Effect of Different pH Beverages on the Color Stability of Smart Monochromatic Composite
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paolone, G.; Scolavino, S.; Gherlone, E.; Spagnuolo, G. Direct Esthetic Composite Restorations in Anterior Teeth: Managing Symmetry Strategies. Symmetry 2021, 13, 797. [Google Scholar] [CrossRef]
- Amin, M.; Naz, F.; Sheikh, A.; Ahmed, M.A. Post Operative Sensitivity in Teeth Restored with Posterior Dental Composite Using Self Etch and Total Etch Adhesives. J. Pak. Dent. Assoc. 2015, 24, 22–28. [Google Scholar]
- Peng, P.W.; Huang, C.F.; Hsu, C.Y.; Chen, A.; Ng, H.H.; Cheng, M.S.; Tsay, S.; Lai, J.Y.; Yang, T.S.; Lee, W.F. Color Stability and Staining Susceptibility of Direct Resin-Based Composites after Light-Activated in-Office Bleaching. Polymers 2021, 13, 2941. [Google Scholar] [CrossRef]
- Adresi, Y.; Eroğlu, E.; Çetin, C.; Küçükeşmen, C.; Özişçi, Ö. CIE L*a*b* Color Analyses of Anterior Maxillary Teeth According to Gender and Localization Variables Maksiller Anterior Dişlerin Cinsiyet ve Lokalizasyon Değişkenlerine Göre CIE L*a*b* Renk Analizi. Süleyman Demirel Üniversitesi Sağlık Bilimleri Dergisi 2019, 10, 448–453. [Google Scholar]
- Al Badr, R.M.; Hassan, H.A. Effect of Immersion in Different Media on the Mechanical Properties of Dental Composite Resins. Int. J. Appl. Dent. Sci. 2017, 3, 81–88. [Google Scholar]
- Jouhar, R. Effect of Sonic Activation on Push-out Bond Strength of Fiber Post: An in Vitro Study. Materials 2021, 14, 5038. [Google Scholar] [CrossRef]
- Shamszadeh, S.; Sheikh-Al-Eslamian, S.M.; Hasani, E.; Abrandabadi, A.N.; Panahandeh, N. Color Stability of the Bulk-Fill Composite Resins with Different Thickness in Response to Coffee/Water Immersion. Int. J. Dent. 2016, 2016, 7186140. [Google Scholar] [CrossRef]
- Alawjali, S.S.; Lui, J.L. Effect of One-Step Polishing System on the Color Stability of Nanocomposites. J. Dent. 2013, 41, e53–e61. [Google Scholar] [CrossRef]
- Alnasser, H.A.; Elhejazi, A.A.; Al-Abdulaziz, A.A.; Alajlan, S.S.; Habib, S.R. Effect of Conventional and Electronic Cigarettes Smoking on the Color Stability and Translucency of Tooth Colored Restorative Materials: An in Vitro Analysis. Coatings 2021, 11, 1568. [Google Scholar] [CrossRef]
- Freitas, F.; de Melo, T.P.; Delgado, A.H.S.; Monteiro, P.; Rua, J.; Proença, L.; Caldeira, J.; Azul, A.M.; Mendes, J.J. Varying the Polishing Protocol Influences the Color Stability and Surface Roughness of Bulk-Fill Resin-Based Composites. J. Funct. Biomater. 2021, 12, 1. [Google Scholar] [CrossRef]
- Barutcigil, Ç.; Yildiz, M. Intrinsic and Extrinsic Discoloration of Dimethacrylate and Silorane Based Composites. J. Dent. 2012, 40 (Suppl. 1), e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Gregorius, W.C.; Kattadiyil, M.T.; Goodacre, C.J.; Roggenkamp, C.L.; Powers, J.M.; Paravina, R.D. Effects of Ageing and Staining on Color of Acrylic Resin Denture Teeth. J. Dent. 2012, 40 (Suppl. 2), e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.K.; Sunarintyas, S.; Irnawati, D. Color Stability of Visible Light Cured Composite Resin after Soft Drink Immersion. Dent. J. (Majalah Kedokteran Gigi) 2009, 42, 123. [Google Scholar] [CrossRef][Green Version]
- Özdaş, D.Ö.; Kazak, M.; Çilingir, A.; Subaşı, M.G.; Tiryaki, M.; Günal, Ş. Color Stability of Composites After Short-Term Oral Simulation: An in Vitro Study. Open Dent. J. 2016, 10, 431–437. [Google Scholar] [CrossRef]
- Raja, K.K.; Hari, P.; Chin, M.Q.K.; Singbal, K.; Fareez, I.M. Color Stability of a New Rice Husk Composite in Comparison with Conventional Composites after Exposure to Commonly Consumed Beverages in Malaysia. Int. J. Dent. 2019, 2019, 9753431. [Google Scholar] [CrossRef]
- Alhamdan, E.M.; Bashiri, A.; Alnashmi, F.; Al-Saleh, S.; Al-Shahrani, K.; Al-Shahrani, S.; Alsharani, A.; Alzahrani, K.M.; Alqarawi, F.K.; Vohra, F.; et al. Evaluation of Smart Chromatic Technology for a Single-Shade Dental Polymer Resin: An in Vitro Study. Appl. Sci. 2021, 11, 10108. [Google Scholar] [CrossRef]
- Gamal, W.; Safwat, A.; Abdou, A. Effect of Coloring Beverages on Color Stability of Single Shade Restorative Material: An In Vitro Study. Open Access Maced. J. Med. Sci. 2022, 10, 28–32. [Google Scholar]
- Sensi, L.; Winkler, C.; Geraldeli, S. Accelerated Aging Effects on Color Stability of Potentially Color Adjusting Resin-based Composites. Oper Dent. 2021, 46, 188–196. [Google Scholar] [CrossRef]
- Ertaş, E.; Güler, A.U.; Yücel, A.Ç.; Köprülü, H.; Güler, E. Color Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef]
- Um, C.M.; Ruyter, I.E. Staining of Resin-Based Veneering Materials with Coffee and Tea. Quintessence Int. 1991, 22, 377–386. [Google Scholar]
- Poggio, C.; Ceci, M.; Beltrami, R.; Mirando, M.; Wassim, J.; Colombo, M. Color Stability of Esthetic Restorative Materials: A Spectrophotometric Analysis. Acta Biomater. Odontol. Scand. 2016, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of Food-Simulating Solutions and Surface Finish on Susceptibility to Staining of Aesthetic Restorative Materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Janda, R.; Roulet, J.F.; Kaminsky, M.; Steffin, G.; Latta, M. Color Stability of Resin Matrix Restorative Materials as a Function of the Method of Light Activation. Eur. J. Oral Sci. 2004, 112, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, T.C.; Gusson, J.M.; Bortolatto, J.F.; Pigossi, S.; Oliveira, J.O.B.; Ricci, W.A. Color Stability of Conventional and Bulk Fill Composite Resins. RGO-Rev. Gaúcha Odontol. 2018, 66, 15–20. [Google Scholar] [CrossRef]
- Lopes-Rocha, L.; Mendes, J.M.; Garcez, J.; Sá, A.G.; Pinho, T.; Souza, J.C.M.; Torres, O. The Effect of Different Dietary and Therapeutic Solutions on the Color Stability of Resin-Matrix Composites Used in Dentistry: An in Vitro Study. Materials 2021, 14, 6267. [Google Scholar] [CrossRef]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and Wear Resistance of Dental Biomedical Nanomaterials in a Humid Environment with Non-Stationary Temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef]
- Scribante, A.; Bollardi, M.; Chiesa, M.; Poggio, C.; Colombo, M. Flexural Properties and Elastic Modulus of Different Esthetic Restorative Materials: Evaluation after Exposure to Acidic Drink. Biomed Res. Int. 2019, 2019, 5109481. [Google Scholar] [CrossRef]
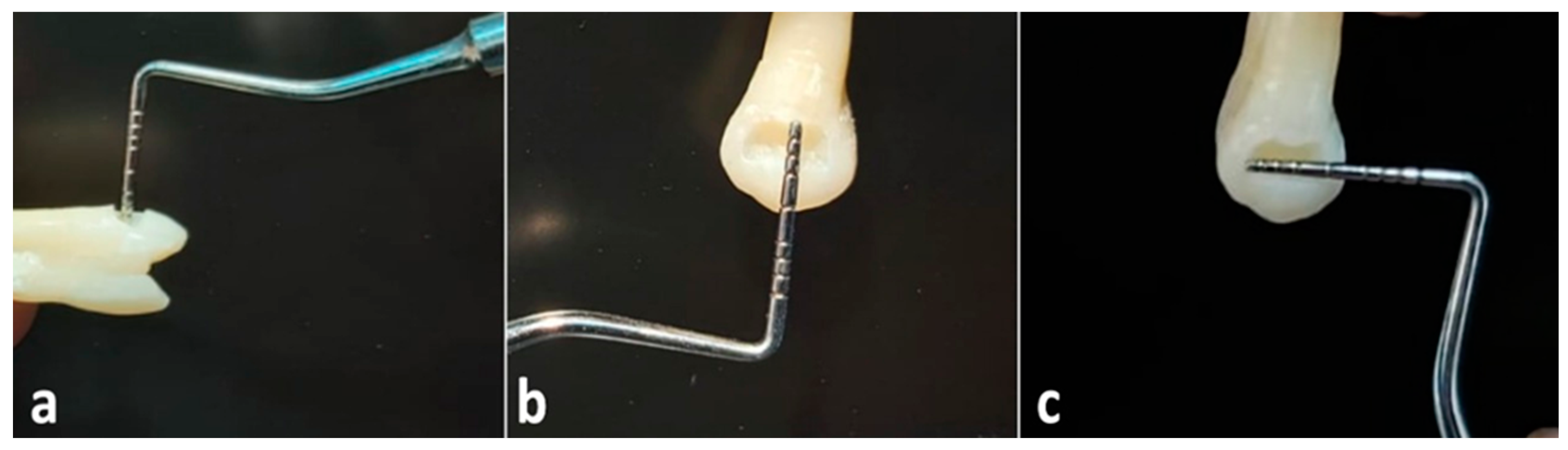
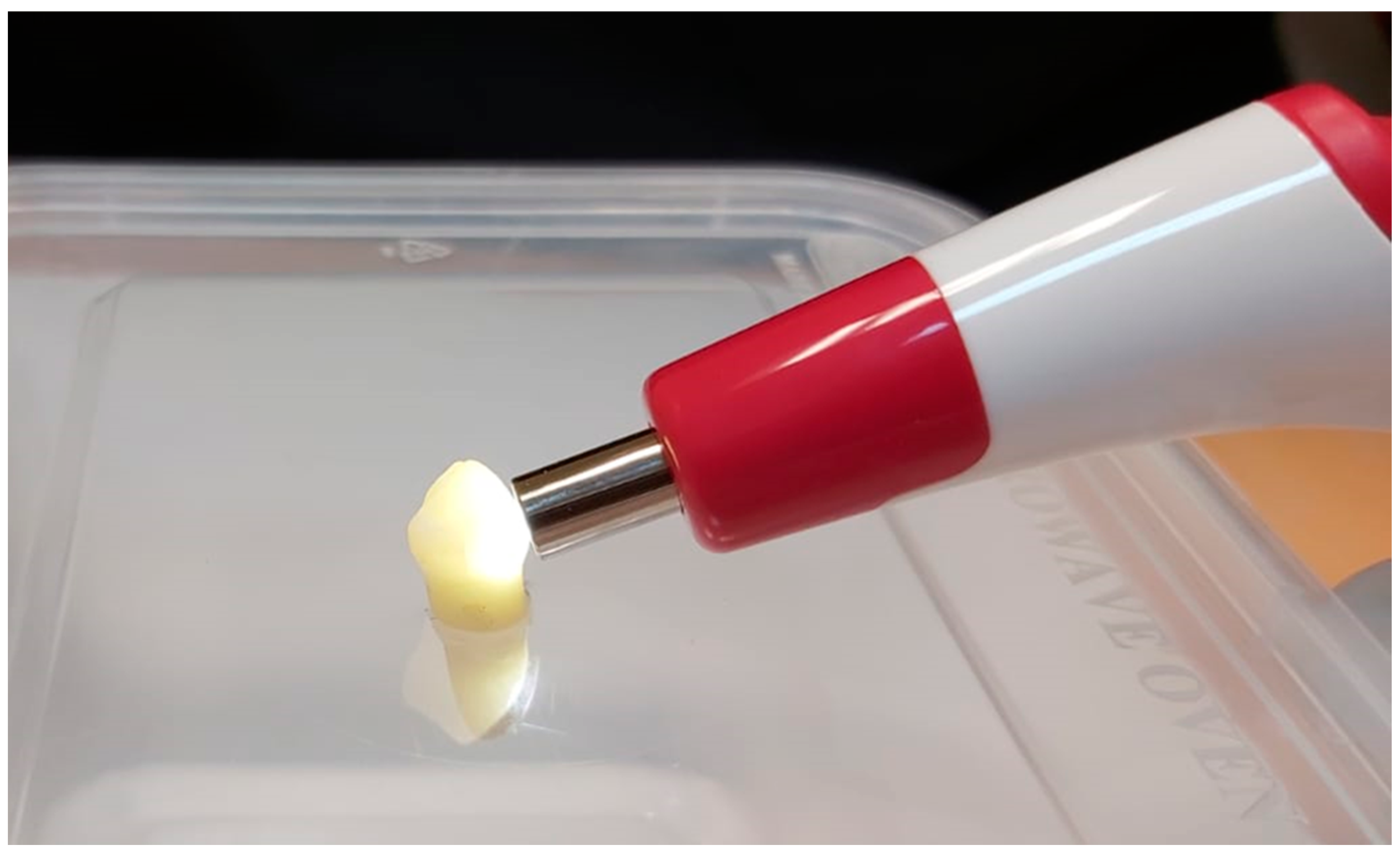

| Filtek Z250 Composite | Omnichroma Composite |
|---|---|
| BIS-GMA (Bisphenol A diglycidyl ether dimethacrylate), UDMA (urethane dimethacrylate), and Bis-EMA (Bisphenol A polyethylene glycol diether dimethacrylate) | UDMA, TEGDMA |
| 60% (volume) silica/zirconia fillers | Uniform-sized supra-nano spherical filler (260 nm spherical SiO2-ZrO2) |
| The filler particle size distribution is from 0.01 µm to 3.5 µm with an average particle size of 0.6 µm | Composite filler (include 260 nm spherical SiO2-ZrO2). Filler loading 79 wt% (68 vol%) |
| Comparison among Groups | Shades | p Value | ||||
|---|---|---|---|---|---|---|
| A2 | A3 | B2 | B3 | |||
| Mean (SD) | ||||||
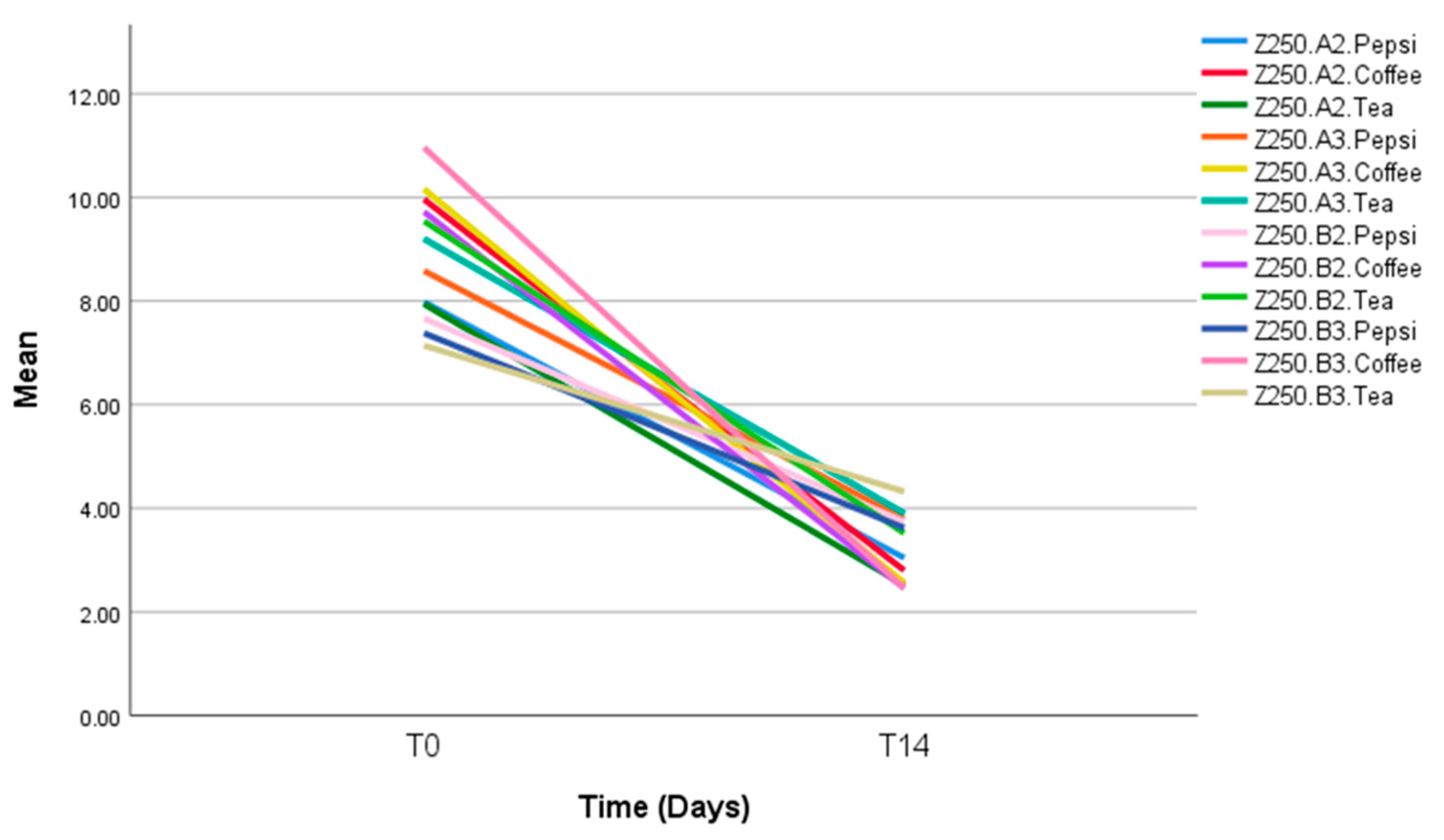
| Pepsi | Omnnichroma | 7.26 (2.64) | 6.26 (1.51) | 6.68 (1.41) | 4.58 (2.01) | 0.202 |
| Filtek Z250 | 5.14 (2.69) | 4.78 (2.54) | 3.92 (2.44) | 3.76 (1.56) | 0.754 | |
| p-value | 0.245 | 0.296 | 0.060 | 0.493 | ||
| Coffee | Omnichroma | 5.34 (3.32) | 5.94 (1.45) | 5.54 (2.06) | 6.54 (1.63) | 0.841 |
| Filtek Z250 | 7.16 (4.21) | 7.60 (2.62) | 7.26 (1.02) | 8.50 (3.22) | 0.891 | |
| p-value | 0.470 | 0.251 | 0.134 | 0.260 | ||
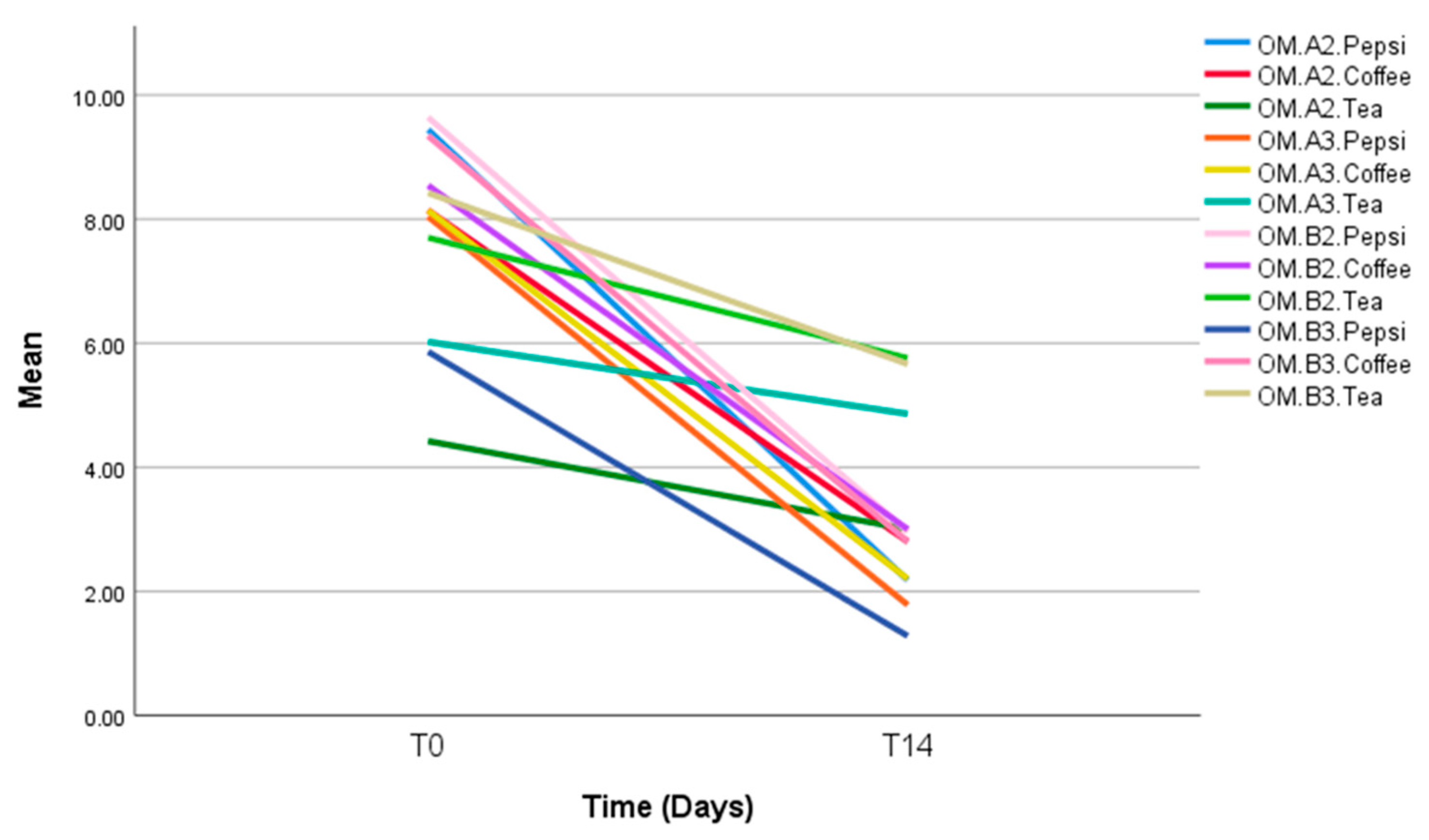
| Milk Tea | Omnichroma | 1.42 (1.69) | 1.16 (0.30) | 1.94 (0.48) | 2.76 (0.96) | 0.104 |
| Filtek Z250 | 5.42 (2.13) | 5.30 (1.90) | 6.02 (1.32) | 2.82 (2.18) | 0.079 | |
| p-value | 0.011 | 0.001 | 0.000 | 0.957 | ||
| Dependent Variable | Shades | Shades | Mean Difference | Std. Error | Sig. |
|---|---|---|---|---|---|
| OM Pepsi | A1 | A2 | 1.00000 | 1.23956 | 0.432 |
| B2 | 0.58000 | 1.23956 | 0.646 | ||
| B3 | 2.68000 * | 1.23956 | 0.046 | ||
| A2 | A1 | −1.00000 | 1.23956 | 0.432 | |
| B2 | −0.42000 | 1.23956 | 0.739 | ||
| B3 | 1.68000 | 1.23956 | 0.194 | ||
| B2 | A1 | −0.58000 | 1.23956 | 0.646 | |
| A2 | 0.42000 | 1.23956 | 0.739 | ||
| B3 | 2.10000 | 1.23956 | 0.110 | ||
| B3 | A1 | −2.68000 * | 1.23956 | 0.046 | |
| A2 | −1.68000 | 1.23956 | 0.194 | ||
| B2 | −2.10000 | 1.23956 | 0.110 | ||
| FT Pepsi | A1 | A2 | 0.36000 | 1.48829 | 0.812 |
| B2 | 1.22000 | 1.48829 | 0.424 | ||
| B3 | 1.38000 | 1.48829 | 0.368 | ||
| A2 | A1 | −0.36000 | 1.48829 | 0.812 | |
| B2 | 0.86000 | 1.48829 | 0.571 | ||
| B3 | 1.02000 | 1.48829 | 0.503 | ||
| B2 | A1 | −1.22000 | 1.48829 | 0.424 | |
| A2 | −0.86000 | 1.48829 | 0.571 | ||
| B3 | 0.16000 | 1.48829 | 0.916 | ||
| B3 | A1 | −1.38000 | 1.48829 | 0.368 | |
| A2 | −1.02000 | 1.48829 | 0.503 | ||
| B2 | −0.16000 | 1.48829 | 0.916 | ||
| OM Coffee | A1 | A2 | −0.60000 | 1.41958 | 0.678 |
| B2 | −0.20000 | 1.41958 | 0.890 | ||
| B3 | −1.20000 | 1.41958 | 0.410 | ||
| A2 | A1 | 0.60000 | 1.41958 | 0.678 | |
| B2 | 0.40000 | 1.41958 | 0.782 | ||
| B3 | −0.60000 | 1.41958 | 0.678 | ||
| B2 | A1 | 0.20000 | 1.41958 | 0.890 | |
| A2 | −0.40000 | 1.41958 | 0.782 | ||
| B3 | −1.00000 | 1.41958 | 0.491 | ||
| B3 | A1 | 1.20000 | 1.41958 | 0.410 | |
| A2 | 0.60000 | 1.41958 | 0.678 | ||
| B2 | 1.00000 | 1.41958 | 0.491 | ||
| FT Coffee | A1 | A2 | −0.44000 | 1.89937 | 0.820 |
| B2 | −0.10000 | 1.89937 | 0.959 | ||
| B3 | −1.34000 | 1.89937 | 0.491 | ||
| A2 | A1 | 0.44000 | 1.89937 | 0.820 | |
| B2 | 0.34000 | 1.89937 | 0.860 | ||
| B3 | −0.90000 | 1.89937 | 0.642 | ||
| B2 | A1 | 0.10000 | 1.89937 | 0.959 | |
| A2 | −0.34000 | 1.89937 | 0.860 | ||
| B3 | −1.24000 | 1.89937 | 0.523 | ||
| B3 | A1 | 1.34000 | 1.89937 | 0.491 | |
| A2 | 0.90000 | 1.89937 | 0.642 | ||
| B2 | 1.24000 | 1.89937 | 0.523 | ||
| OM Milk Tea | A1 | A2 | 0.26000 | 0.64195 | 0.691 |
| B2 | −0.52000 | 0.64195 | 0.430 | ||
| B3 | −1.34000 | 0.64195 | 0.053 | ||
| A2 | A1 | −0.26000 | 0.64195 | 0.691 | |
| B2 | −0.78000 | 0.64195 | 0.242 | ||
| B3 | −1.60000 * | 0.64195 | 0.024 | ||
| B2 | A1 | 0.52000 | 0.64195 | 0.430 | |
| A2 | 0.78000 | 0.64195 | 0.242 | ||
| B3 | −0.82000 | 0.64195 | 0.220 | ||
| B3 | A1 | 1.34000 | 0.64195 | 0.053 | |
| A2 | 1.60000 * | 0.64195 | 0.024 | ||
| B2 | 0.82000 | 0.64195 | 0.220 | ||
| FT Milk Tea | A1 | A2 | 0.12000 | 1.21227 | 0.922 |
| B2 | −0.60000 | 1.21227 | 0.627 | ||
| B3 | 2.60000 * | 1.21227 | 0.048 | ||
| A2 | A1 | −0.12000 | 1.21227 | 0.922 | |
| B2 | −0.72000 | 1.21227 | 0.561 | ||
| B3 | 2.48000 | 1.21227 | 0.058 | ||
| B2 | A1 | 0.60000 | 1.21227 | 0.627 | |
| A2 | 0.72000 | 1.21227 | 0.561 | ||
| B3 | 3.20000 * | 1.21227 | 0.018 | ||
| B3 | A1 | −2.60000 * | 1.21227 | 0.048 | |
| A2 | −2.48000 | 1.21227 | 0.058 | ||
| B2 | −3.20000 * | 1.21227 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.A.; Jouhar, R.; Vohra, F. Effect of Different pH Beverages on the Color Stability of Smart Monochromatic Composite. Appl. Sci. 2022, 12, 4163. https://doi.org/10.3390/app12094163
Ahmed MA, Jouhar R, Vohra F. Effect of Different pH Beverages on the Color Stability of Smart Monochromatic Composite. Applied Sciences. 2022; 12(9):4163. https://doi.org/10.3390/app12094163
Chicago/Turabian StyleAhmed, Muhammad Adeel, Rizwan Jouhar, and Fahim Vohra. 2022. "Effect of Different pH Beverages on the Color Stability of Smart Monochromatic Composite" Applied Sciences 12, no. 9: 4163. https://doi.org/10.3390/app12094163
APA StyleAhmed, M. A., Jouhar, R., & Vohra, F. (2022). Effect of Different pH Beverages on the Color Stability of Smart Monochromatic Composite. Applied Sciences, 12(9), 4163. https://doi.org/10.3390/app12094163








