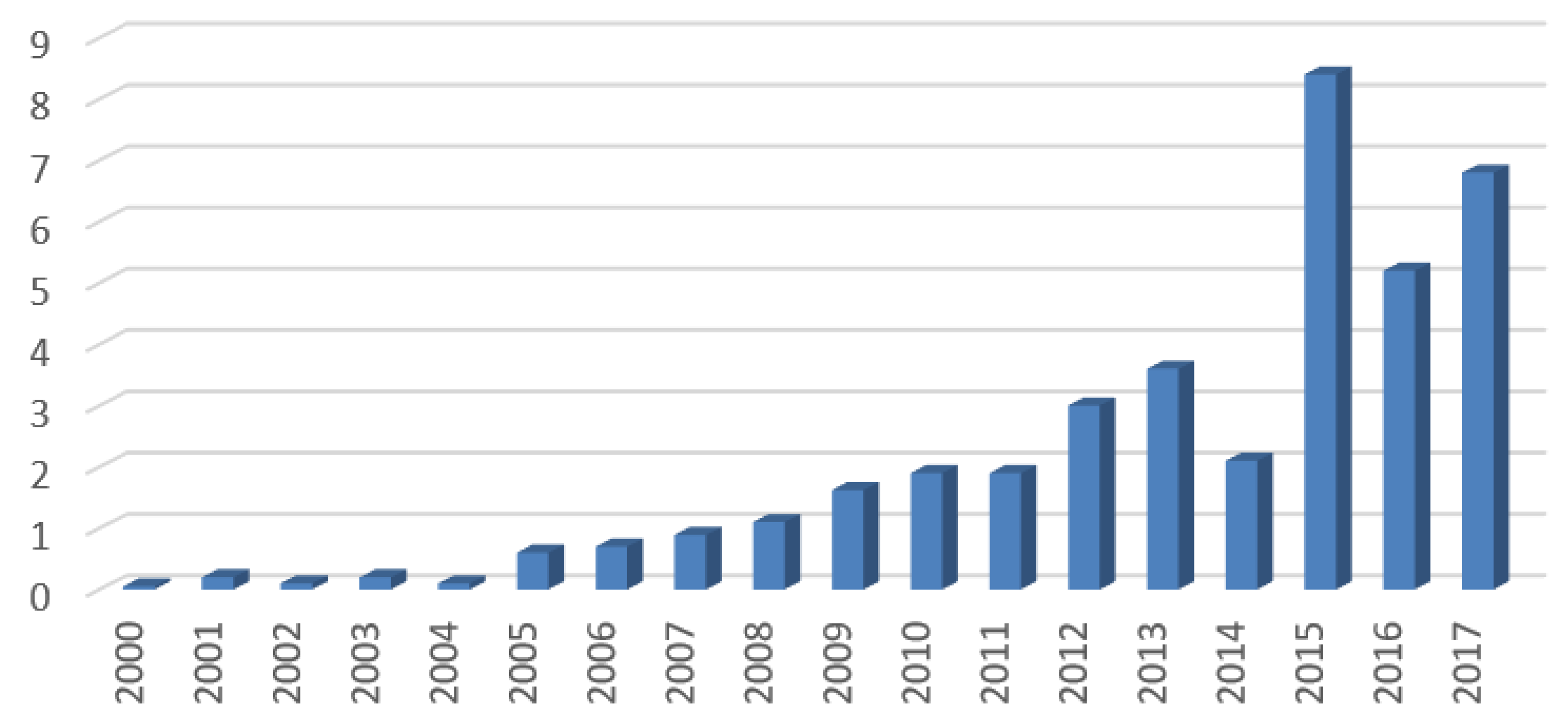
3.2. Data Analysis
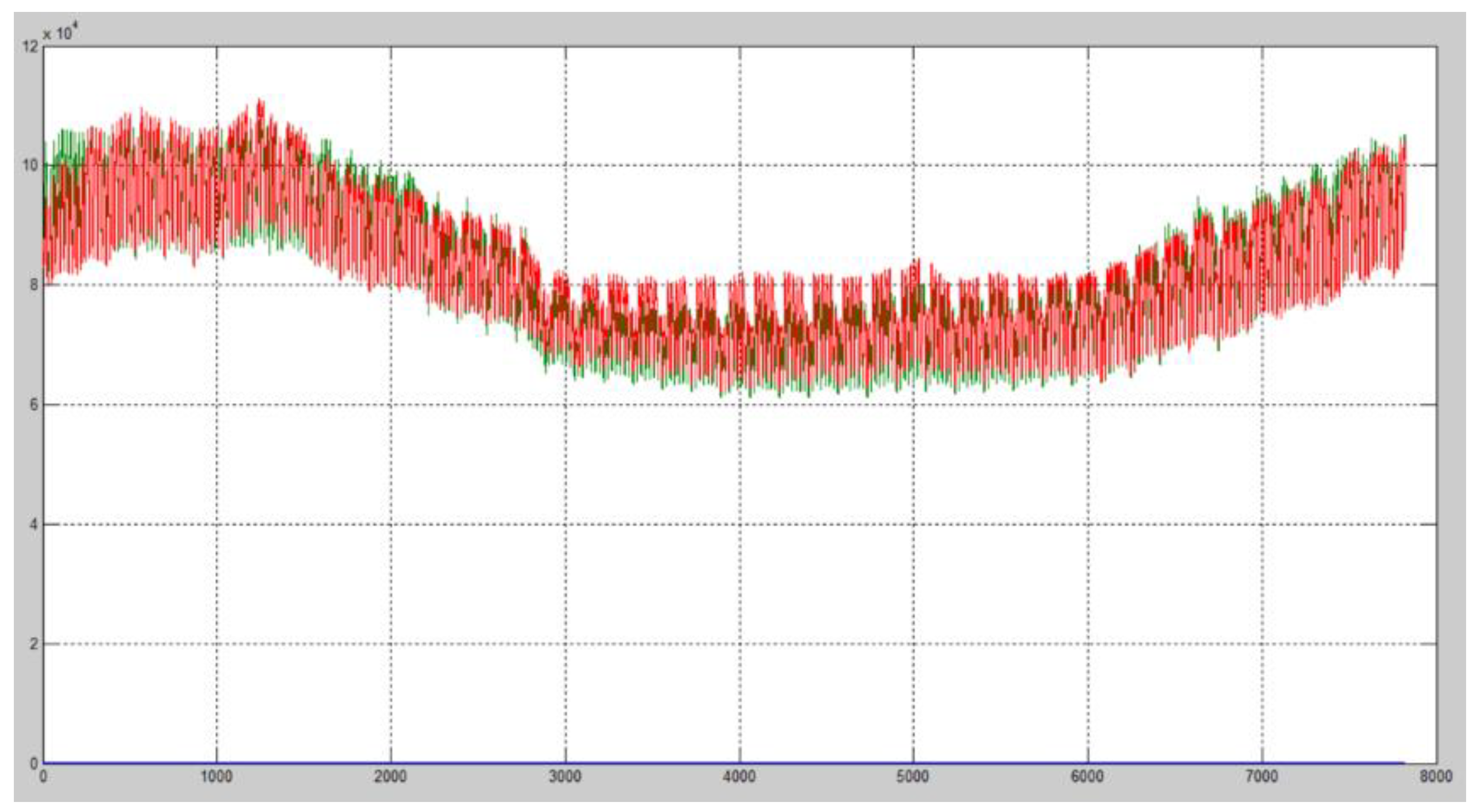
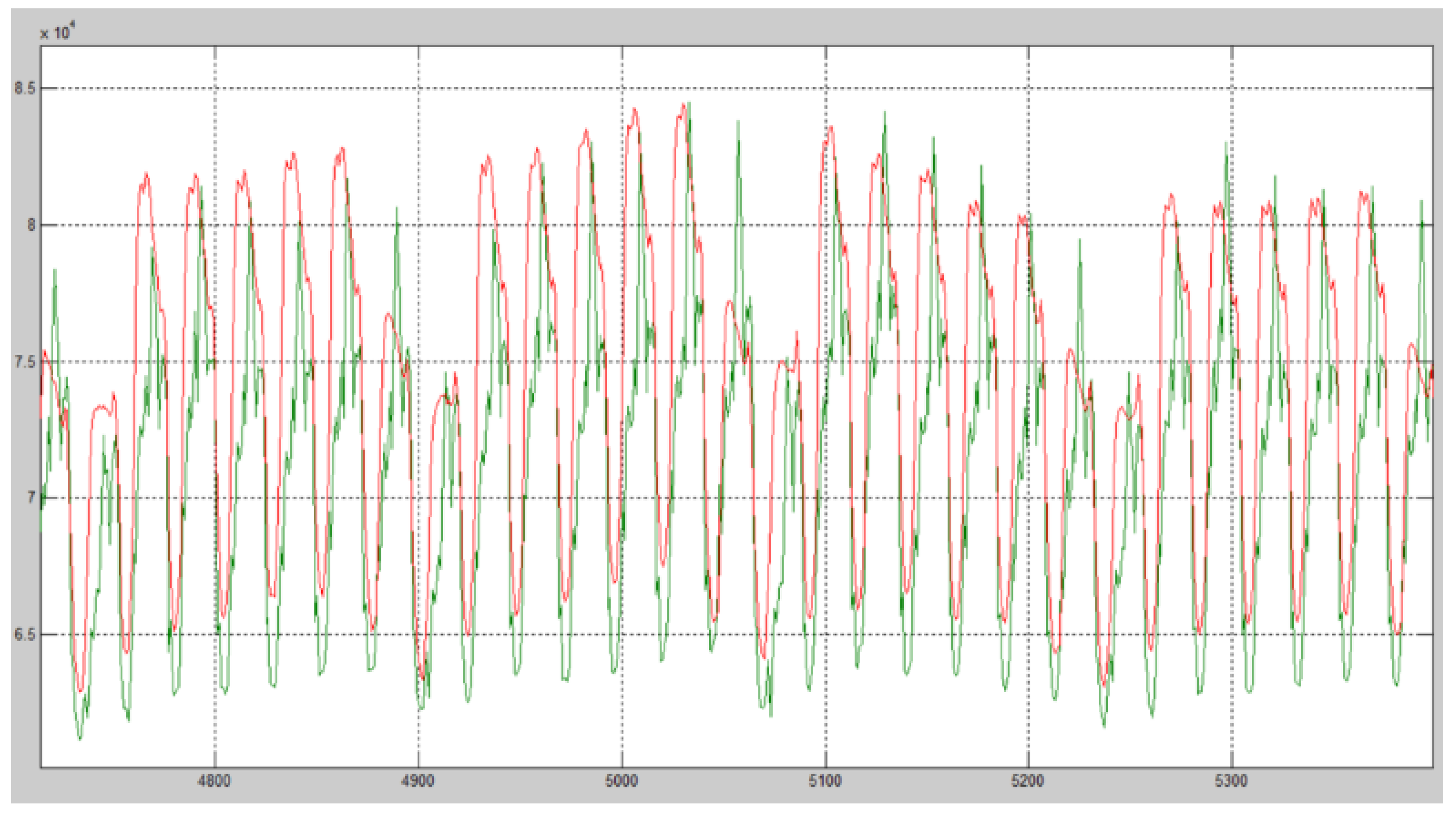
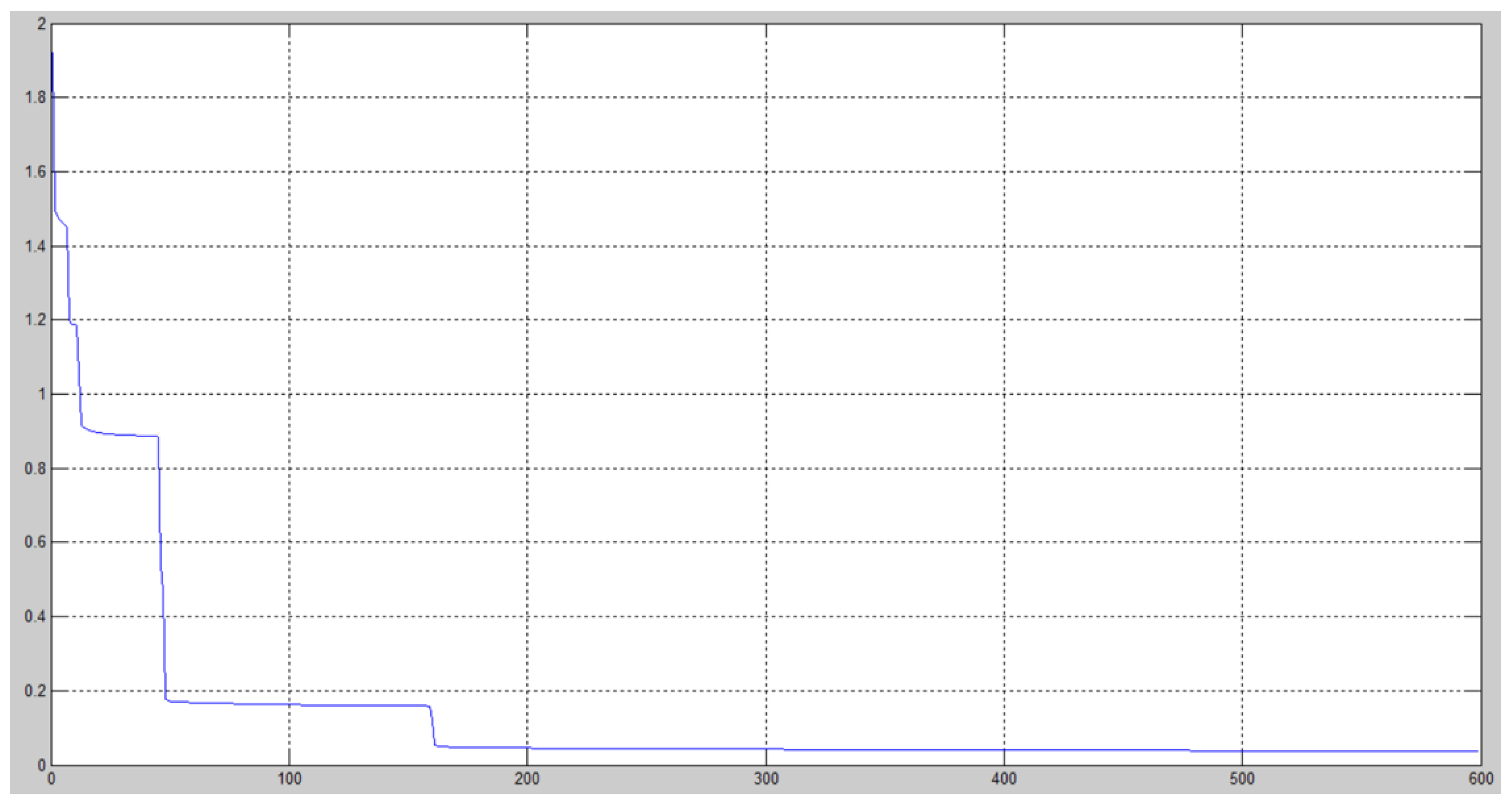
4. Results
- Creation of unfavorable conditions for the habitat of carriers of Lyme disease—clearing and landscaping of forest areas (clearing debris, removing deadwood, brushwood, undersized shrubs, mowing grass).
- Carrying out extermination measures (disinfestation, the use of a chemical method of combating ticks, deratization, the destruction of hosts of tick larvae and nymphs).
- systematic self- and mutual examination of clothing and body, which is extremely important for preventing ticks from being sucked. They are held every two hours of being in a natural focus without taking off their clothes;
- timely and correct removal of sucked ticks, if possible, in a medical facility;
- wearing protective clothing while staying in dangerous areas of the natural focus;
- impregnation of clothing with repellents or insect repellents. After contact with the treated fabric, after 3–5 min, all attached ticks become incapable of suction and fall off the clothes. You can scare away many ticks by applying repellent aerosols to clothing with encircling stripes.
- formation among the population of an understanding of the severity of the course of the disease and its consequences;
- instilling basic knowledge about the ways of infection, methods of collective and individual protection against ticks, the importance of emergency prevention of Lyme disease;
- developing the population’s skills to conduct self- and mutual examinations in endemic foci and use protective clothing, special dressing of ordinary clothing, individual protection against ticks, including the use of repellents.
- features of the attack of ixodid ticks on humans;
- the importance of measures of individual protection against ticks and the order of their application;
- the need to quickly remove a tick attached to the body by a medical professional or on their own, and in this case, immediately contact a doctor.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model | Task | Outcome | Approach |
|---|---|---|---|
| Porto T. (1999) [47] | To define the threshold condition for a disease to enter a non-enzootic area depending on the various possible chains of transmission operating during the year. | Mathematical model of the life history of the tick to quantify the relative contributions of various transmission chains throughout the year. | Differential equations |
| Kurtenback K., et al., (2006) [48] | To study how external factors and internal dynamics shape populations of B. burgdorferi sl. | Possible epidemiological parallels between B. burgdorferi sl and other transmissible zoonotic pathogens are described. | Compartmental model |
| Wang W., Zhao X.Q. (2015) [49] | To formulate a mathematical model of Lyme disease, including a spatially heterogeneous structure. | It has been shown that the basic reproduction number R0 serves as a threshold value between extinction and persistence in the evolution of Lyme disease. | Differential equations |
| Bisanzio D., et al., (2010) [50] | To describe the distribution of Ixodes ricinus ticks on mice and lizards from two independent studies. | The extreme aggregation of vectors on hosts, described by the power-law decay of the degree distribution, makes the epidemic threshold decrease with the size of the network and vanish asymptotically. | Bipartite networks |
| Gaff H., et al., (2020) [51] | To upgrade the LYMESIM model to mimic the I. scapularis life cycle and transmission dynamics of B. burgdorferi.ss, which includes several modifications to increase the biological realism of the model and produce results that are easier to measure in the field. | The model showed the importance of temperature in host detection for nymph density, the importance of transmission from small mammals to ticks for the density of infected nymphs, and the survival of ticks as a function of temperature. | Compartmental model |
| Hart T.M., et al., (2021) [52] | To reveal the mechanisms of tropism of the host of Lyme disease. | Different types of Lyme disease bacteria differ in their ability to survive in mice and quails, as well as in ticks that feed on human or quail blood after transmission. | Molecular study |
| Lou Y, Wu J., Wu X. (2014) [53] | To understand the combined effect of seasonal temperature fluctuations and host community composition on transmission of the Lyme disease pathogen. | The relationship between host community biodiversity and disease risk varies, requiring more accurate measurements of the local environment, both biotic and abiotic. | Compartmental model |
| Belli A., et al., (2017) [54] | To test whether ticks that acquire Lyme disease pathogens through co-feeding are infective to vertebrate hosts. | B. afzelii may use a co-feeding transfer to complete its life cycle. | Vector study |
| Lis S., et al., (2016) [55] | To study the temperature-driven seasonality of Ixodes ricinus ticks and the transmission of B. burgdorferi sl in mainland Scotland. | The risk of Lyme disease currently peaks in the fall, about six weeks after the temperature peaks. | Multiagent model |
| Zhang Y., Zhao X.Q. (2013) [56] | To study the reaction-diffusion model of Lyme disease taking into account seasonality. | In the case of a bounded habitat, we obtain a threshold result on the global stability of either disease-free or endemic periodic solution. In the case of an unbounded habitat, we establish the existence of the disease spreading speed and its coincidence with the minimal wave speed for time-periodic traveling wave solutions. | Compartmental model |
| Imai C., et al., (2015) [57] | To study changes in immune population, strong autocorrelations, a wide range of plausible lag structures and association patterns, seasonality adjustments, large overdispersion. | For overdispersed models, alternative distribution models such as quasi-Poisson and negative binomial should be considered. | Time series regression |
| Dumic I., Severnini E. (2018) [58] | To investigate how the activity of ticks and their survival depend on temperature and humidity. | A significant effect of temperature on the incidence of Lyme disease has been found. These impacts can be roughly described by an inverted U-shaped relationship consistent with tick survival patterns and host-seeking behavior. | Panel regression model |
| Ogden N.H., et al., (2018) [59] | To study the factors that determine seasonality in a multi-year study in seven areas of the geographic range of I. scapularis | Temperature-independent diapause mechanisms explain some key observed variations in I. scapularis seasonality, and are responsible in part for geographic variations in I. scapularis seasonality in the United States. | Binomial regression model |
| Zhao G.P., et al., (2021) [60] | To understand the ecological niches of major tick species and common tick-borne pathogens. | Suitable habitats for the 19 tick species are 14–476% larger in size than the geographic areas where these species were detected, indicating severe under-detection | Machine learning |
| Nguyen A., Mahaffy J., Vaidya N.K. (2019) [61] | To study interactions between the main Lyme disease vectors involved: black-footed ticks (I. scapularis), white-footed mice (Peromyscus leucopus) and white-tailed deer (Odocoileus virginianus). | The presence of multiple vectors can have a significant impact on the dynamics and spread of Lyme disease. | Compartmental model |
| Bobe J.R., et al., (2021) [62] | To investigate the prevention, diagnosis and treatment of Lyme disease during COVID-19 pandemic. | COVID-19 may further complicate diagnosis of Lyme disease since non-specific symptoms in these two conditions overlap and people may be spending more time outdoors. | Various approaches |
References
- Sprong, H.; Azagi, T.; Hoornstra, D.; Nijhof, A.; Knorr, S.; Baarsma, M.; Hovius, J. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasites Vectors 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, R.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Jutras, B.; Lochhead, R.; Kloos, Z.; Biboy, J.; Strle, K.; Booth, C.J.; Govers, S.K.; Gray, J.; Schumann, P.; Vollmer, W.; et al. Borrelia burgdorferipeptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc. Natl. Acad. Sci. USA 2019, 116, 13498–13507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 2016. Available online: https://apps.who.int/iris/handle/10665/246208 (accessed on 18 November 2021).
- Ben, I.; Lozynskyi, I. Prevalence of Anaplasma phagocytophilumin Ixodes ricinusand Dermacentor reticulatusand Coinfection with Borrelia burgdorferiand Tick-Borne Encephalitis Virus in Western Ukraine. Vector-Borne Zoonotic Dis. 2019, 19, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Best, S.; Tschaepe, M.; Wilson, K. Investigation of the performance of serological assays used for Lyme disease testing in Australia. PLoS ONE 2019, 14, e0214402. [Google Scholar] [CrossRef]
- Shapiro, E.; Wormser, G. Lyme Disease in 2018. JAMA 2018, 320, 635–636. [Google Scholar] [CrossRef]
- Rogovskyy, A.; Biatov, A.; Davis, M.; Liu, S.; Nebogatkin, I. Upsurge of Lyme borreliosis in Ukraine: A 20-year survey. J. Travel Med. 2020, 27, taaa100. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Lindgren, P.E. Detection of a novel Lyme borreliosis pathogen. Lancet. Infect. Dis. 2016, 16, 511–512. [Google Scholar] [CrossRef]
- Bobbi, S.P.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet. Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Kullberg, B.J.; Vrijmooeth, H.D.; Schoor, F.V.D.; Hovius, J.W. Lyme borreliosis: Diagnosis and management. BMJ 2020, 369, m1041. [Google Scholar] [CrossRef]
- Schotthoefer, A.M.; Frost, H.M. Ecology and Epidemiology of Lyme Borreliosis. Clin. Lab. Med. 2015, 35, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.L. Clinical manifestations and treatment of Lyme disease. Clin. Lab. Med. 2015, 35, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, C.C.V.D.; Hofhuis, A.; Simoes, M.; Rood, E.; Pelt, W.V.; Zeller, H.; Bortel, W.V. Surveillance perspective on Lyme borreliosis across the European Union and European economic area. Euro Surveill. Eur. Commun. Dis. Bull. 2017, 22, 30569. [Google Scholar] [CrossRef] [PubMed]
- Lindergen, E.; Jaenson, T.G.T. Lyme Borreliosis in Europe. Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures; World Health Organization: Copenhagen, Denmark, 2006; p. 34. [Google Scholar]
- Kugeler, K.J.; Farley, G.M.; Forrester, J.D.; Mead, P.S. Geographic distribution and expansion of human Lyme disease, United States. Emerg. Infect. Dis. 2015, 21, 1455–1457. [Google Scholar] [CrossRef]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef]
- Petrulioniene, A.; Radzisauskiene, D.; Ambrozaitis, A.; Caplinskas, S.; Paulauskas, A.; Venalis, A. Epidemiology of Lyme disease in highly endemic European zone. Medicina 2020, 56, 115. [Google Scholar] [CrossRef] [Green Version]
- Rizolli, A.; Hauffe, H.; Carpi, G.; Vourc, G.H.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Euro Surveill. Eur. Commun. Dis. Bull. 2011, 16, 19906. [Google Scholar] [CrossRef]
- Bacon, R.M.; Kugeler, K.J.; Mead, P.S. Surveillance for Lyme disease—United States, 1992–2006. Morbidity and mortality weekly report. Surveill. Summ. 2008, 57, 1–9. [Google Scholar]
- Cook, M.J.; Puri, B.K. Estimates for Lyme borreliosis infections based on models using sentinel canine and human seroprevalence data. Infect. Dis. Model. 2020, 5, 871–888. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; Cutler, S.; Potkonjak, A.; Vassier-Tussaut, M.; Bortel, W.V.; Zeller, H.; Fernandez-Ruiz, N.; Mihalca, A.D. An updated meta-analysis of the distribution of Borrelia burgorferi s.l. in ticks in Europe. Int. J. Health Geogr. 2018, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Bedson, J.; Skrip, L.A.; Pedi, D.; Abramowitz, S.; Carter, S.; Jalloh, M.F.; Funk, S.; Gobat, N.; Giles-Vernick, T.; Chowell, G.; et al. A review and agenda for integrated disease models including social and behavioral factors. Nat. Hum. Behav. 2021, 5, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. An application of the theory of probabilities to the study of a priori pathometry. Proc. R. Soc. Lond. 1916, 92, 204–230. [Google Scholar] [CrossRef] [Green Version]
- Hamer, W. The Milroy lectures on epidemic disease in England—The evidence of variability and of persistency of type. Lancet 1906, 167, 569–574. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. Contribution to the mathematical theory to epidemics. Proc. R. Soc. Lond. 1927, 115, 700–721. [Google Scholar] [CrossRef] [Green Version]
- Dietz, K.; Heesterbeek, J. Daniel Bernoulli’s epidemiological model revisited. Math. Biosci. 2002, 180, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Fine, P. John Brownlee and the Measurement of Infectiousness: An Historical Study in Epidemic Theory. J. R. Stat. Soc. 1979, 142, 347. [Google Scholar] [CrossRef]
- Santillana, M.; Tuite, A.; Nasserie, T.; Fine, P.; Champredon, D.; Chindelevitch, L.; Dushoff, J.; Fisman, D. Relatedness of the incidence decay with exponential adjustment (IDEA) model, “Farr’s law” and SIR compartmental difference equation models. Infect. Dis. Model. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Urabe, C.; Tanaka, G.; Aihara, K.; Mimura, M. Parameter Scaling for Epidemic Size in a Spatial Epidemic Model with Mobile Individuals. PLoS ONE 2016, 11, e0168127. [Google Scholar] [CrossRef] [Green Version]
- Baroyan, O.; Rvachev, L.; Basilevsky, U.; Ermakov, V.; Frank, K.; Rvachev, M.; Shashkov, V. Computer modelling of influenza epidemics for the whole country (USSR). Adv. Appl. Probab. 1971, 3, 224–226. [Google Scholar] [CrossRef]
- Brauer, F. The Kermack–McKendrick epidemic model revisited. Math. Biosci. 2005, 198, 119–131. [Google Scholar] [CrossRef]
- Douglas, J.; Bianco, S.; Edlund, S.; Engelhardt, T.; Filter, M.; Gunther, T.; Hu, K.M.; Nixon, E.J.; Sevilla, N.L.; Swaid, A.; et al. STEM: An Open Source Tool for Disease Modeling. Health Secur. 2019, 17, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Eichner, M.; Schwehm, M.; Duerr, H.; Brockmann, S. The influenza pandemic preparedness planning tool InfluSim. BMC Infect. Dis. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, D. Deterministic and Stochastic Epidemics in Closed Populations. In Proceedings of the Third Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, July–August 1955; pp. 149–165. [Google Scholar]
- Smith, D.; Battle, K.; Hay, S.; Barker, C.; Scott, T.; McKenzie, F. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, N. The simulation of stochastic epidemics in two dimensions. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, June–July 1967; pp. 237–257. [Google Scholar]
- Becker, N. A General Chain Binomial Model for Infectious Diseases. Biometrics 1981, 37, 251. [Google Scholar] [CrossRef]
- Chao, D.; Halloran, M.; Obenchain, V.; Longini, I. FluTE, a Publicly Available Stochastic Influenza Epidemic Simulation Model. PLoS Comput. Biol. 2010, 6, e1000656. [Google Scholar] [CrossRef] [Green Version]
- Longini, I.; Halloran, M.E.; Nizam, A.; Yang, Y.; Xu, S.; Burke, D.S.; Cummings, D.A.T.; Epstein, J.M. Containing a large bioterrorist smallpox attack: A computer simulation approach. Int. J. Infect. Dis. 2007, 11, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Cooley, P.; Roberts, D.; Bakalov, V.; Bikmal, S.; Cantor, S.; Costandine, T.; Ganapathi, L.; Golla, B.J.; Grubbs, G.; Hollingsworth, C.; et al. The Model Repository of the Models of Infectious Disease Agent Study. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Deguchi, H.; Ichikawa, M. Agent-based simulation on avian influenza in Vietnam: Basic characteristics of the epidemic and efficiency evaluation of control measures. In Proceedings of the 7Th International Conference on Service Systems and Service Management, Tokyo, Japan, 28–30 June 2010. [Google Scholar] [CrossRef]
- Ohkusa, Y.; Sugawara, T.; Taniguchi, K.; Okabe, N. Real-time estimation and prediction for pandemic A/H1N1(2009) in Japan. J. Infect. Chemother. 2011, 17, 468–472. [Google Scholar] [CrossRef]
- Zhu, G.; Fu, X.; Chen, G. Global attractivity of a network-based epidemic SIS model with nonlinear infectivity. Commun. Nonlinear Sci. Numer. Simul. 2012, 17, 2588–2594. [Google Scholar] [CrossRef]
- Ni, P.H.; Ye, X.W. Nonlinear finite element model updating with a decentralized approach. Smart Struct. Syst. 2019, 24, 683–692. [Google Scholar] [CrossRef]
- Ni, P.; Han, Q.; Du, X.; Cheng, X. Bayesian Model Updating of Civil Structures with Likelihood-Free Inference Approach and Response Reconstruction Technique. Mech. Syst. Signal Processing 2022, 164, 108204. [Google Scholar] [CrossRef]
- Porto, T.C. A mathematical model of the ecology of Lyme disease. IMA J. Math. Appl. Med. Biol. 1999, 16, 261–296. [Google Scholar]
- Kurtenback, K.; Hanincova, K.; Tsao, J.I.; Margos, G.; Fish, D.; Ogden, N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 2006, 4, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, X.Q. Spatial invasion threshold of Lyme disease. SIAM J. Appl. Math. 2015, 75, 1142–1170. [Google Scholar] [CrossRef]
- Bisanzio, D.; Bertolotti, L.; Tomassone, L.; Amore, G.; Ragagli, C.; Mannelli, A.; Giacobini, M.; Provero, P. Modeling the Spread of Vector-Borne Diseases on Bipartite Networks. PLoS ONE 2010, 5, e13796. [Google Scholar] [CrossRef] [PubMed]
- Gaff, H.; Eisen, R.J.; Eisen, L.; Nadolny, R.; Bjork, J.; Monaghan, A.J. LYMESIM 2.0: An Updated Simulation of Blacklegged Tick (Acari: Ixodidae) Population Dynamics and Enzootic Transmission of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae). J. Med. Entomol. 2020, 57, 715–727. [Google Scholar] [CrossRef]
- Hart, T.M.; Dupuis, A.P., II; Tufts, D.M.; Blom, A.M.; Starkey, S.R.; Rego, R.O.M.; Ram, S.; Kraiczy, P.; Kramer, L.D.; Diuk-Wasser, M.A.; et al. Host tropism determination by convergent evolution of immunological evasion in the Lyme disease system. PLoS Pathog. 2021, 17, e1009801. [Google Scholar] [CrossRef]
- Lou, Y.; Wu, J.; Wu, X. Impact of biodiversity and seasonality on Lyme-pathogen transmission. Theor. Biol. Med. Model. 2014, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Belli, A.; Sarr, A.; Rais, O.; Rego, R.O.M.; Voordouw, M.J. Ticks infected via co-feeding transmission can transmit Lyme borreliosis to vertebrate hosts. Sci. Rep. 2017, 7, 5006. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Harrison, P.A.; Rounsevell, M.D.A. Modelling the seasonality of Lyme disease risk and the potential impacts of a warming climate within the heterogeneous landscapes of Scotland. J. R. Soc. Interface 2016, 13, 20160140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, X.Q. A reaction-diffusion Lyme disease model with seasonality. SIAM J. Appl. Math. 2013, 73, 2077–2099. [Google Scholar] [CrossRef]
- Imai, C.; Armstrong, B.; Chalabi, Z.; Mangtani, P.; Hashizume, M. Time series regression model for infectious disease and weather. Environ. Res. 2015, 142, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumic, I.; Severnini, E. “Ticking Bomb”: The impact of climate change on the incidence of Lyme disease. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5719081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, N.H.; Pang, G.; Ginsberg, H.S.; Hickling, G.J.; Burke, R.L.; Beati, L.; Tsao, J.I. Evidence for geographic variation in life-cycle processes affecting phenology of the Lyme disease vector Ixodes scapularis (Acari: Ixodidae) in the United States. J. Med. Entomol. 2018, 55, 1386–1401. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.P.; Wang, Y.X.; Fan, Z.W.; Ji, Y.; Liu, M.-J.; Zhang, W.-H.; Li, X.-L.; Zhou, S.-X.; Li, H.; Liang, S.; et al. Mapping ticks and tick-borne pathogens in China. Nat. Commun. 2021, 12, 1075. [Google Scholar] [CrossRef]
- Nguyen, A.; Mahaffy, J.; Vaidya, N.K. Modeling transmission dynamics of Lyme disease: Multiple vectors, seasonality, and vector mobility. Infect. Dis. Model. 2019, 4, 28–43. [Google Scholar] [CrossRef]
- Bobe, J.R.; Jutras, B.L.; Horn, E.J. Recent Progress in Lyme Disease and Remaining Challenges. Front. Med. 2021, 8, 666554. [Google Scholar] [CrossRef]
- Waits, A.; Emelyanova, A.; Oksanen, A.; Abass, K.; Rautio, A. Human infectious diseases and the changing climate in the Arctic. Environ. Int. 2018, 121, 703–713. [Google Scholar] [CrossRef]
- Why is CDC concerned about Lyme disease? Atlanta: Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/lyme/why-is-cdc-concerned-about-lyme-disease.html (accessed on 16 February 2022).
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Rebman, A.W.; Aucott, J.N. Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease. Front. Med. 2020, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Predkele, N.; Mednieks, J. Lyme disease and associated NMDAR Encephalitis: A case report and literature review. Neurol. Int. 2021, 13, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Evans, G.A.; Patel, S.N.; Pullenayegum, E.M.; Sander, B. Estimating the population health burden of Lyme disease in Ontario, Canada: A microsimulation modelling approach. CMAJ Open 2021, 9, e1005–e1012. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Silva, S.R.D.; Sander, B. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: A scoping review. PLoS ONE 2019, 14, e0210280. [Google Scholar] [CrossRef] [PubMed]
- Rogalska, A.M.; Pawelczyk, O.; Solarz, K.; Holecki, T. What are the cost of diagnostics and treatment of Lyme Borreliosis in Poland? Front. Public Health 2021, 8, 599239. [Google Scholar] [CrossRef]
- Porta, M. A Dictionary of Epidemiology; Oxford University Press: New York, NY, USA, 2014; p. 376. [Google Scholar]
- Yakovlev, S.; Bazilevych, K.; Chumachenko, D.; Chumachenko, T.; Hulianytskyi, L.; Meniailov, I.; Tkachenko, A. The concept of developing a decision support system for the epidemic morbidity control. CEUR Workshop Proc. 2020, 2753, 265–274. [Google Scholar]
- Pages, F.; Faulde, M.; Orlandi-Pradines, E.; Parola, P. The past and present threat of vector-borne diseases in deployed troops. Clin. Microbiol. Infect. 2010, 16, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Tarnas, M.C.; Desai, A.N.; Lassmann, B.; Abbara, A. Increase in vector-borne disease reporting affecting humans and animals in Syria and neighboring countries after the onset of conflict: A ProMED analysis 2003–2018. Int. J. Infect. Dis. 2020, 102, 103–109. [Google Scholar] [CrossRef]
| Approach | Average Absolute Prediction Error |
|---|---|
| Differential equations | 21.4% |
| Binomial chains | 18.3% |
| Cellular automata | 17.2% |
| Moving average method | 14.6% |
| Exponential smoothing | 11.2% |
| Brown polynomial model | 10.8% |
| Adaptive Holt model | 10.2% |
| Multiagent approach | 5.0% |
| Neural network approach | 3.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumachenko, D.; Piletskiy, P.; Sukhorukova, M.; Chumachenko, T. Predictive Model of Lyme Disease Epidemic Process Using Machine Learning Approach. Appl. Sci. 2022, 12, 4282. https://doi.org/10.3390/app12094282
Chumachenko D, Piletskiy P, Sukhorukova M, Chumachenko T. Predictive Model of Lyme Disease Epidemic Process Using Machine Learning Approach. Applied Sciences. 2022; 12(9):4282. https://doi.org/10.3390/app12094282
Chicago/Turabian StyleChumachenko, Dmytro, Pavlo Piletskiy, Marya Sukhorukova, and Tetyana Chumachenko. 2022. "Predictive Model of Lyme Disease Epidemic Process Using Machine Learning Approach" Applied Sciences 12, no. 9: 4282. https://doi.org/10.3390/app12094282
APA StyleChumachenko, D., Piletskiy, P., Sukhorukova, M., & Chumachenko, T. (2022). Predictive Model of Lyme Disease Epidemic Process Using Machine Learning Approach. Applied Sciences, 12(9), 4282. https://doi.org/10.3390/app12094282







