Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Design of Experiment
2.3. Samples Preparation
2.4. Biometric Measurements
2.5. Physiological—Chlorophyll Content
2.6. Organoleptic Properties—Firmness, Total Soluble Solids and Acidity
2.7. Antioxidant Status—Lycopene, β-Carotene, Phenolic Compounds and Antioxidant Activity
2.8. The Proximate Composition—Ash, Crude Lipid, Crude Protein, Crude Fibre, Dietary Fibre, Calorific Value
2.9. The Antinutritional Composition—Phytate, Tannins, Oxalates, Saponins, Trypsin Inhibitors, α-Amylase Inhibitors
2.10. The Mineral Content
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoleru, V.; Munteanu, N.; Sellitto, V.M. New Approach of Organic Vegetable Systems; Aracnee: Rome, Italy, 2014; pp. 36–39. [Google Scholar]
- FAOSTAT. Production/Yield Quantities of Tomatoes in World. 2018. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 15 May 2020).
- FAOSTAT. Food Supply Quantity (kg/capita/year)—Tomatoes and Products. 2018. Available online: http://www.fao.org/faostat/en/#data/FBS/visualize (accessed on 26 January 2021).
- INS. Vegetable consumption in Romania. 2018. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/disponibilitatile_de_consum_ale_populatiei_anul_2018.pdf (accessed on 21 December 2020).
- Wu, J.; Lin, L. Elicitor-like effects of low-energy ultrasound on plant (Panax ginseng) cells: Induction of plant defense responses and secondary metabolite production. Appl. Microbiol. Biotechnol. 2002, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D. Effects of electricity on plant responses. Sci. Hortic. 2018, 234, 382–392. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Gimeno-Añó, V.; Martín-Belloso, O. Lycopene, Vitamin C, and Antioxidant Capacity of Tomato Juice as Affected by High-Intensity Pulsed Electric Fields Critical Parameters. J. Agric. Food Chem. 2007, 55, 9036–9042. [Google Scholar] [CrossRef]
- Volkov, A.G.; Shtessel, Y.B. Electrical signal propagation within and between tomato plants. Bioelectrochemistry 2018, 124, 195–205. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Garcí, D.; Sueiro, L.; Gilart, F.; Porras, E.; Licea, L. Pre-sowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 2006, 27, 247–257. [Google Scholar] [CrossRef]
- Ahmad, P.; Wani, M.R. Growth Patterns of Tomato Plants Subjected to Two Non-Conventional Abiotic Stresses: UV-C Irradiations and Electric Fields. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2014; Volume 2, pp. 285–297. [Google Scholar]
- Nechitailo, G.; Gordeev, A. The use of an electric field in increasing the resistance of plants to the action of unfavorable space flight factors. Adv. Space Res. 2004, 34, 1562–1565. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Mykhailyk, V.; Samborska, K.; Gondek, E.; Witrowa-Rajchert, D.; Toepfl, S.; Parniakov, O. Pulsed electric field pre-treatment improves microstructure and crunchiness of freeze-dried plant materials: Case of strawberry. LWT 2020, 134, 1–8. [Google Scholar] [CrossRef]
- Mihalache, G.; Peres, C.I.; Bodale, I.; Achitei, V.; Gheorghitoaie, M.V.; Teliban, G.C.; Cojocaru, A.; Butnariu, M.; Muraru, V.; Stoleru, V. Tomato Crop Performances under Chemical Nutrients Monitored by Electric Signal. Agronomy 2020, 10, 1915. [Google Scholar] [CrossRef]
- Gheorghitoaie, M.-V.; Achiței, V.; Teliban, G.-C.; Stoleru, V. Optimization of tomato cultivation technology under greenhouse through the use of continuous electric current. Sci. Papers Ser. B Hortic. 2020, 64, 379–383. [Google Scholar]
- Black, J.D.; Forsyth, F.R.; Fensom, D.S.; Ross, R.B. Electrical stimulation and its effects on growth and ion accumulation in tomato plants. Can. J. Bot. 1971, 49, 1809–1815. [Google Scholar] [CrossRef]
- Moon, J.-D.; Chung, H.-S. Acceleration of germination of tomato seed by applying AC electric and magnetic fields. J. Electrost. 2000, 48, 103–114. [Google Scholar] [CrossRef]
- De Souza, A.; Sueiro, L.; García, D.; Porras, E. Extremely low frequency non-uniform magnetic fields improve tomato seed germination and early seedling growth. Seed Sci. Technol. 2010, 38, 61–72. [Google Scholar] [CrossRef]
- Aroca-Delgado, R.; Pérez-Alonso, J.; Callejón-Ferre, Á.J.; Díaz-Pérez, M. Morphology, yield and quality of greenhouse tomato cultivation with flexible photovoltaic rooftop panels (Almería-Spain). Sci. Hortic. 2019, 257, 1–12. [Google Scholar] [CrossRef]
- Angersbach, A.; Heinz, V.; Knorr, D. Effects of pulsed electric fields on cell membranes in real food systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 135–149. [Google Scholar] [CrossRef]
- Joey, A.; Jane, Z.; Bo, L. Automated Pruning of Greenhouse Indeterminate Tomato Plants. In Proceedings of the 2nd International Conference on Vision, Image and Signal Processing (ICVISP), Las Vegas, NV, USA, 27–29 August 2018; pp. 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Zhao, T. High Throughput Sequencing of circRNAs in Tomato Leaves Responding to Multiple Stresses of Drought and Heat. Hortic. Plant J. 2020, 6, 34–38. [Google Scholar] [CrossRef]
- Mbonihankuye, C.; Kusolwa, P.; Msogoya, T.J. Assessment of the effect of pruning systems on plant developmental cycle—Yield and quality of selected indeterminate tomato lines. Acta Hortic. 2013, 1007, 535–542. [Google Scholar] [CrossRef]
- Munteanu, N. Tomatoes, Peppers and Eggplants; Ion Ionescu de la Brad: Iaşi, Romania, 2003; pp. 123–131. [Google Scholar]
- Stoleru, V.; Inculet, S.-C.; Mihalache, G.; Cojocaru, A.; Teliban, G.-C.; Caruso, G. Yield and Nutritional Response of Greenhouse Grown Tomato Cultivars to Sustainable Fertilization and Irrigation Management. Plants 2020, 9, 1053. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The Effects of a Microorganisms-Based Commercial Product on the Morphological, Biochemical and Yield of Tomato Plants under Two Different Water Regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef] [Green Version]
- Alenazi, M.M.; Shafiq, M.; Alsadon, A.A.; Alhelal, I.M.; Alhamdan, A.M.; Solieman, T.H.I.; Ibrahim, A.A.; Shady, M.R.; Saad, M.A.O. Non-destructive assessment of flesh firmness and dietary antioxidants of greenhouse-grown tomato (Solanum lycopersicum L.) at different fruit maturity stages. Saudi J. Biol. Sci. 2020, 27, 2839–2846. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Baldwin, E.A.; Plotto, A.; Rosskopf, E.; Hong, J.C.; Bai, J. Electronic tongue discrimination of four tomato cultivars harvested at six maturities and exposed to blanching and refrigeration treatments. Postharvest Biol. Technol. 2018, 136, 42–49. [Google Scholar] [CrossRef]
- Davis, A.R.; Fish, W.W.; Perkins-Veazie, P. A rapid spectrophotometric method for analyzing lycopene content in tomato and tomato products. Postharvest Biol. Technol. 2003, 28, 425–430. [Google Scholar] [CrossRef]
- Cadoni, E.; De Giorgi, M.R.; Medda, E.; Poma, G. Supercritical CO2 extraction of lycopene and b-carotene from ripe tomatoes. Dye. Pigment. 2000, 44, 27–32. [Google Scholar] [CrossRef]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crisan, O.; Gheldiu, A.M.; Crisan, G. Phytochemical characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and their antioxidant and antimicrobial properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ouis, N.; Hariri, A. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. Banat. J. Biotechnol. 2017, 8, 93–104. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC International. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Lolas, G.M.; Markakis, P. Phytic acid and other phosphorus compounds of beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 1975, 23, 13–15. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin Determination and Biological Activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Liebman, M. Oxalate content of legumes, nuts, and grain-based flours. J. Food Compos. Anal. 2005, 18, 723–729. [Google Scholar] [CrossRef]
- Umogbai, K.; Tiamiyu, L.O.; Anunne, P.A. Proximate and phytochemical changes in hydrothermally processed orange (Citrus sinensis) pulp meal. Banat. J. Biotechnol. 2016, 7, 68–72. [Google Scholar] [CrossRef]
- Attalla, K.; De, S.; Monga, M. Oxalate Content of Food: A Tangled Web. Urology 2014, 84, 555–560. [Google Scholar] [CrossRef]
- Chai, W.; Liebman, M. Effect of Different Cooking Methods on Vegetable Oxalate Content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 15th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Korsinczky, M.L.J.; Schirra, H.J.; Craik, D.J. Sunflower Trypsin Inhibitor-1. Curr. Protein Pept. Sci. 2004, 5, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Lukanc, T.; Brzin, J.; Kos, J.; Sabotič, J. Trypsin-specific Inhibitors from the Macrolepiota procera, Armillaria mellea and Amanita phalloides wild mushrooms. Acta Biochim. Pol. 2017, 64, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr. J. 2011, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and Nutritional Quality of Vesuvian Piennolo Tomato PDO as Affected by Farming System and Biostimulant Application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Al-Asheh, S.; Jumah, R.; Banat, F.; Al-Zou’bi, K. Direct Current Electroosmosis Dewatering of Tomato Paste Suspension. Food Bioprod. Process. 2004, 82, 193–200. [Google Scholar] [CrossRef]
- Miri, H.R. Grain yield and morpho-physiological changes from 60 years of genetic improvement of wheat in iran. Exp. Agric. 2009, 45, 149–163. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Ren, X.L.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A Reevaluation of the Key Factors That Influence Tomato Fruit Softening and Integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ma, X.; Zou, M.; Jiang, P.; Hu, W.; Li, J.; Zhi, Z.; Chen, J.; Li, S.; Ding, T.; et al. Effects of Ultrasound on Spoilage Microorganisms, Quality, and Antioxidant Capacity of Postharvest Cherry Tomatoes. J. Food Sci. 2015, 80, C2117–C2126. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martinez, P.; Soliva-Fortuny, R. Enhancing the carotenoid content of tomato fruit with pulsed electric field treatments: Effects on respiratory activity and quality attributes. Postharvest Biol. Technol. 2018, 137, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Asavasanti, S.; Ersus, S.; Ristenpart, W.; Stroeve, P.; Barrett, D.M. Critical Electric Field Strengths of Onion Tissues Treated by Pulsed Electric Fields. J. Food Sci. 2010, 75, E433–E443. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Foof. Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Sammi, S.; Masud, T. Effect of different packaging systems on the quality of tomato (Lycopersicon esculentum var. Rio Grande) fruits during storage. Int. J. Food Sci. Technol. 2009, 44, 918–926. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventos, R.M.; Martín-Belloso, O.; Elez-Martinez, P. Effects of Pulsed Electric Fields on the Bioactive Compound Content and Antioxidant Capacity of Tomato Fruit. J. Agric. Food Chem. 2012, 60, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D.; Huyskens-Keil, S.; Eichholz, I.; Ulrichs, C.; Schmidt, U. Effects of intermittent-direct-electric-current (IDC) on polyphenols and antioxidant activity in radish (Raphanus sativus L.) during growth. J. Appl. Bot. Food Qual. 2009, 83, 54–59. [Google Scholar]
- Yu, J.; Engeseth, N.J.; Feng, H. High Intensity Ultrasound as an Abiotic Elicitor—Effects on Antioxidant Capacity and Overall Quality of Romaine Lettuce. Food Bioprocess Technol. 2016, 9, 262–273. [Google Scholar] [CrossRef]
- Dannehl, D.; Huyskens-Keil, S.; Wendorf, D.; Ulrichs, C.; Schmidt, U. Influence of intermittent-direct-electric-current (IDC) on phytochemical compounds in garden cress during growth. Food Chem. 2012, 131, 239–246. [Google Scholar] [CrossRef]
- John, F.M.; Patrick, O.A.; Moses, S.A. Effect of maturity stage on quality and shelf life of tomato (Lycopersicon esculentum Mill) using refrigerator storage system. EJAR 2020, 4, 23–44. [Google Scholar]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Lee, S.; Oh, M.-M. Electric stimulation promotes growth, mineral uptake, and antioxidant accumulation in kale (Brassica oleracea var. acephala). Bioelectrochemistry 2021, 138, 107727. [Google Scholar] [CrossRef] [PubMed]
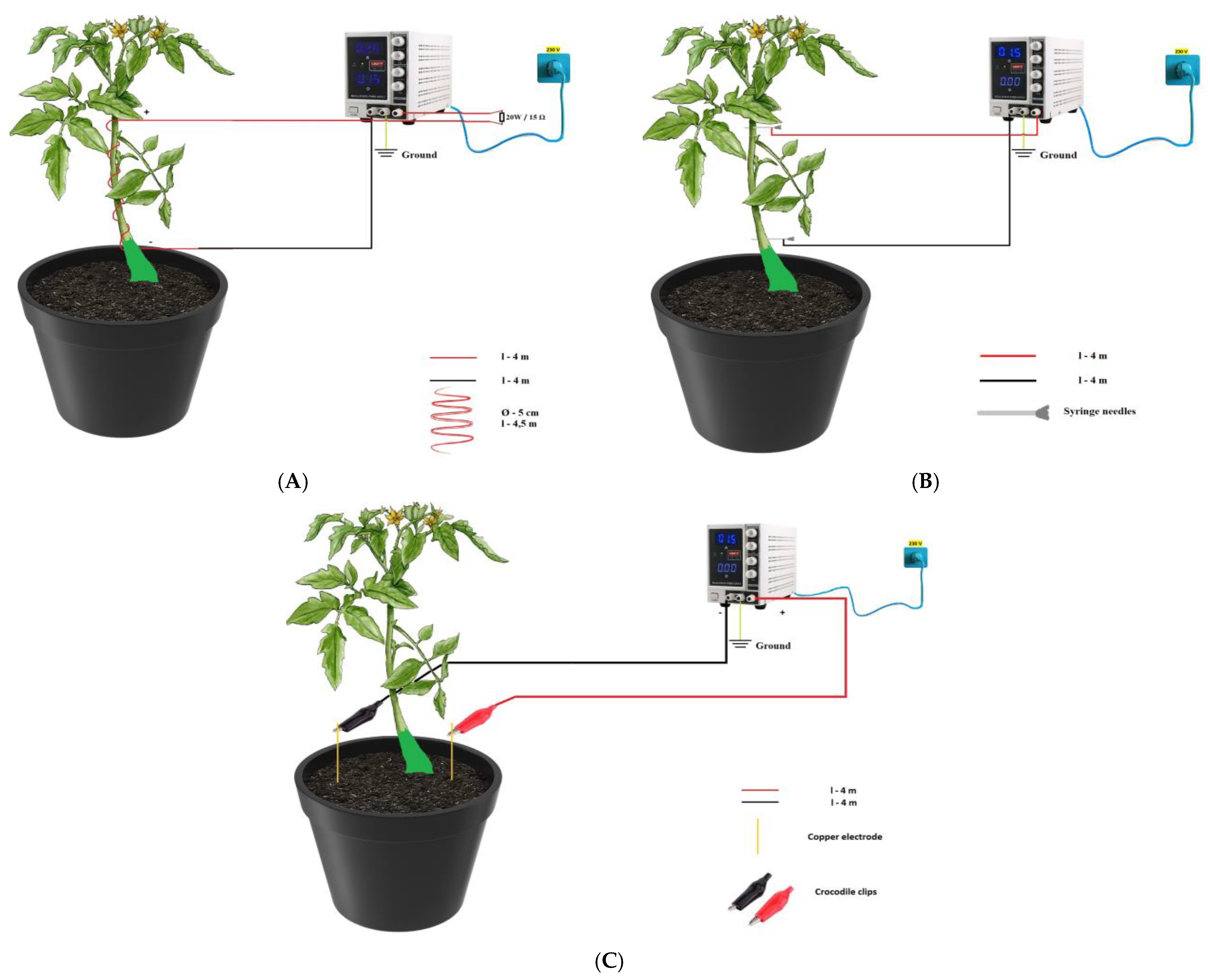

| Treatment | Description of Treatment |
|---|---|
| Treatment 1 (T1) | Applied electric current crossed the circuit, created using a current with an intensity of 0.15 A, using an electrical conductor with a length of 4.5 m, arranged in the form of a spiral with a diameter of 5 cm. |
| Treatment 2 (T2) | Applied electric current crossed the circuit, created using a current with an intensity of 0.30 A, using an electrical conductor with a length of 4.5 m, arranged in the form of a spiral with a diameter of 5 cm. |
| Treatment 3 (T3) | Applied electrical current that crossed the circuit, created using a current with an intensity of 0.45 A, using an electrical conductor with a length of 4.5 m, arranged in the form of a spiral with a diameter of 5 cm. |
| Treatment 4 (T4) | In this case, syringe needles were used that crossed the plant stem, these being inserted twice for each plant, one being inserted in the apical area, and the other at the base of the stem. The electrical conductors were connected to the syringe needles with the help of crocodile clips. The DC power applied was 1.5 V, DC (polarity). |
| Treatment 5 (T5) | In this case, for each plant we inserted two copper electrodes into the soil which were connected to a DC of 1.5 V using crocodile clips (soil version). |
| Control (C) | Control without electric current applied. |
| Months | Temperature (°C) | Relative Humidity (%) | Sunlight (kl) | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| February | 18.1 | 18.7 | 75.9 | 76.4 | 24.6 | 23.9 |
| March | 20.0 | 18.7 | 73.1 | 71.9 | 39.8 | 35.6 |
| April | 20.3 | 18.9 | 69.3 | 68.4 | 44.6 | 58.4 |
| May | 20.9 | 19.7 | 65.7 | 66.2 | 48.5 | 45.7 |
| June | 26.1 | 24.8 | 65.8 | 71.2 | 60.4 | 53.8 |
| July | 27.5 | 25.6 | 66.2 | 67.2 | 61.9 | 61.7 |
| Variant | Plant Height (cm) | Number of Fruits | Average Fruits Weight (g) | Average Fruits Diameter (mm) | Yield (t∙ha−1) |
|---|---|---|---|---|---|
| T1 | 161.33 ± 4.48 a | 33.00 ± 2.31 ns | 164.38 ± 11.98 ns | 69.29 ± 1.79 ns | 142.47 ± 35.03 a |
| T2 | 160.33 ± 8.41 a | 36.67 ± 3.76 ns | 148.00 ± 10.65 ns | 68.16 ± 3.31 ns | 138.40 ± 12.89 ab |
| T3 | 154.33 ± 6.96 ab | 38.33 ± 4.37 ns | 132.35 ± 9.52 ns | 64.07 ± 1.70 ns | 133.60 ± 42.76 ab |
| T4 | 156.67 ± 4.91 ab | 36.33 ± 5.21 ns | 147.30 ± 10.59 ns | 67.42 ± 3.49 ns | 138.01 ± 39.45 ab |
| T5 | 155.67 ± 6.12 ab | 36.00 ± 2.52 ns | 124.55 ± 8.96 ns | 63.62 ± 1.18 ns | 115.50 ± 6.32 ab |
| C | 125.67 ± 9.94 b | 27.67 ± 3.48 ns | 118.89 ± 8.55 ns | 62.01 ± 1.91 ns | 83.49 ± 8.30 b |
| Variant | Firmness (kg∙cm−2) | Acidity (g Citric Acid∙100 g−1 f.w.) | Total Soluble Solids (° Brix) |
|---|---|---|---|
| T1 | 3.45 ± 0.25 b | 0.53 ± 0.03 a | 5.23 ± 0.03 a |
| T2 | 3.82 ± 0.28 ab | 0.53 ± 0.01 a | 5.07 ± 0.03 ab |
| T3 | 4.02 ± 0.29 ab | 0.55 ± 0.01 a | 5.03 ± 0.03 b |
| T4 | 4.56 ± 0.33 ab | 0.43 ± 0.02 b | 4.83 ± 0.03 c |
| T5 | 3.87 ± 0.28 ab | 0.40 ± 0.01 b | 5.07 ± 0.03 ab |
| C | 4.93 ± 0.35 a | 0.44 ± 0.02 b | 5.10 ± 0.06 ab |
| Variant | Lycopene (mg·100 g−1 d.w.) | β-Carotene (mg·100 g−1 d.w.) | Polyphenols (mg·100 g−1 d.w.) | Antioxidant Activity (mmol Trol·100 g−1 d.w.) |
|---|---|---|---|---|
| T1 | 8.21 ± 0.68 ns | 7.46 ± 0.49 ns | 1698.45 ± 83.50 ns | 83.05 ± 0.51 a |
| T2 | 9.16 ± 0.81 ns | 7.69 ± 0.50 ns | 1722.42 ± 199.81 ns | 77.03 ± 0.56 b |
| T3 | 10.52 ± 0.82 ns | 7.47 ± 0.49 ns | 1863.18 ± 226.02 ns | 72.84 ± 0.15 c |
| T4 | 9.78 ± 1.32 ns | 7.58 ± 0.50 ns | 1766.87 ± 100.22 ns | 73.28 ± 0.34 c |
| T5 | 9.43 ± 0.53 ns | 7.77 ± 0.51 ns | 1772.43 ± 104.44 ns | 73.91 ± 0.68 bc |
| C | 7.85 ± 0.66 ns | 7.00 ± 0.46 ns | 1735.11 ± 33.10 ns | 81.35 ± 1.35 a |
| Variant | Ash (g·100 g−1 d.w.) | Crude Lipid (g·100 g−1 d.w.) | Crude Protein (g·100 g−1 d.w.) | Crude Fibre (g·100 g−1 d.w.) | Dietary Fibre (g·100 g−1 d.w.) | Calorific Value kJ/g (kcal/100 g) |
|---|---|---|---|---|---|---|
| T1 | 4.51 ± 0.29 ns | 11.29 ± 0.74 ns | 33.44 ± 2.19 ns | 15.43 ± 1.01 bc | 9.52 ± 0.62 a | 441.95 ± 28.97 ns |
| T2 | 4.11 ± 0.27 ns | 11.41 ± 0.75 ns | 31.96 ± 2.09 ns | 13.43 ± 0.88 c | 6.74 ± 0.44 b | 436.42 ± 28.60 ns |
| T3 | 4.83 ± 0.32 ns | 11.90 ± 0.78 ns | 31.27 ± 2.05 ns | 23.07 ± 1.51 a | 8.30 ± 0.54 ab | 423.91 ± 27.78 ns |
| T4 | 4.34 ± 0.28 ns | 12.25 ± 0.80 ns | 29.49 ± 1.93 ns | 20.02 ± 1.31 ab | 8.71 ± 0.57 ab | 423.98 ± 27.78 ns |
| T5 | 4.76 ± 0.31 ns | 11.70 ± 0.77 ns | 32.54 ± 2.13 ns | 15.83 ± 1.04 bc | 9.55 ± 0.63 a | 452.68 ± 29.67 ns |
| C | 5.12 ± 0.34 ns | 11.21 ± 0.74 ns | 30.96 ± 2.03 ns | 13.43 ± 0.88 c | 6.74 ± 0.44 b | 445.75 ± 29.21 ns |
| Variant | Phytate (g·100 g−1 d.w.) | Tannins (g·100 g−1 d.w.) | Oxalates (g·100 g−1 d.w.) | Saponins (g·100 g−1 d.w.) | Trypsin Inhibitors (TUI mg−1) | α-Amylase Inhibitors IC50 (mg ml−1) |
|---|---|---|---|---|---|---|
| T1 | 3.75 ± 0.25 ns | 3.96 ± 0.26 a | 2.14 ± 0.14 bc | 2.63 ± 0.17 bc | 9.26 ± 0.61 ns | 0.77 ± 0.05 a |
| T2 | 3.59 ± 0.23 ns | 2.46 ± 0.16 cd | 1.36 ± 0.09 c | 1.66 ± 0.11 d | 9.95 ± 0.65 ns | 0.43 ± 0.03 bc |
| T3 | 3.43 ± 0.23 ns | 1.77 ± 0.12 de | 3.84 ± 0.25 a | 3.94 ± 0.26 a | 9.75 ± 0.64 ns | 0.35 ± 0.02 c |
| T4 | 3.27 ± 0.22 ns | 1.18 ± 0.08 e | 4.00 ± 0.26 a | 3.24 ± 0.21 ab | 9.42 ± 0.62 ns | 0.54 ± 0.03 b |
| T5 | 4.02 ± 0.27 ns | 3.46 ± 0.23 ab | 2.48 ± 0.16 b | 2.82 ± 0.18 b | 9.49 ± 0.62 ns | 0.82 ± 0.05 a |
| C | 3.89 ± 0.25 ns | 2.65 ± 0.17 bc | 1.57 ± 0.10 c | 1.78 ± 0.12 cd | 10.07 ± 0.66 ns | 0.53 ± 0.03 bc |
| Variant | Dry Matter (%) | Moisture (%) | K (ppm) | Ca (ppm) | Mg (ppm) | Fe (ppm) | Zn (ppm) | Na (ppm) |
|---|---|---|---|---|---|---|---|---|
| T1 | 5.82 ± 0.14 ns | 94.18 ± 0.14 ns | 2.02 ± 0.13 d | 61.33 ± 4.02 ns | 30.08 ± 1.97 c | 1.89 ± 0.12 b | 0.59 ± 0.04 bc | 1.33 ± 0.09 ns |
| T2 | 5.95 ± 0.15 ns | 94.05 ± 0.15 ns | 2.73 ± 0.18 cd | 65.56 ± 4.30 ns | 33.47 ± 2.19 bc | 2.35 ± 0.16 b | 0.33 ± 0.02 d | 1.35 ± 0.09 ns |
| T3 | 6.17 ± 0.13 ns | 93.83 ± 0.13 ns | 3.17 ± 0.21 bc | 69.88 ± 4.58 ns | 34.50 ± 2.26 bc | 2.59 ± 0.17 b | 0.53 ± 0.03 bc | 1.37 ± 0.09 ns |
| T4 | 5.79 ± 0.14 ns | 94.21 ± 0.14 ns | 5.55 ± 0.36 a | 75.58 ± 4.95 ns | 45.82 ± 3.00 ab | 4.89 ± 0.32 a | 0.89 ± 0.06 a | 1.52 ± 0.10 ns |
| T5 | 5.89 ± 0.14 ns | 94.11 ± 0.14 ns | 3.96 ± 0.26 b | 71.86 ± 4.71 ns | 49.54 ± 3.25 a | 4.39 ± 0.29 a | 0.67 ± 0.04 b | 1.34 ± 0.09 ns |
| C | 5.91 ± 0.14 ns | 94.09 ± 0.14 ns | 1.72 ± 0.11 d | 76.13 ± 4.99 ns | 43.55 ± 2.85 ab | 2.63 ± 0.17 b | 0.42 ± 0.03 cd | 1.11 ± 0.07 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghitoaie, M.-V.; Bodale, I.; Achitei, V.; Teliban, G.-C.; Cojocaru, A.; Caruso, G.; Mihalache, G.; Stoleru, V. Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato. Appl. Sci. 2022, 12, 4211. https://doi.org/10.3390/app12094211
Gheorghitoaie M-V, Bodale I, Achitei V, Teliban G-C, Cojocaru A, Caruso G, Mihalache G, Stoleru V. Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato. Applied Sciences. 2022; 12(9):4211. https://doi.org/10.3390/app12094211
Chicago/Turabian StyleGheorghitoaie, Madalin-Vasile, Ilie Bodale, Vladut Achitei, Gabriel-Ciprian Teliban, Alexandru Cojocaru, Gianluca Caruso, Gabriela Mihalache, and Vasile Stoleru. 2022. "Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato" Applied Sciences 12, no. 9: 4211. https://doi.org/10.3390/app12094211
APA StyleGheorghitoaie, M.-V., Bodale, I., Achitei, V., Teliban, G.-C., Cojocaru, A., Caruso, G., Mihalache, G., & Stoleru, V. (2022). Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato. Applied Sciences, 12(9), 4211. https://doi.org/10.3390/app12094211









