Natural Salicylaldehyde for Fungal and Pre- and Post-Emergent Weed Control
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
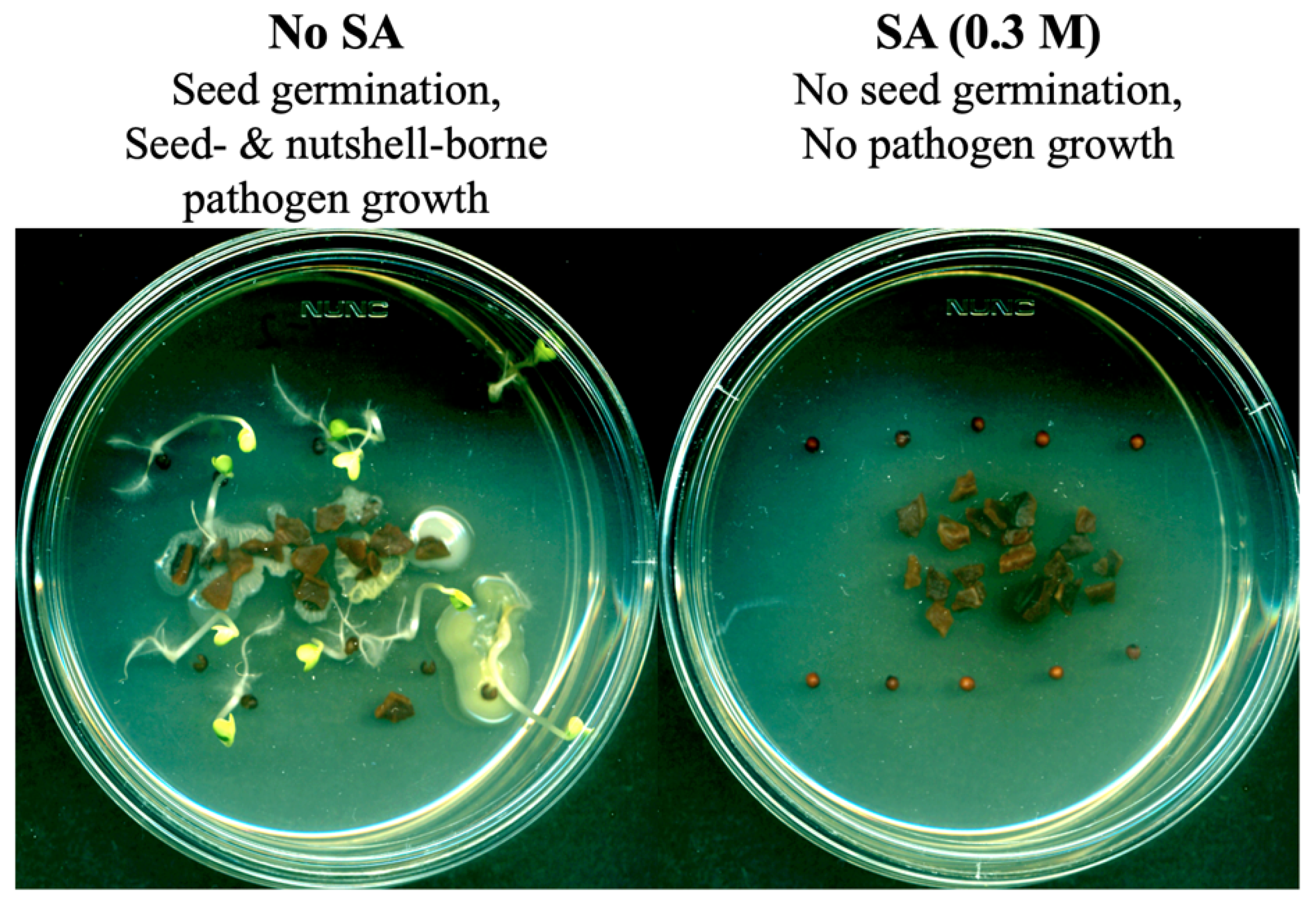
2.1. Testing the Level of Fungal Contamination on the Surfaces of Weed Seeds or Nutshell Particles
2.2. Optimization of Nutshell Particles as Salicylaldehyde (SA) Delivery Vehicles
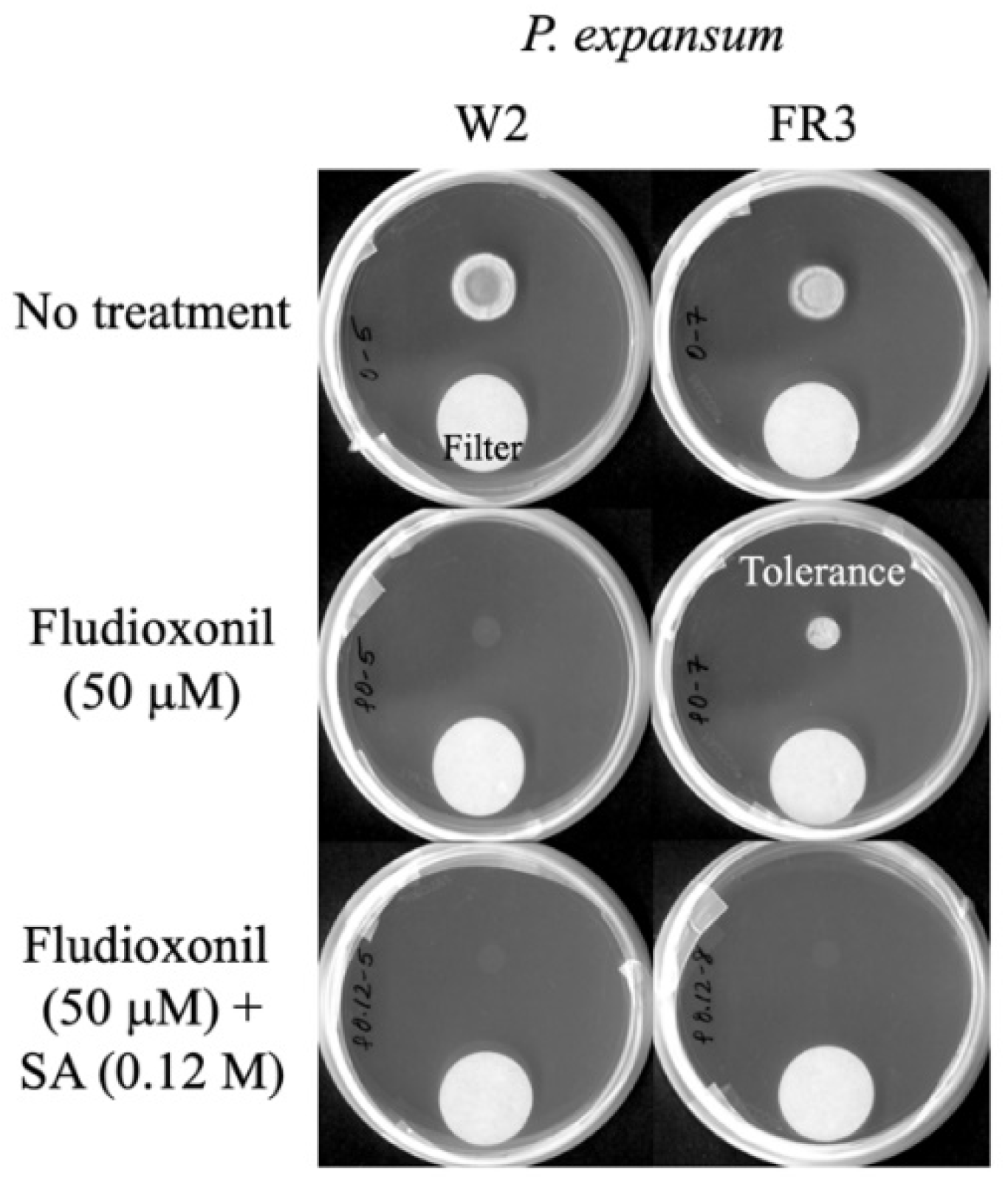
2.3. Overcoming Fludioxonil Tolerance of Mitogen-Activated Protein Kinase (MAPK) Mutants of Fungi
2.4. Antifungal Synergism between SA and Mild Heat (42 °C)
2.5. Pre- and Post-Emergent Herbicidal Activity of SA
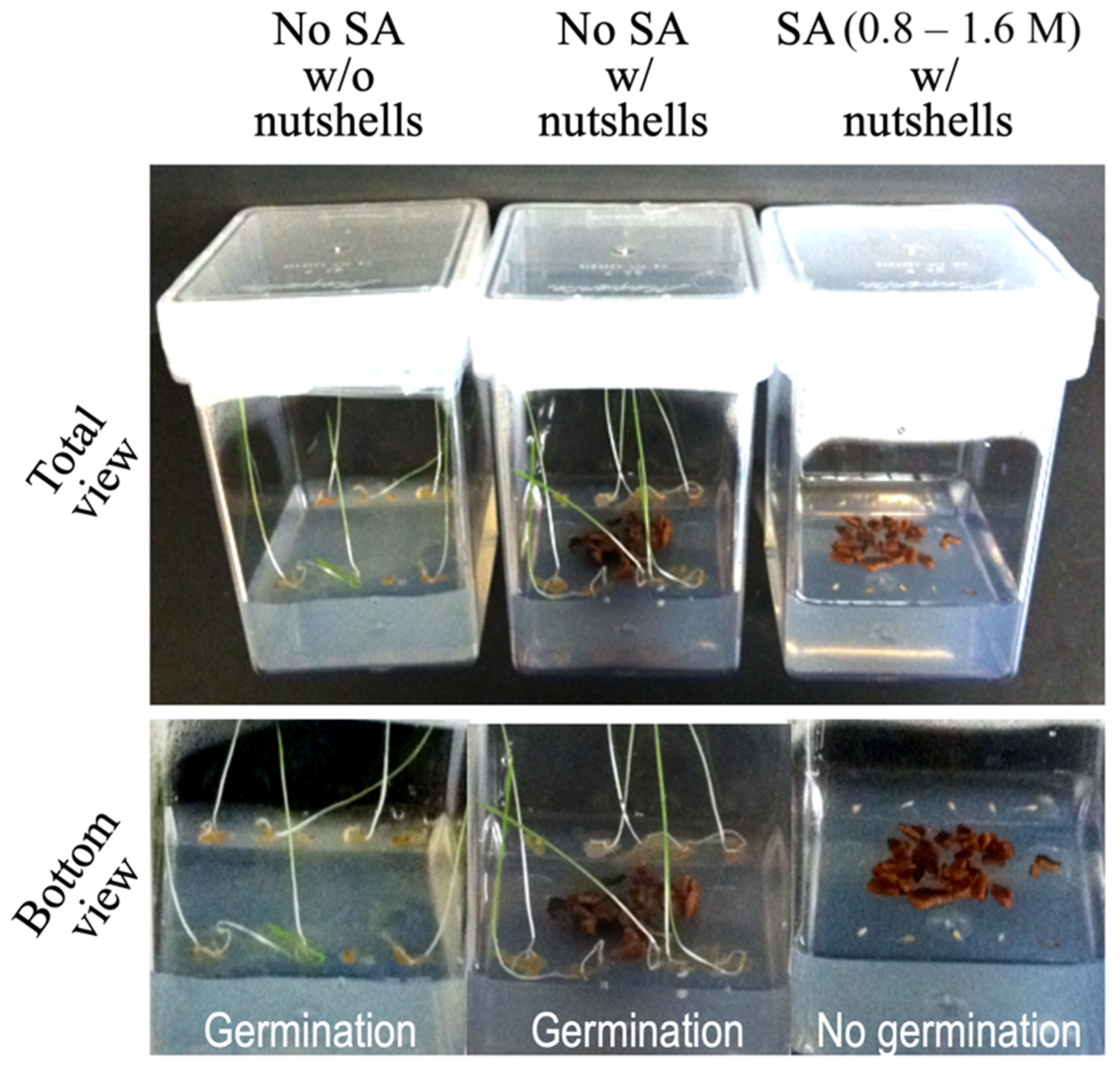
2.5.1. Petri Plate Assay
2.5.2. Magenta Vessel Assay
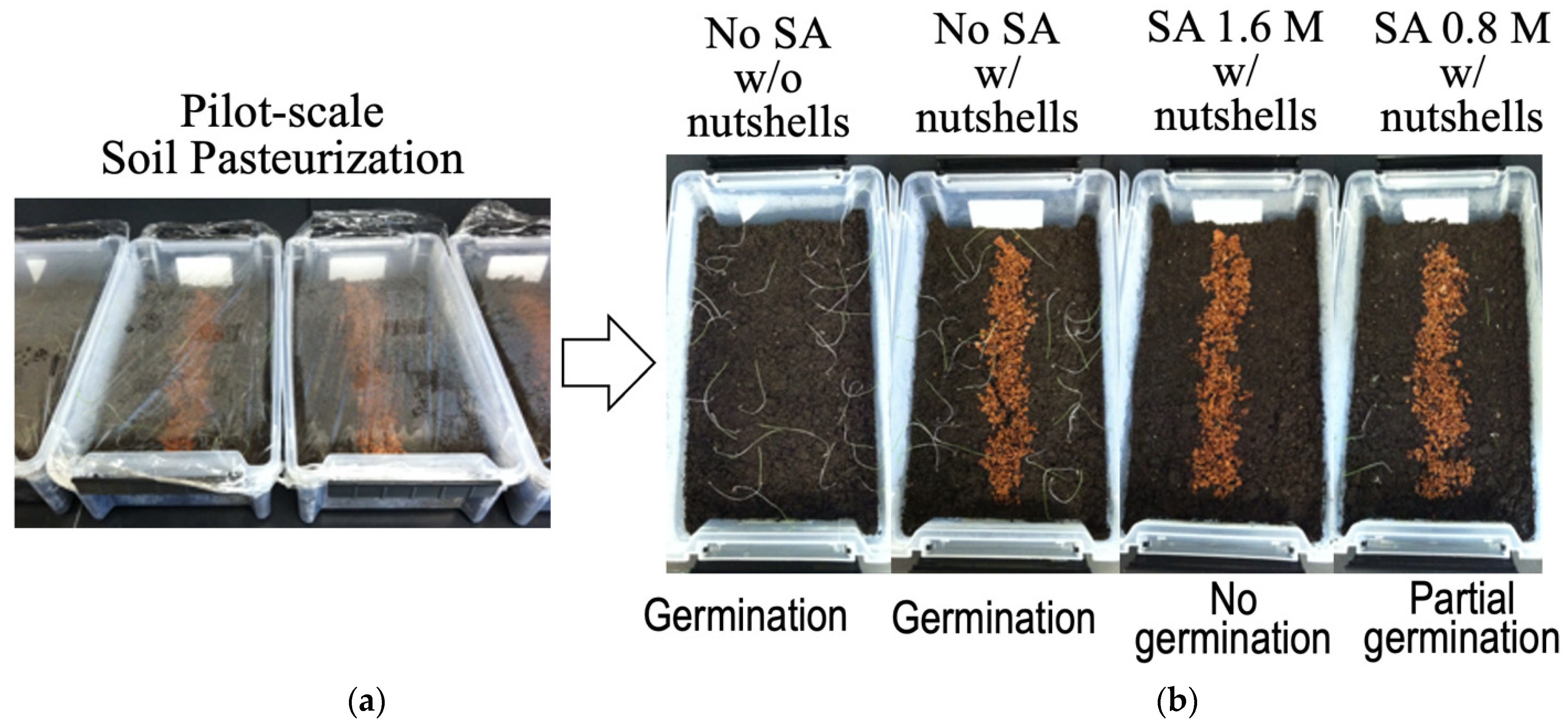
2.6. SA Herbicidal Efficacy in Soil
2.7. Statistical Analysis
3. Results and Discussion
3.1. Agricultural Byproducts (Walnut Shell Particles) as SA Delivery Vehicles: Intrinsic Antifungal Activity of SA as a Fumigant
3.2. Overcoming Fludioxonil Tolerance of Mitogen-Activated Protein Kinase (MAPK) Mutants of Aspergillus fumigatus (sakAΔ, mpkCΔ) or Penicillium expansum (FR2, FR3) by SA
3.3. Pre- and Post-Emergent Herbicidal Activity of SA
3.3.1. Pre-Emergent Herbicidal Activity of SA
3.3.2. Post-Emergent Herbicidal Activity of SA
3.4. SA Herbicidal Efficacy during Soil Pasteurization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reboud, X.; Eychenne, N.; Délos, M.; Folcher, L. Withdrawal of maize protection by herbicides and insecticides increases mycotoxins contamination near maximum thresholds. Agron. Sustain. Dev. 2016, 36, 43. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, B.D.; Wright, S.D.; Sosnoskie, L.M.; Fischer, A.; Jasieniuk, M.A.; Roncoroni, J.A.; Hembree, K.J.; Orloff, S.; Shrestha, A.; Al-Khatib, K. Maintaining long-term management: Herbicide-resistant weeds challenge some signature cropping systems. Calif. Agric. 2014, 68, 142–152. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance glufosinate. Eur. Food Saf. Auth. J. 2005, 3, 27r. [Google Scholar] [CrossRef]
- Anastassiadou, M.; Brancato, A.; Cabrera, L.C.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Evaluation of confirmatory data following the Article 12 MRL review for fludioxonil. EFSA J. 2019, 17, 5812. [Google Scholar] [CrossRef] [Green Version]
- Medline Plus. US National Library of Medicine. Paraquat Poisoning. Available online: https://medlineplus.gov/ency/article/001085.htm (accessed on 1 March 2022).
- Pest Management Regulatory Agency (PMRA) Canada. Re-Evaluation Decision RVD2018-38, Thiram and Its Associated End-use Products. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2018/thiram.html (accessed on 1 March 2022).
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [Green Version]
- University of California Integrated Pest Management (UC IPM). Bee Precaution Pesticide Ratings. Available online: https://www2.ipm.ucanr.edu/beeprecaution/ (accessed on 17 March 2022).
- Davis, A.S.; Frisvold, G.B. Are herbicides a once in a century method of weed control? Pest Manag. Sci. 2017, 73, 2209–2220. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L.; Cheng, L.W.; Tell, L.A.; Byrne, B.A.; Clothier, K.; Land, K.M. High efficiency drug repurposing design for new antifungal agents. Methods Protoc. 2019, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J. Chemosensitization of aflatoxigenic fungi to antimycin A and strobilurin using salicylaldehyde, a volatile natural compound targeting cellular antioxidation system. Mycopathologia 2011, 171, 291–298. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.; Faria, N.C.G.; Martins, M.D.L.; Campbell, B. Targeting the oxidative stress response system of fungi with redox-potent chemosensitizing agents. Front. Microbiol. 2012, 3, 88. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters and acetals (chemical group 23) when used as flavourings for all animal species. EFSA J. 2012, 10, 2785. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Substances Added to Food (Formerly EAFUS). Salicylaldehyde. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FoodSubstances&id=SALICYLALDEHYDE (accessed on 17 March 2022).
- California Department of Food and Agriculture (CDFA). California Agricultural Statistics Review 2017–2018. Available online: https://www.cdfa.ca.gov/statistics/PDFs/2017-18AgReport.pdf (accessed on 17 March 2022).
- Almond Board of California. Using Everything the Orchard Grows: Our Commitment to Zero Waste. Available online: https://www.almonds.com/why-almonds/almond-living-magazine/using-everything-orchard-grows-our-commitment-zero-waste (accessed on 17 March 2022).
- Barbu, M.C.; Sepperer, T.; Tudor, E.M.; Petutschnigg, A. Walnut and hazelnut shells: Untapped industrial resources and their suitability in lignocellulosic composites. Appl. Sci. 2020, 10, 6340. [Google Scholar] [CrossRef]
- Xue, T.; Nguyen, C.K.; Romans, A.; May, G.S. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell 2004, 3, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, G.; Romans, A.; Nguyen, C.K.; May, G.S. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 2006, 5, 1934–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.X.; Xiao, C.L. Characterization of fludioxonil-resistant and pyrimethanil-resistant phenotypes of Penicillium expansum from apple. Phytopathology 2008, 98, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkman, T.W. Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 17 March 2022).
- Abdullah, I.; Ahmad, N.; Hussain, M.; Ahmed, A.; Ahmed, U.; Park, Y.-K. Conversion of biomass blends (walnut shell and pearl millet) for the production of solid biofuel via torrefaction under different conditions. Chemosphere 2022, 295, 133894. [Google Scholar] [CrossRef]
- Figueira, P.; Vale, C.; Pereira, E. Factors influencing sorption of trace elements in contaminated waters onto ground nut shells. J. Environ. Manag. 2022, 308, 114618. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Tong, Z.; Jiang, N.; Chen, W. Adsorption of Rhodamine B from an aqueous solution by acrylic-acid-modified walnut shells: Characterization, kinetics, and thermodynamics. Environ. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Dovi, E.; Aryee, A.A.; Li, J.; Li, Z.; Qu, L.; Han, R. Amine-grafted walnut shell for efficient removal of phosphate and nitrate. Environ. Sci. Pollut. Res. 2022, 29, 20976–20995. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Kashaninejad, M.; Motlagh, M.B. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int. J. Biol. Macromol. 2018, 120, 1216–1224. [Google Scholar] [CrossRef]
- Campbell, B.; Chan, K.; Kim, J.H. Chemosensitization as a means to augment commercial antifungal agents. Front. Microbiol. 2012, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the post-harvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Fludioxonil; Pesticide Tolerances. 87 FR 7388. Available online: https://www.federalregister.gov/d/2022-02560 (accessed on 16 March 2022).
- Kojima, K.; Takano, Y.; Yoshimi, A.; Tanaka, C.; Kikuchi, T.; Okuno, T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 2004, 53, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Young-Matthews, A. Plant Guide for Field Mustard (Brassica rapa var. rapa); USDA-Natural Resources Conservation Service, Corvallis Plant Materials Center: Corvallis, OR, USA, 2012. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_brrar.pdf (accessed on 15 March 2022).
- Adorada, D.L.; Stodart, B.J.; Pangga, I.B.; Ash, G.J. Implications of bacterial contaminated seed lots and endophytic colonization by Pseudomonas fuscovaginae on rice establishment. Plant Pathol. 2015, 64, 43–50. [Google Scholar] [CrossRef]
- Munkvold, G.P. Seed pathology progress in academia and industry. Annu. Rev. Phytopathol. 2009, 47, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; Chase, C.A.; Chellemi, D.O.; Fornari, F. Noxious Weed Control by Soil Solarization. U.S. Patent 6,189,466, 20 February 2001. [Google Scholar]
- Stapleton, J.J.; Wilen, C.A.; Molinar, R.H. Soil Solarization for Gardens & Landscapes Management; University of California, Agricultural and Natural Resources: Davis, CA, USA, 2019; Available online: http://ipm.ucanr.edu/PMG/PESTNOTES/pn74145.html (accessed on 17 March 2022).
- United States Department of Agriculture; Agricultural Research Service. Dr. Duke’s Phytochemical and Ethnobotanical Databases. Salicylaldehyde. Available online: https://phytochem.nal.usda.gov/phytochem/chemicals/show/15858?qlookup=salicylaldehyde&offset=0&max=20&et= (accessed on 17 March 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6998, Salicylaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/salicylaldehyde (accessed on 17 March 2022).
- Kim, J.H.; Chan, K.L.; Mahoney, N.; Campbell, B.C. Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Vogelbacher, U.J.; Eicken, K.; Rheinheimer, J.; Goetz, N.; Harreus, A.; Paul, G.; Westphalen, K.O.; Wuerzer, B. Salicylaldehyde Derivatives and Salicyclic Acid Derivatives and Their Sulfur Analogs and Their Use as Herbicides. U.S. Patent 5,085,686, 4 February 1992. [Google Scholar]
- Andolfi, A.; Cimmino, A.; Vurro, M.; Berestetskiy, A.; Troise, C.; Zonno, M.C.; Motta, A.; Evidente, A. Agropyrenol and agropyrenal, phytotoxins from Ascochyta agropyrina var. nana, a fungal pathogen of Elitrigia repens. Phytochemistry 2012, 79, 102–108. [Google Scholar] [CrossRef]
- Gallardo, M.T.; Martin, B.B.; Martin, D.F. Inhibition of water fern Salvinia minima by cattail (Typha domingensis) extracts and by 2-chlorophenol and salicylaldehyde. J. Chem. Ecol. 1998, 24, 1483–1490. [Google Scholar] [CrossRef]
- Huang, W.; Sun, D.; Wang, R.; An, Y. Integration of transcriptomics and metabolomics reveals the responses of sugar beet to continuous cropping obstacle. Front. Plant Sci. 2021, 12, 711333. [Google Scholar] [CrossRef]
- Caro, A.A.; Commissariat, A.; Dunn, C.; Kim, H.; García, S.L.; Smith, A.; Strang, H.; Stuppy, J.; Desrochers, L.P.; Goodwin, T.E. Prooxidant and antioxidant properties of salicylaldehyde isonicotinoyl hydrazone iron chelators in HepG2 cells. Biochim. Biophys. Acta 2015, 1850, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006, 273, 3106–3117. [Google Scholar] [CrossRef]
- Potůčková, E.; Hrušková, K.; Bureš, J.; Kovarikova, P.; Špirková, I.A.; Pravdíková, K.; Kolbabová, L.; Hergeselová, T.; Haskova, P.; Jansova, H.; et al. Structure-activity relationships of novel salicylaldehyde isonicotinoyl hydrazone (SIH) analogs: Iron chelation, anti-oxidant and cytotoxic properties. PLoS ONE 2014, 9, e112059. [Google Scholar] [CrossRef]
- Guillén, F.; Evans, C.S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994, 60, 2811–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- RASFF—The Rapid Alert System for Food and Feed. Food and Food Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff-food-and-feed-safety-alerts_en (accessed on 15 March 2022).
- Sosnoskie, L. On the Hunt for Alkaliweed (Cressa truxilensis). Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=29855 (accessed on 17 March 2022).


| Fungi | Characteristics | References/Source |
|---|---|---|
| Aspergillus fumigatus AF293 | Human pathogen (aspergillosis), Reference clinical strain | The University of Texas, MD Andersen Cancer Center, Houston, TX, USA [19] |
| A. fumigatus sakAΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | The University of Texas, MD Andersen Cancer Center, Houston, TX, USA [19] |
| A. fumigatus mpkCΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | The University of Texas, MD Andersen Cancer Center, Houston, TX, USA [20] |
| Aspergillus flavus 3357 | Plant pathogen (aflatoxigenic), Human pathogen (aspergillosis), Reference aflatoxigenic strain used for genome sequencing | National Center for Agricultural Utilization and Research, USDA-ARS, Peoria, IL, USA |
| Penicillium expansum W1 | Plant pathogen (Patulin-producing, Parental strain) | Washington State University, Wenatchee, WA, USA [21] |
| P. expansum FR2 | Plant pathogen, Fludioxonil resistant mutant derived from P. expansum W1 | Washington State University, Wenatchee, WA, USA [21] |
| Penicillium expansum W2 | Plant pathogen (Patulin-producing, Parental strain) | Washington State University, Wenatchee, WA, USA [21] |
| P. expansum FR3 | Plant pathogen, Fludioxonil resistant mutant derived from P. expansum W2 | Washington State University, Wenatchee, WA, USA [21] |
| Plant Seeds | Characteristics | References/Source |
| Brassica rapa var. pekinensis (dicot) | Chinese cabbage; field weed | Plant nursery, Oakland, CA, USA |
| Centaurea solstitialis (dicot) (Yellow starthistle) | California invasive weed | USDA-ARS, Albany, CA, USA |
| Salsola tragus (dicot) (Russian thistle) | California invasive weed | USDA-ARS, Albany, CA, USA |
| Genista monspessulana (dicot) (French broom) | California invasive weed | USDA-ARS, Albany, CA, USA |
| Lagurus ovatus (monocot) (Bunny Tails Grass) | Ornamental grass | Plant nursery, Berkeley, CA, USA |
| Schizachyrium scoparium (monocot) (Little Bluestem) | USA native grass | Plant nursery, Berkeley, CA, USA |
| SA (M) | # of Seeds Germinated | # of Seeds Contaminated w/ Fungi | Level of Nutshell Contamination w/ Fungi 1 |
|---|---|---|---|
| 0.00 | 10, 10 | 2, 1 | +++, +++ |
| 0.05 | 4, 3 | 1, 0 | +++, ++ |
| 0.10 | 1, 0 | 1, 0 | +, - |
| 0.15 | 0, 0 | 2, 0 | +, + |
| 0.20 | 0, 0 | 0, 0 | +, - |
| 0.25 | 0, 0 | 1, 0 | +, - |
| 0.30 | 0, 0 | 0, 0 | -, - |
| 0.35 | 0, 0 | 0, 0 | -, - |
| 0.40 | 0, 0 | 0, 0 | -, - |
| 0.45 | 0, 0 | 0, 0 | -, - |
| 0.50 | 0, 0 | 0, 0 | -, - |
| Plants | SA MIC (M) |
|---|---|
| S. scoparium (Little Bluestem) | 0.25 |
| S. tragus (Russian thistle) | 0.40 |
| G. monspessulana (French broom) | 0.40 |
| L. ovatus (Bunny Tails Grass) | 0.80 |
| C. solstitialis (Yellow starthistle) | 1.60 |
| Average | 0.60 ± 0.54 (p = 0.021) 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Chan, K.L. Natural Salicylaldehyde for Fungal and Pre- and Post-Emergent Weed Control. Appl. Sci. 2022, 12, 3749. https://doi.org/10.3390/app12083749
Kim JH, Chan KL. Natural Salicylaldehyde for Fungal and Pre- and Post-Emergent Weed Control. Applied Sciences. 2022; 12(8):3749. https://doi.org/10.3390/app12083749
Chicago/Turabian StyleKim, Jong H., and Kathleen L. Chan. 2022. "Natural Salicylaldehyde for Fungal and Pre- and Post-Emergent Weed Control" Applied Sciences 12, no. 8: 3749. https://doi.org/10.3390/app12083749
APA StyleKim, J. H., & Chan, K. L. (2022). Natural Salicylaldehyde for Fungal and Pre- and Post-Emergent Weed Control. Applied Sciences, 12(8), 3749. https://doi.org/10.3390/app12083749







