Use of Raman Spectroscopy, Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy in a Multi-Technique Approach for Physical Characterization of Purple Urine Bag Syndrome
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.W.; Su, Y.J. Trends in the epidemiology of purple urine bag syndrome: A systematic review. Biomed. Rep. 2018, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Aycock, R. A Case of Purple Urine Bag Syndrome in a Patient with an Ileal Conduit. Int. J. Nephrol. Urol. 2010, 2, 580–583. [Google Scholar]
- Al-Sardar, H.; Haroon, D. Purple urinary bag syndrome. Am. J. Med. 2009, 122, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.; Merlo, J.; Beech, N.; Giles, C.; Boon, B.; Parker, B.; Dancer, C.; Munckhof, W.; Teng, H.S. The purple urine bag syndrome: A visually striking side effect of a highly alkaline urinary tract infection. Can. Urol. Assoc. J. 2011, 5, 233–234. [Google Scholar] [CrossRef]
- Wattanapisit, S.; Wattanapisit, A.; Meepuakmak, A.; Rakkapan, P. Purple urine bag syndrome in palliative care. BMJ Support. Palliat. Care. 2019, 9, 155–157. [Google Scholar] [CrossRef]
- Worku, D.A. Purple urine bag syndrome: An unusual but important manifestation of urinary tract infection. Case report and literature review. SAGE Open Med. Case Rep. 2019, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Austin, L.A.; Osseiran, S.; Evans, C.L. Raman technologies in cancer diagnostics. Analyst 2016, 141, 476–503. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Cristiano, M.C.; Venuti, V.; Crupi, V.; Majolino, D.; Paladini, G.; Acri, G.; Testagrossa, B.; Irrera, A.; Paolino, D.; et al. Rutin-Loaded Solid Lipid Nanoparticles: Characterization and In Vitro Evaluation. Molecules 2021, 26, 1039. [Google Scholar] [CrossRef]
- Giannetto, C.; Acri, G.; Giudice, E.; Arfuso, F.; Testagrossa, B.; Piccione, G. Quantifying serum total lipids and tryptophan cocentrations by Raman spectroscopy during standardized obstacle course in horses. J. Equine Vet. Sci. 2021, 108, 103820. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.-S.; Wang, P.; Tong, Y.; Huang, Y. Charge Relays via Dual Carbon-Actions on Nanostructured BiVO 4 for High Performance Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mi, X.; Tan, X.; Xiang, R. Recent Progress on Liquid Biopsy Analysis using Surface-Enhanced Raman Spectroscopy. Theranostics 2019, 9, 491–525. [Google Scholar] [CrossRef]
- Acri, G.; Romano, C.; Costa, S.; Pellegrino, S.; Testagrossa, B. Raman Spectroscopy Technique: A Non-Invasive Tool in Celiac Disease Diagnosis. Diagnostics 2021, 11, 1277. [Google Scholar] [CrossRef]
- Saatkamp, C.J.; De Almeida, M.L.; Bispo, J.A.M.; Pinheiro, A.L.B.; Fernandes, A.B.; Silveira, L. Quantifying creatinine and urea in human urine through Raman spectroscopy aiming at diagnosis of kidney disease. J. Biomed. Opt. 2016, 21, 037001. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.B.; Nogueira, M.S.; Canevari, R.A.; Carvalho, L.F.C.S. Vibration spectroscopy and body biofluids: Literature review for clinical applications. Photodiagnosis Photodyn. Ther. 2018, 24, 237–244. [Google Scholar] [CrossRef]
- Kušnír, J.; Dubayová, K.; Lešková, L.; Lajtár, M. Concentration matrices-solutions for fluorescence definition of urine. Anal. Lett. 2005, 38, 1559–1567. [Google Scholar] [CrossRef]
- Saude, E.J.; Adamko, D.; Rowe, B.H.; Marrie, T.; Sykes, B.D. Variation of metabolites in normal human urine. Metabolomics 2007, 3, 439–451. [Google Scholar] [CrossRef]
- Moreira, L.P.; Silveira, L.; Pacheco, M.T.T.; da Silva, A.G.; Rocco, D.D.F.M. Detecting urine metabolites related to training performance in swimming athletes by means of Raman spectroscopy and principal component analysis. J. Photochem. Photobiol. B: Biol. 2018, 185, 223–234. [Google Scholar] [CrossRef]
- Bispo, J.A.M.; De Sousa Vieira, E.E.; Silveira, L.; Fernandes, A.B. Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J. Biomed. Opt. 2013, 18, 087004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acri, G.; Testagrossa, B.; Faenza, P.; Caridi, F. Spectroscopic analysis of pigments of the Antonello Gagini annunciation’s sculptural marble group, church of st. Theodore martyr (Bagaladi, Reggio Calabria, Italy): Case study. Mediterr. Archaeol. Archaeom. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Meduri, A.; Severo, A.A.; De Maria, A.; Perroni, P.; Acri, G.; Testagrossa, B.; Puzzolo, D.; Montalbano, G.; Aragona, P.; Micali, A.G. PMMA intraocular lenses changes after treatment with Nd:Yag Laser: A scanning electron microscopy and X-ray spectrometry study. Appl. Sci. 2020, 10, 6321. [Google Scholar] [CrossRef]
- Mijangos, F.; Celaya, M.A.; Gainza, F.J.; Imaz, A.; Arana, E. SEM-EDX linear scanning: A new tool for morpho-compositional analysis of growth bands in urinary stones. J. Biol. Inorg. Chem. 2020, 25, 705–715. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Oh, S.W.; Kim, Y.J. Power of Scanning Electron Microscopy and Energy Dispersive X-Ray Analysis in Rapid Microbial Detection and Identification at the Single Cell Level. Sci. Rep. 2020, 10, 2368. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, C.S.; Miller, S.E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 2009, 22, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Tatsch, E.; Schrader, B. Near-infrared Fourier Transform Raman spectroscopy of indigoids. J. Raman Spectrosc. 1995, 26, 467–473. [Google Scholar] [CrossRef]
- Acri, G.; Venuti, V.; Costa, S.; Testagrossa, B.; Pellegrino, S.; Crupi, V.; Majolino, D. Raman Spectroscopy as Noninvasive Method of Diagnosis of Pediatric Onset Inflammatory Bowel Disease. Appl. Sci. 2020, 10, 6974. [Google Scholar] [CrossRef]
- Silveira, L.J.; de Cassia Fernandes Borges, R.; Navarro, R.S.; Giana, H.E.; Zangaro, R.A.; Pacheco, M.T.T.; Fernandes, A.B. Quantifying glucose and lipid components in human serum by Raman spectroscopy and multivariate statistc. Laseres Med. Sci. 2017, 32, 787–795. [Google Scholar] [CrossRef]
- Acri, G.; Micali, A.; D’Angelo, R.; Puzzolo, D.; Aragona, P.; Testagrossa, B.; Aragona, E.; Wylegala, E.; Nowinska, A. Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy. J. Clin. Med. 2022, 11, 1403. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, G.B.; Dickson, J.A.S. Purple urine bags. Lancet 1978, 311, 220–221. [Google Scholar] [CrossRef]
- Dealler, S.F.; Hawkey, P.M.; Millar, M.R. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J. Clin. Microbiol. 1988, 26, 2152–2156. [Google Scholar] [CrossRef] [Green Version]
- Dealler, S.F.; Belfield, P.W.; Bedford, M.; Whitley, A.J.; Mulley, G.P. Purple urine bags. J. Urol. 1989, 142, 769–770. [Google Scholar] [CrossRef]
- Willett, J.L.E.; Ji, M.M.; Dunny, G.M. Exploiting biofilm phenotypes for functional characterization of hypothetical genes in Enterococcus faecalis. NPJ Biofilms Microbiomes 2019, 5, 23. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, S.J.; Choi, S.; Uh, Y.; Park, J.Y.; Han, K.-H. The Influence of Urinary Catheter Materials on Forming Biofilms of Microorganisms. J. Bacteriol. Virol. 2017, 47, 32–40. [Google Scholar] [CrossRef] [Green Version]
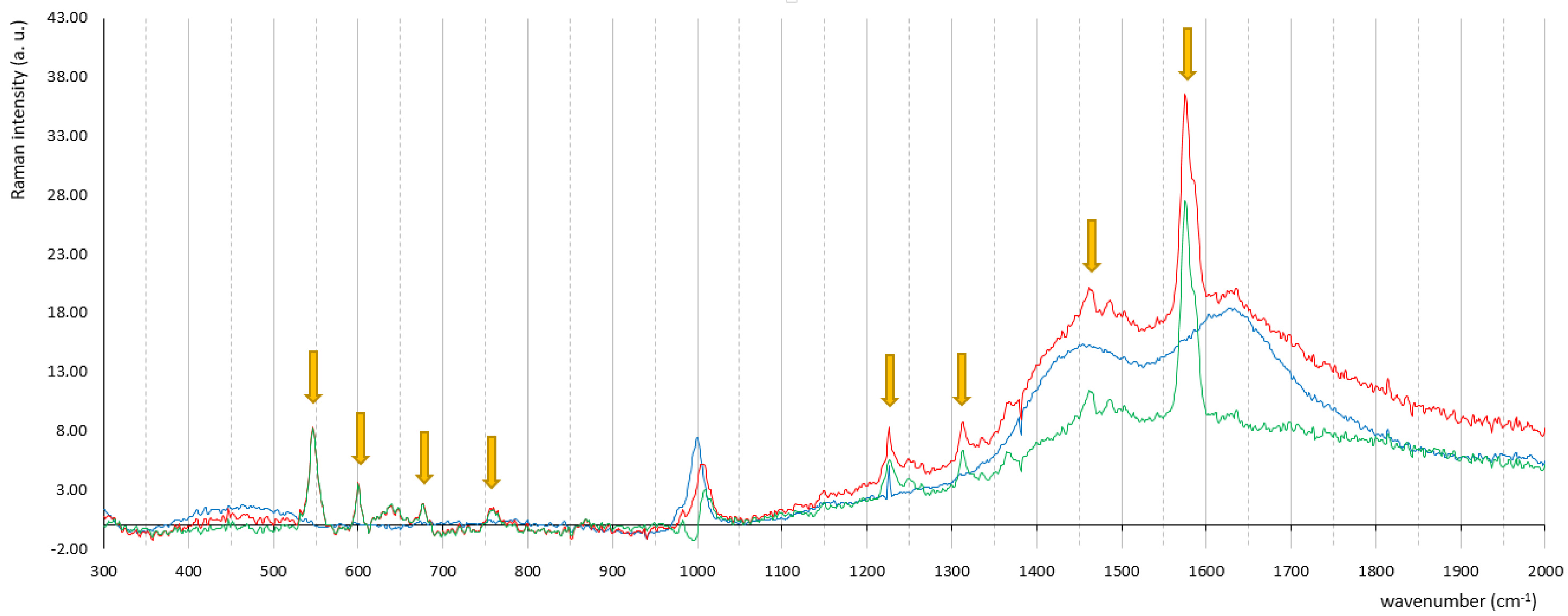

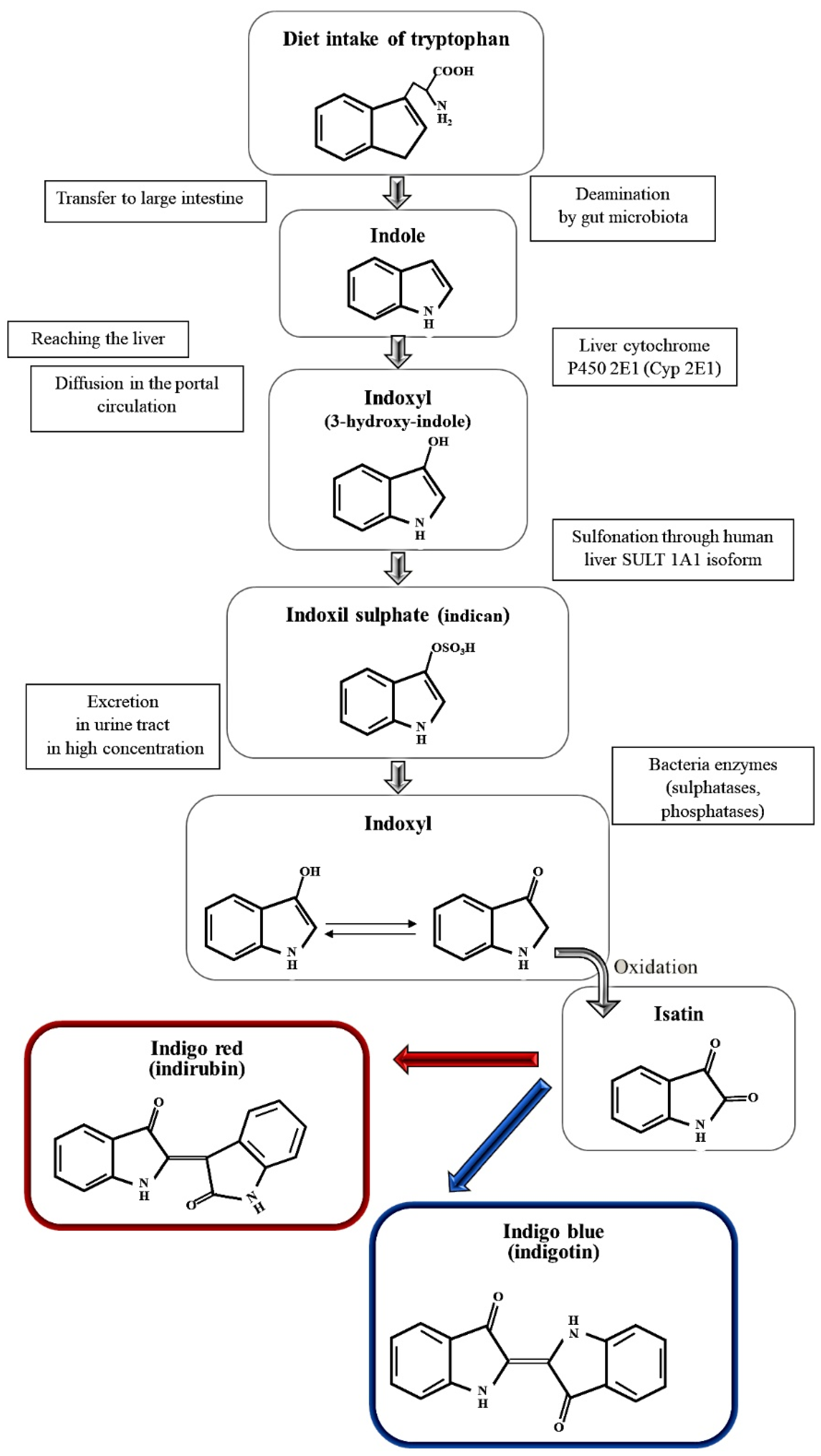
| Experimental Peaks (cm−1) | Ref Peaks (cm−1) [25] | Intensity | Assignment |
|---|---|---|---|
| 469.2 | 467 | vw | γ C-C |
| 546.7 | 544 | m | δ C=C-CO-C |
| 600.0 | 598 | vw | δ C=O δ C-H δ C-NH-C |
| 639.6 | 635 | w | γ N-H |
| 676.3 | 674 | vw | δ C-C |
| 759.5 | 758 | vw | δ C-H δ N-C-C |
| 867.9 | 868 | vw | ν C-N |
| 948.9 | 940 | vw | γ C-H |
| 1008.8 | 1015 | m | δ C-H |
| 1149.1 | 1147 | vw | δ C-C |
| 1226.2 | 1224 | s | δ C-H ν C-N |
| 1248.9 | 1248 | m | δ C-H δ C=O |
| 1312.7 | 1310 | s | ν C-C |
| 1364.6 | 1365 | s | δ N-H δ C-H |
| 1461.8 | 1460 | s | ν C-C δ C-H |
| 1486.2 | 1482 | s | ν C-C δ C-H |
| 1575.1 | 1582 | vs | ν C=C ν C=O |
| 1624.4 | 1625 | s | ν C-C δ C-H |
| 1697.9 | 1701 | w | ν C=C ν C=O |
| Element | Control (abs) | “Blue Bags” (abs) | Control Wt [Error] (%) | “Blue Bag” Wt [Error] (%) |
|---|---|---|---|---|
| Chlorine | 25.25 | 33.34 | 16.63 [2.64] | 25.17 [3.47] |
| Carbon | 97.15 | 57.12 | 63.98 [32.64] | 43.13 [20.96] |
| Oxygen | 28.01 | 37.32 | 18.45 [10.09] | 28.18 [13.12] |
| Sodium | - | 1.91 | - | 1.44 [0.46] |
| Calcium | 0.03 | 0.23 | 0.02 [0.08] | 0.18 [0.10] |
| Potassium | - | 0.08 | - | 0.06 [0.08] |
| Nickel | 0.02 | 0.03 | 0.02 [0.08] | 0.02 [0.08] |
| Aluminum | 0.43 | 0.71 | 0.28 [0.14] | 0.54 [0.18] |
| Magnesium | 0.66 | 1.09 | 0.44 [0.19] | 0.82 [0.26] |
| Phosphorus | - | 0.19 | - | 0.14 [0.10] |
| Silicon | 0.16 | 0.30 | 0.10 [0.10] | 0.13 [0.12] |
| Neodymium | 0.13 | 0.12 | 0.08 [0.09] | 0.09 [0.09] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acri, G.; Sansotta, C.; Salmeri, F.M.; Romeo, M.; Ruello, E.V.; Denaro, L.; Testagrossa, B. Use of Raman Spectroscopy, Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy in a Multi-Technique Approach for Physical Characterization of Purple Urine Bag Syndrome. Appl. Sci. 2022, 12, 4034. https://doi.org/10.3390/app12084034
Acri G, Sansotta C, Salmeri FM, Romeo M, Ruello EV, Denaro L, Testagrossa B. Use of Raman Spectroscopy, Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy in a Multi-Technique Approach for Physical Characterization of Purple Urine Bag Syndrome. Applied Sciences. 2022; 12(8):4034. https://doi.org/10.3390/app12084034
Chicago/Turabian StyleAcri, Giuseppe, Carlo Sansotta, Francesca Maria Salmeri, Marco Romeo, Elisa V. Ruello, Lucia Denaro, and Barbara Testagrossa. 2022. "Use of Raman Spectroscopy, Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy in a Multi-Technique Approach for Physical Characterization of Purple Urine Bag Syndrome" Applied Sciences 12, no. 8: 4034. https://doi.org/10.3390/app12084034
APA StyleAcri, G., Sansotta, C., Salmeri, F. M., Romeo, M., Ruello, E. V., Denaro, L., & Testagrossa, B. (2022). Use of Raman Spectroscopy, Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy in a Multi-Technique Approach for Physical Characterization of Purple Urine Bag Syndrome. Applied Sciences, 12(8), 4034. https://doi.org/10.3390/app12084034






