On the Absorption and Photoluminescence Properties of Pure ZnSe and Co-Doped ZnSe:Eu3+/Yb3+ Crystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Absorption Measurement Energy Band Gap Determination
3.2. Photoluminescence Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgobiani, A.N. The construction of light emitting diodes on the base of wide gap II–VI semiconductor compounds. J. Lumin. 1991, 48, 839–844. [Google Scholar] [CrossRef]
- Tetsuichiro, O.; Kawaguchi, Y.; Ohki, A.; Matsuoka, T. ZnCdSe/ZnSe Quantum-Well Laser Diode on a (711)A GaAs Substrate. Jpn. J. Appl. Phys. 1994, 33, 5766. [Google Scholar]
- Haase, M.A.; Qiu, J.; DePuydt, J.M.; Cheng, H. Blue-green laser diodes. Appl. Phys. Lett. 1991, 59, 1272–1274. [Google Scholar] [CrossRef]
- Xie, W.; Grillo, D.C.; Gunshor, R.L.; Kobayashi, M.; Jaon, H.; Ding, J.; Nurmikko, A.V.; Hua, G.C.; Otauka, N. Room temperature blue light emitting p-n diodes from Zn(S,Se)-based multiple quantum well structures. Appl. Phys. Lett. 1992, 60, 1999–2001. [Google Scholar] [CrossRef]
- Aggarwal, R.L.; Maki, P.A.; Molnar, R.J.; Liau, Z.L.; Melngailis, I. Optically pumped GaN/Al0.1Ga0.9N double-heterostructure ultraviolet laser. J. Appl. Phys. 1995, 79, 2148–2150. [Google Scholar] [CrossRef]
- Nakamura, S.; Senoh, M.; Mukai, T. High-power lnGaN/GaN double-heterostructure violet light emitting diodes. Appl. Phys. Lett. 1993, 62, 2390–2392. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.Y.; Wei, Z.Y.; Zhang, C.; Long, Y.B.; Zhang, Z.G.; Zhang, H.J.; Wang, J.Y. Compact Diode-Pumped Continuous-Wave Nd:LuVO4 Lasers Operated at 916 nm and 458 nm. Chin. Phys. Lett. 2006, 23, 1192. [Google Scholar]
- Ponce, F.A.; Bour, D.P. Nitride-based semiconductors for blue and green light-emitting devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Schmidt, T.; Muller, G.; Spanhel, L. Activation of 1.54 µm Er3+ Fluorescence in Concentrated II-VI Semiconductor Cluster Environments. Chem. Mater. 1998, 10, 65–71. [Google Scholar] [CrossRef]
- Ivashchenko, M.M.; Opanasyuk, A.S.; Buryk, I.P.; Lutsenko, V.A.; Shevchenko, A.V. Optical Properties of Pure and Eu Doped ZnSe Films Deposited by CSVS Technique. J. Nano-Electron. Phys. 2017, 9, 01011-1–01011-5. [Google Scholar] [CrossRef]
- Radevici, I.; Sushkevich, K.; Sirkeli, V.; Huhtinen, H.; Nedeogl, D.; Paturi, P. Luminescent properties of the ZnSe:Yb crystals in the visible spectral range. J. Lumin. 2013, 143, 275–279. [Google Scholar] [CrossRef]
- Brown, M.R.; Cox, A.F.J.; Shand, W.A.; Williams, J.M. Defect configuration for rare earth ions in zinc selenide. J. Phys. C Solid State Phys. 1971, 4, 2550. [Google Scholar] [CrossRef]
- Triboulet, R.; Rabago, F.; Legros, R.; Lozykowski, H.; Didier, D. Low-temperature growth of ZnSe crystals. J. Cryst. Growth. 1982, 59, 172–177. [Google Scholar] [CrossRef]
- Nasiri, S.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Janusas, G. The effect of calcination temperature on the photophysical and mechanical properties of copper iodide (5 mol%)–doped hydroxyapatite. Opt. Mater. 2021, 121, 111559. [Google Scholar] [CrossRef]
- Nasiri, S.; Dashti, A.; Hosseinnezhad, M.; Rabiei, M.; Palevicius, A.; Doustmohammadi, A.; Janusas, G. Mechanochromic and thermally activated delayed fluorescence dyes obtained from D–A–D′ type, consisted of xanthen and carbazole derivatives as an emitter layer in organic light emitting diodes. Chem. Eng. 2022, 430, 131877. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Allegretti, F.; Carrara, A.; Pizzini, S. Growth and characterization of ZnSe for low temperature calorimetry applications. J. Crystal Growth 1993, 128, 646–649. [Google Scholar] [CrossRef]
- Hile, D.D.; Swart, H.C.; Motloung, S.V.; Motaung, T.E.; Kroon, R.E.; Egbo, K.O.; Pawade, V.B.; Koao, L.F. Synthesis and characterization of europium doped zinc selenide thin films prepared by photo-assisted chemical bath technique for luminescence application. Mater. Chem. Phys. 2021, 262, 124303. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R.S. A systematic analysis of the spectra of the lanthanides doped into single crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Judd, B.R. Three-particle operators for equivalent electrons. Phys. Chem. Rev. 1966, 141, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Hufner, S. Optical Spectra of Transparent Rare Earths Compounds; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ions in Crystals; Crosswhite, H.M., Ed.; Wiley: New York, NY, USA, 1968. [Google Scholar]
- Nato, Y.; Shigeo, S.; Takashi, K. Phonon-assisted energy transfer between trivalent Rare Earth ions. J. Phys. Soc. Jap. 1972, 32, 1577. [Google Scholar]
- Loiko, P.A.; Rachkovskaya, G.E.; Zakharevich, G.B.; Kornienko, A.A.; Dunina, E.B.; Yasukevich, A.S.; Yumashev, K.V. Cooperative up−conversion in Eu3+,Yb3+ doped SiO2–PbO–PbF2–CdF2 oxyfluoride glass. J. Non-Cryst. Solids 2014, 392, 39–44. [Google Scholar] [CrossRef]
- Ekambarama, S.; Maaza, M. Combustion synthesis and luminescent properties of Eu3+-activated cheap red phosphors. J. Alloys Compd. 2005, 395, 132–134. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Hkiri, K.; Khenfouch, M.; Dhlamini, S.; Henini, M.; Maaza, M. Optical properties of biosynthesized nanoscaled Eu2O3 for red luminescence applications. JOSA B 2020, 37, C73–C79. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.; Patil, K.C.; Maaza, M. Synthesis of lamp phosphors: Facile combustion approach. J. Alloys Compd. 2005, 393, 81–92. [Google Scholar] [CrossRef] [Green Version]
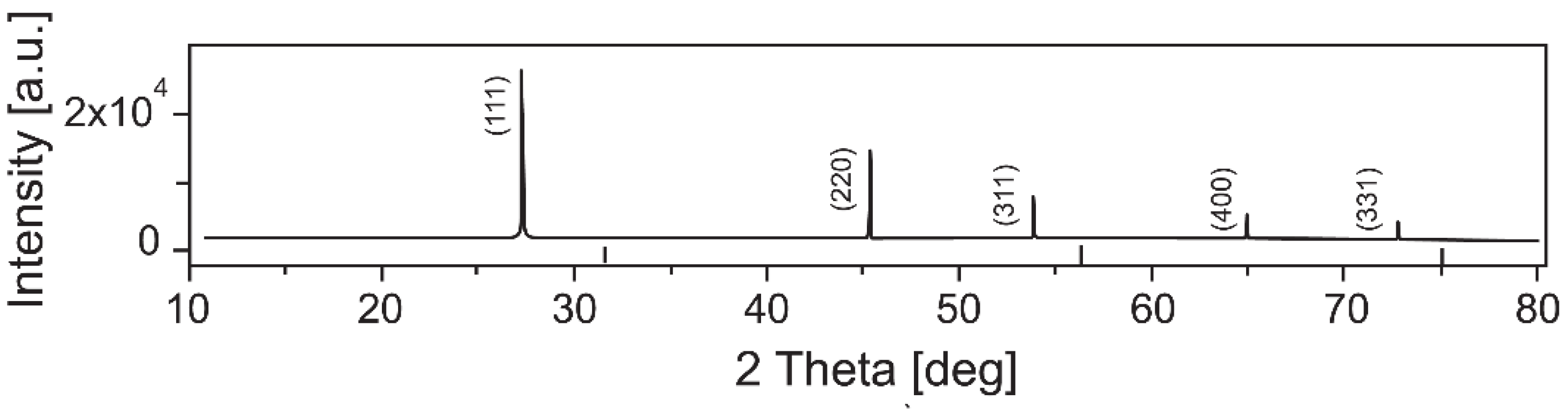
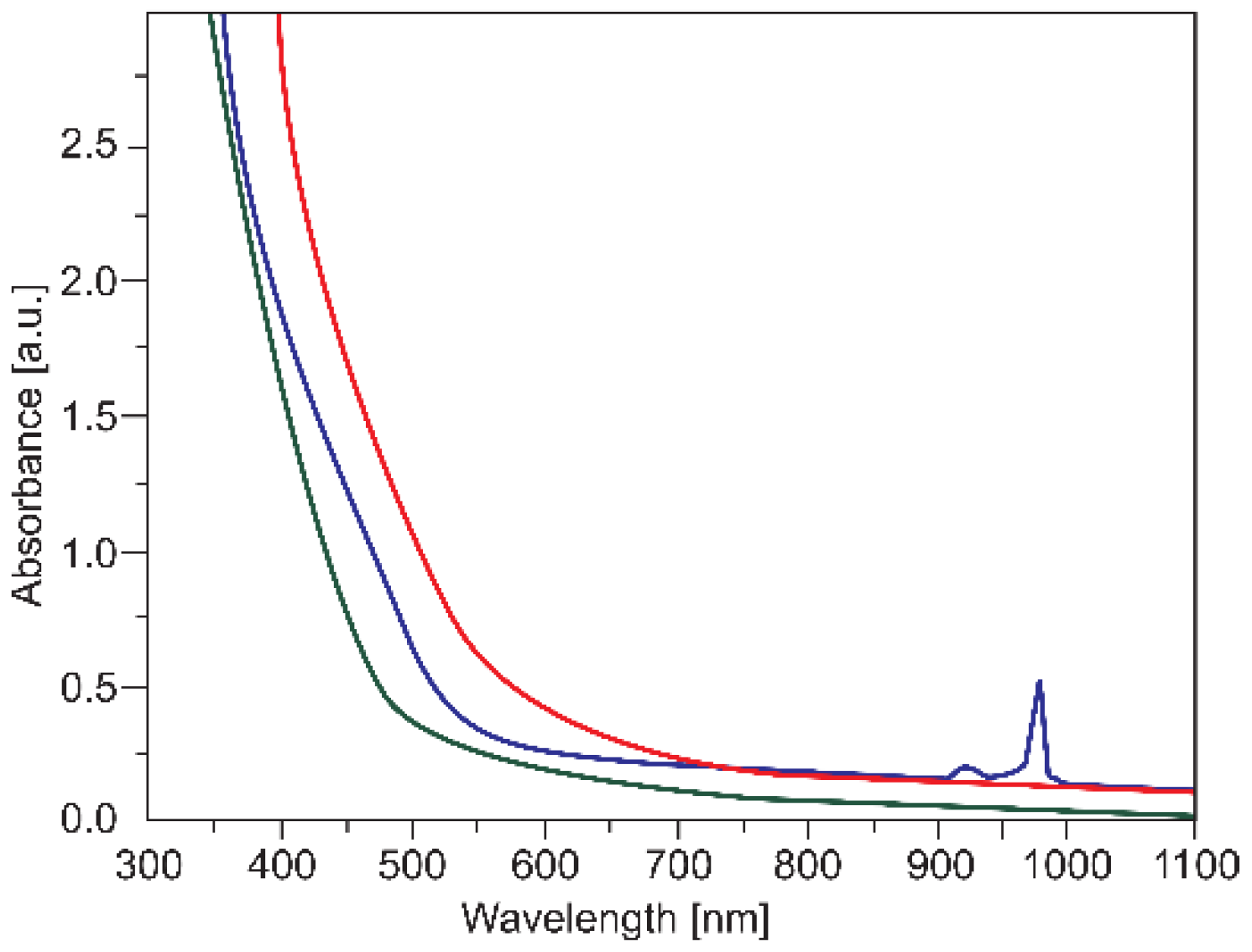
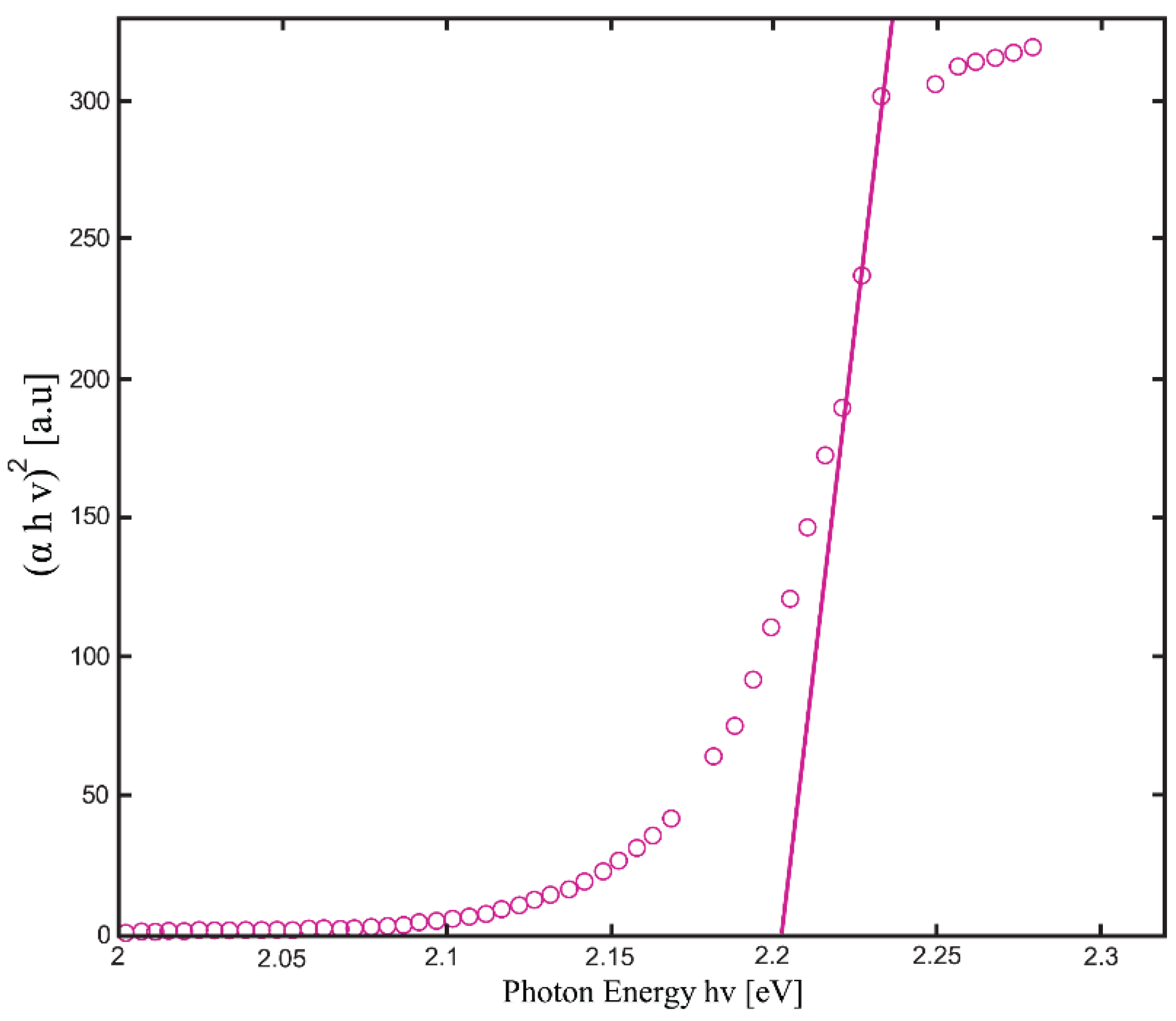
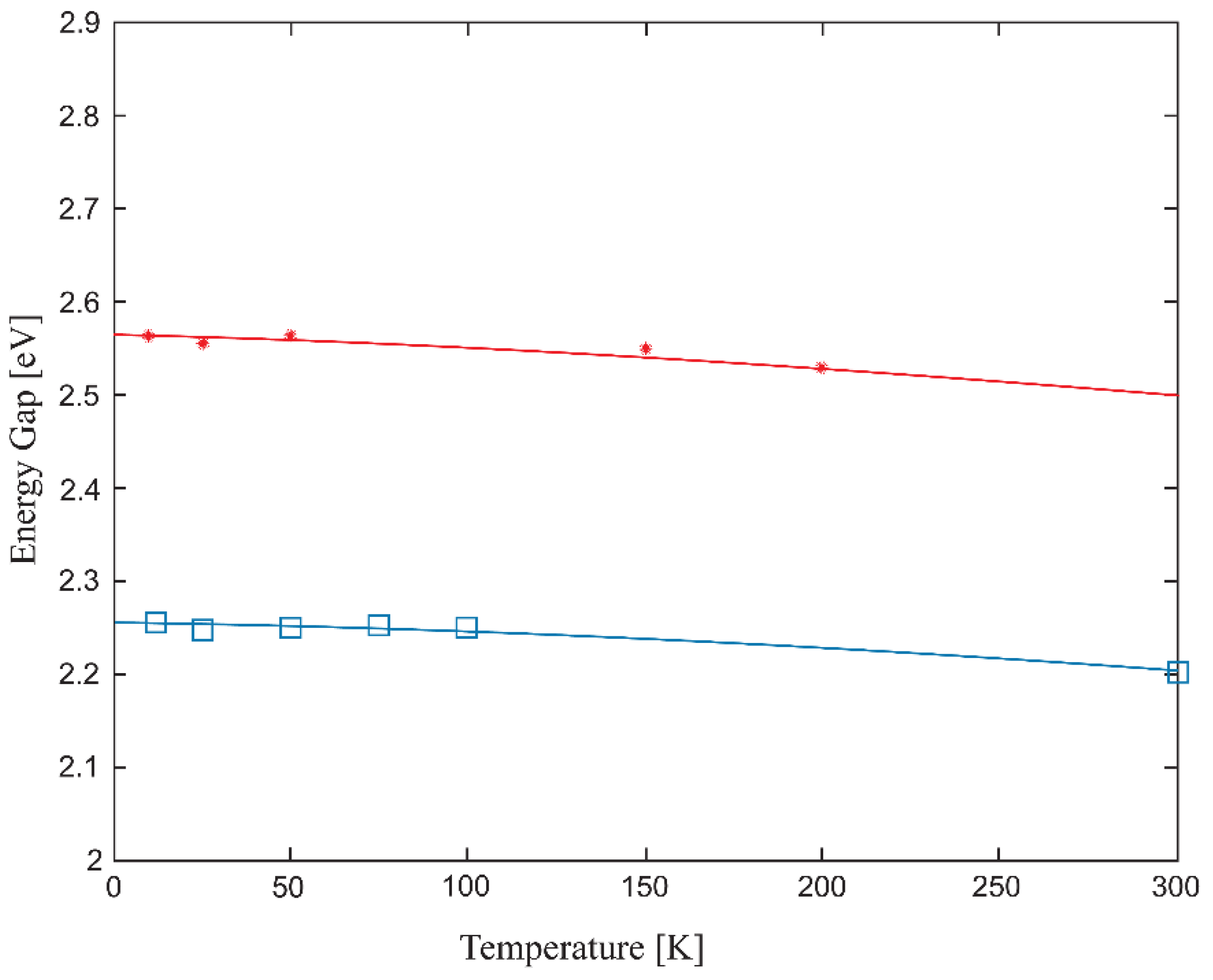

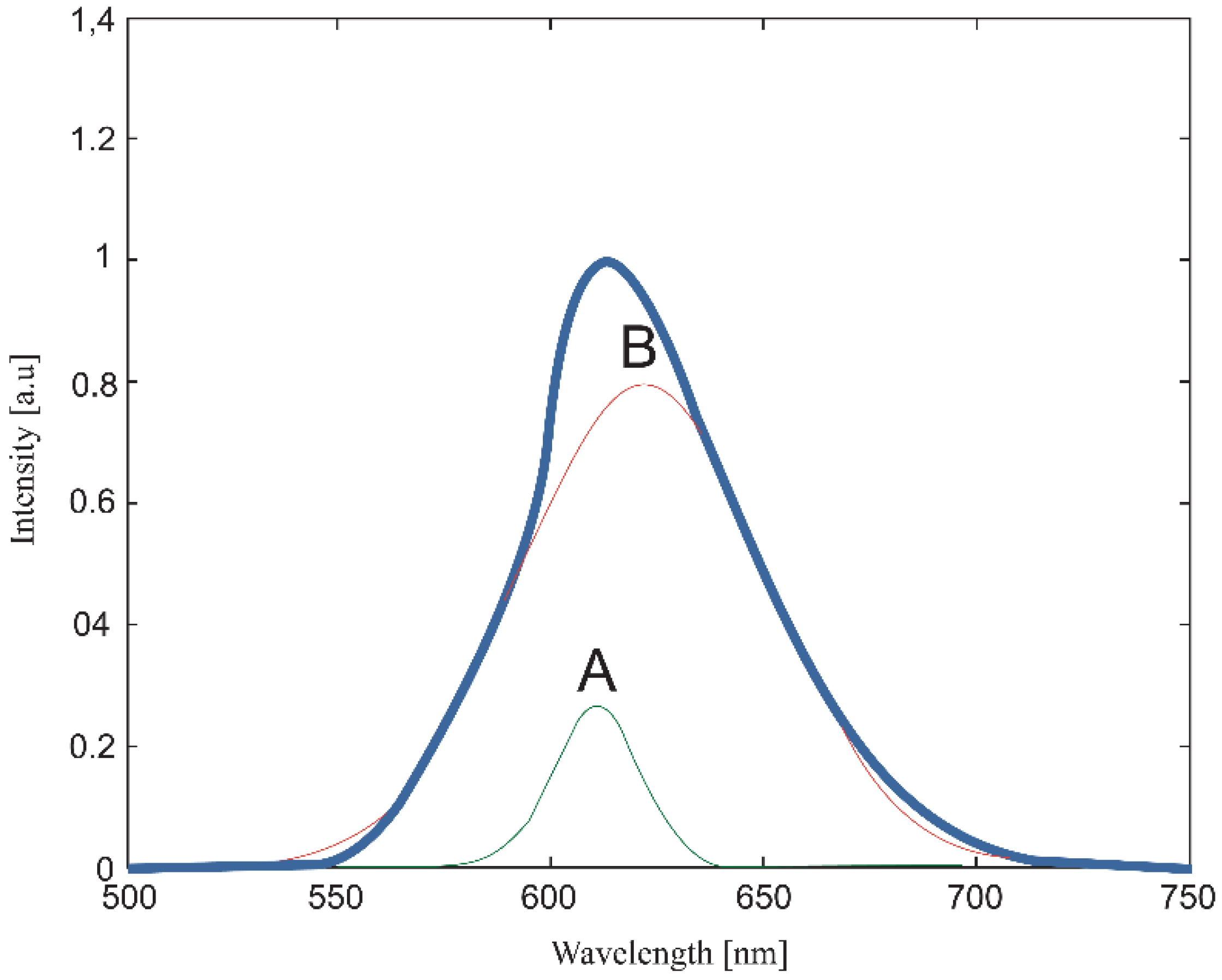
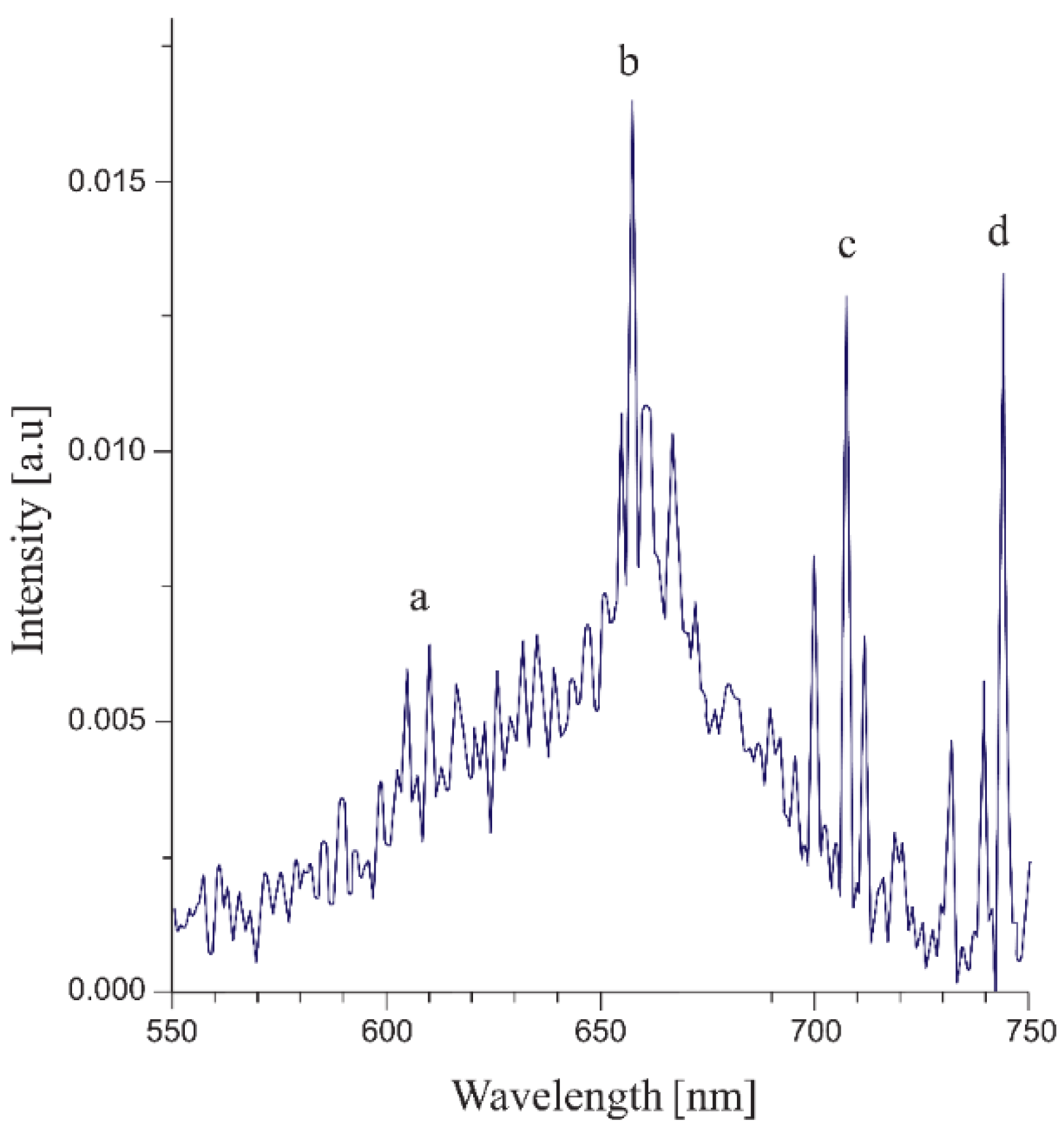

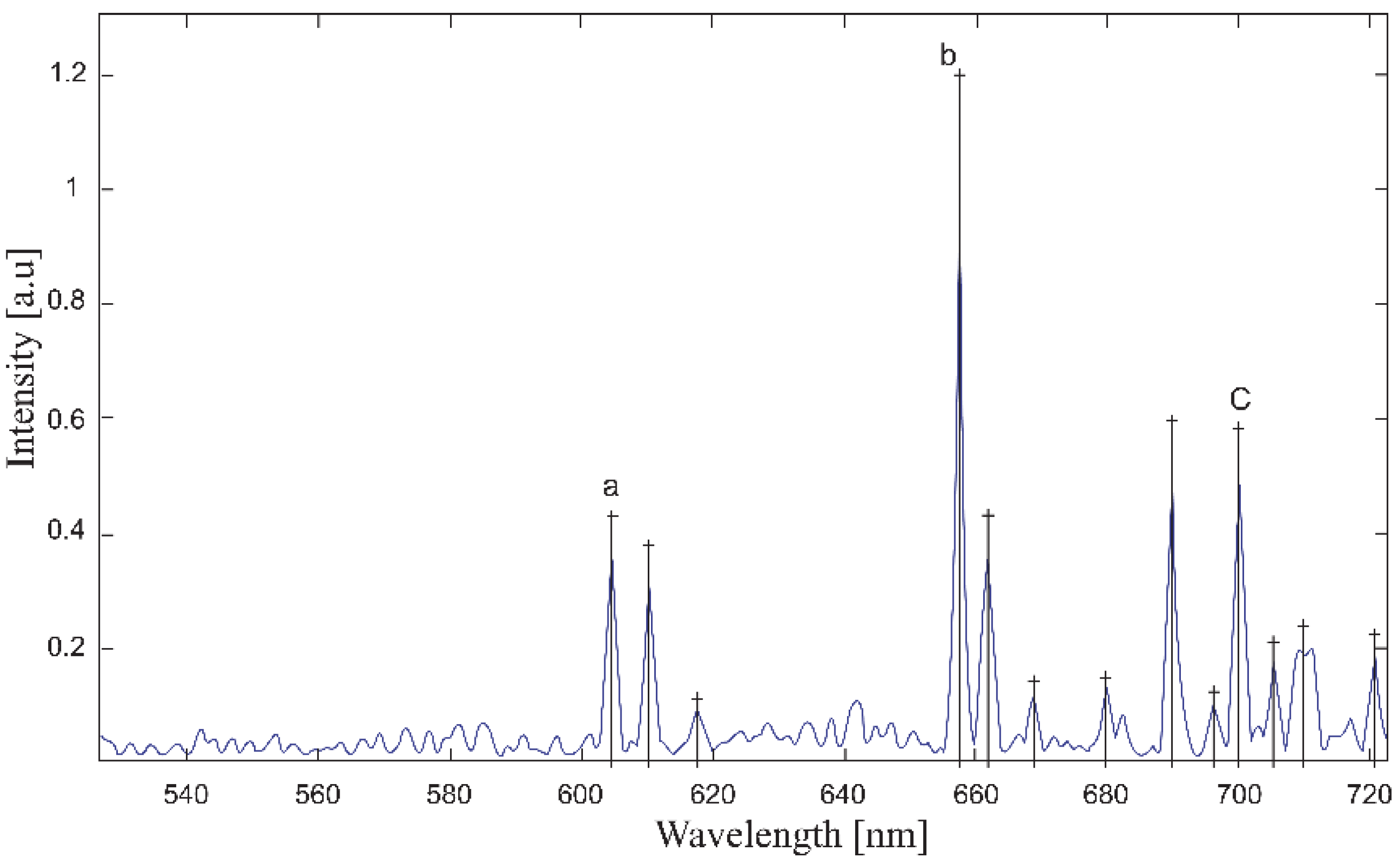

| Temperature, K | Zn:Se:Eu3+, eV | Zn:Se:Eu3+/Yb3+, eV |
|---|---|---|
| 15 | 2.225 | 2.562 |
| 25 | 2.216 | 2.554 |
| 50 | 2.228 | 2.562 |
| 75 | 2.250 | 2.551 |
| 100 | 2.249 | 2.549 |
| 150 | 2.234 | 2.548 |
| 200 | 2.211 | 2.515 |
| 300 | 2.201 | 2.492 |
| Sample | E0(0), eV | γ (×10−6), eV/K | β, K |
|---|---|---|---|
| ZnSe:Eu3+ | 2.25 | 264 | 160.89 |
| Zn:Se:Eu3+/Yb3+ | 2.56 | 260 | 82.75 |
| Temperature | |||||||
|---|---|---|---|---|---|---|---|
| 18 K | 25 K | 50 K | 75 K | 100 K | 150 K | 200 K | Band |
| λ (nm) | |||||||
| 610 | B | 609 | 609 | 609 | 613 | 608 | B |
| 637 | A | 638 | 636 | 627 | 626 | 625 | A |
| Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|
| 18 K | 25 K | 50 K | 75 K | 100 K | 150 K | 200 K | 250 K | Band |
| λ (nm) | ||||||||
| 613.5 | 612.7 | 612.5 | 612.4 | 612.3 | 612.3 | 612.3 | B | |
| 625.2 | 624.5 | 624.0 | 623.7 | 623.4 | 623.1 | 623.0 | 622.1 | A |
| Temperature | Transition | ||||||
|---|---|---|---|---|---|---|---|
| 20 K | 50 K | 75 K | 100 K | 150 K | 300 K | ||
| λ (nm) | ν (cm−1) | ||||||
| 743.9 | 13.443 | 5D0 → 7F5 * | |||||
| 739.6 | 13.521 | * | |||||
| 733.9 | 13.626 | * | |||||
| 720.6 | 720.7 | 720.9 | 720.9 | 720.9 | 13.872 | * | |
| 711.7 | 710.4 | 711.4 | 711.5 | 711.2 | 711.1 | 14.061 | |
| 707.3 | 708.8 | 709.3 | 709.2 | 708.9 | 708.9 | 14.106 | 5D1 → 7F6 |
| 705.1 | 705.7 | 705.7 | 705.7 | 705.8 | 14.170 | 5D1 → 7F6 | |
| 700.0 | 699.9 | 699.9 | 700.5 | 700.0 | 700.2 | 14.286 | |
| 695.7 | 696.4 | 696.2 | 695.9 | 696 | 14.370 | ** | |
| 689.7 | 689.6 | 690.0 | 689.9 | 689.8 | 690 | 14.496 | 5D0 → 7F4 ** |
| 672.3 | 678.1 | 680.2 | 680.0 | 680.2 | 14.706 | ** | |
| 666.9 | 668.5 | 668.5 | 668.7 | 668.8 | 14.957 | ||
| 660.4 | 661.9 | 661.8 | 661.8 | 661.7 | 661.5 | 15.113 | 5D0 → 7F3 |
| 657.6 | 657.5 | 657.6 | 657.6 | 657.7 | 657.8 | 15.205 | 5D1 → 7F5 |
| 610.3 | 610.3 | 610.4 | 610.0 | 610.2 | 610.2 | 16.388 | 5D0 → 7F2 |
| 604.8 | 604.7 | 604.3 | 604.8 | 604.7 | 604.7 | 16.537 | 5D0 → 7F1 |
| 595.9 | 599.2 | 16.781 | 5D2 → 7F6 | ||||
| 544.4 | 544.0 | 18.368 | |||||
| 534.5 | 534.6 | 18.705 | 5D1 → 7F1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasgholi-NA, B.; Aldaghri, O.A.; Ibnouf, K.H.; Madkhali, N.; Cabrera, H. On the Absorption and Photoluminescence Properties of Pure ZnSe and Co-Doped ZnSe:Eu3+/Yb3+ Crystals. Appl. Sci. 2022, 12, 4248. https://doi.org/10.3390/app12094248
Abbasgholi-NA B, Aldaghri OA, Ibnouf KH, Madkhali N, Cabrera H. On the Absorption and Photoluminescence Properties of Pure ZnSe and Co-Doped ZnSe:Eu3+/Yb3+ Crystals. Applied Sciences. 2022; 12(9):4248. https://doi.org/10.3390/app12094248
Chicago/Turabian StyleAbbasgholi-NA, Behnaz, Osamah A. Aldaghri, Khalid Hassan Ibnouf, Nawal Madkhali, and Humberto Cabrera. 2022. "On the Absorption and Photoluminescence Properties of Pure ZnSe and Co-Doped ZnSe:Eu3+/Yb3+ Crystals" Applied Sciences 12, no. 9: 4248. https://doi.org/10.3390/app12094248
APA StyleAbbasgholi-NA, B., Aldaghri, O. A., Ibnouf, K. H., Madkhali, N., & Cabrera, H. (2022). On the Absorption and Photoluminescence Properties of Pure ZnSe and Co-Doped ZnSe:Eu3+/Yb3+ Crystals. Applied Sciences, 12(9), 4248. https://doi.org/10.3390/app12094248









