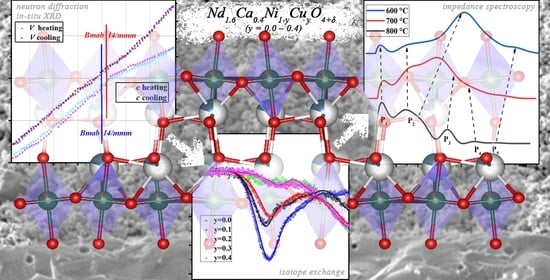
High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Electrode Materials and Their Standard Characterization
2.2. Synthesis of the Electrolite Materials and Preparation of Dense Electrolyte Substrates for the Electrochemical Studies. Chemical Electrode/Electrolyte Compatibility Studies
2.3. High-Resolution Neutron Diffraction and In-Situ XRD of the Electrode Powders
- (1)
- as-synthesized samples, in static air;
- (2)
- samples after heating in static air to 500 °C, annealing at this temperature for 2 h and cooling down to 50 °C with a heating/cooling rate of 300 °C h−1, in static air;
- (3)
- samples after (2), in a vacuum (10−6 bar);
- (4)
- samples after heating in a vacuum to 500 °C, annealing at this temperature for 2 h, heating to 800 °C annealing for 2 h, and cooling down to 50 °C, with a heating/cooling rate of 300 °C h−1.
2.4. X-ray Photoelectron Spectroscopy Studies
2.5. Oxygen Mobility Studies
2.6. Electrode Fabrication and Electrochemical Studies
3. Results and Discussion
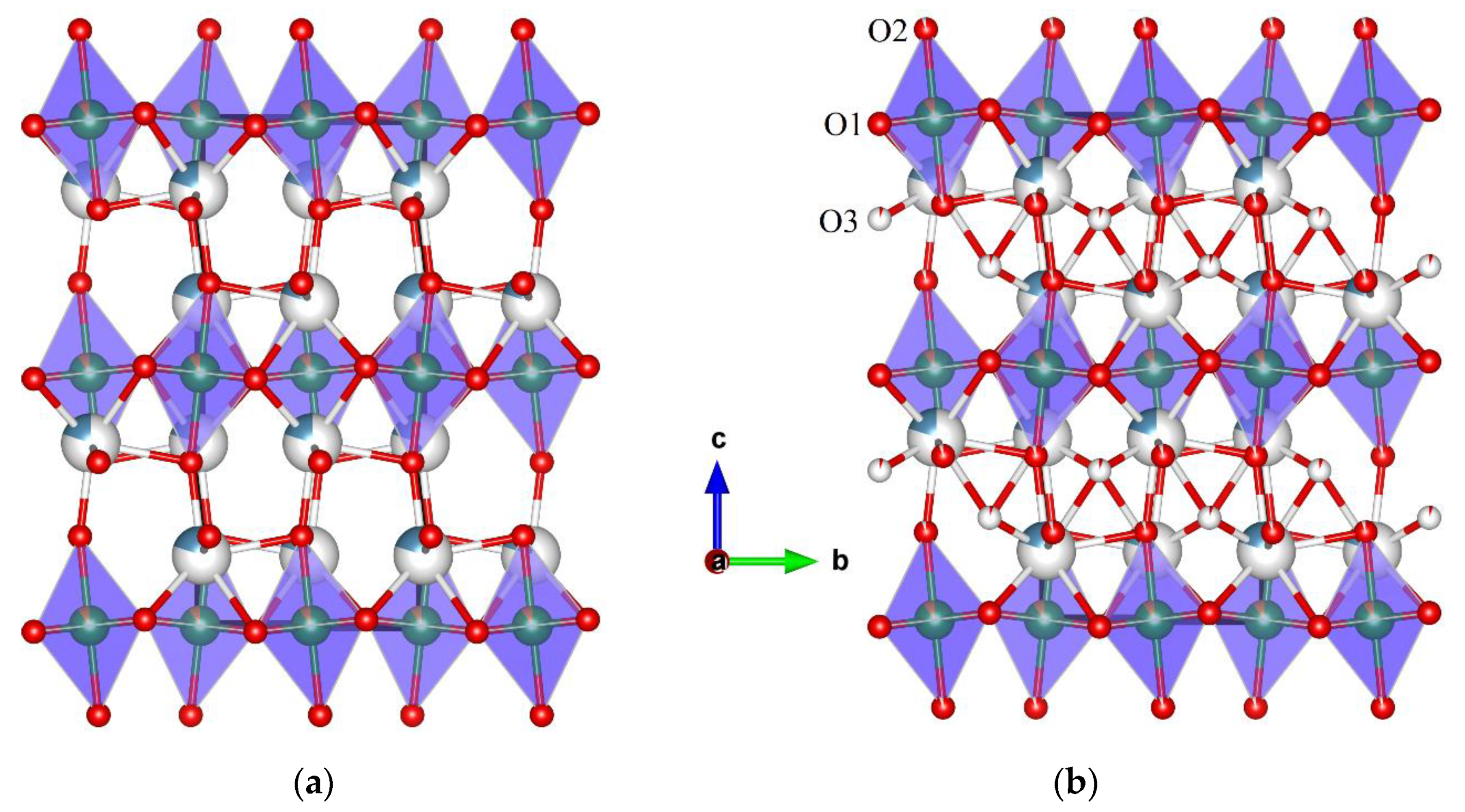
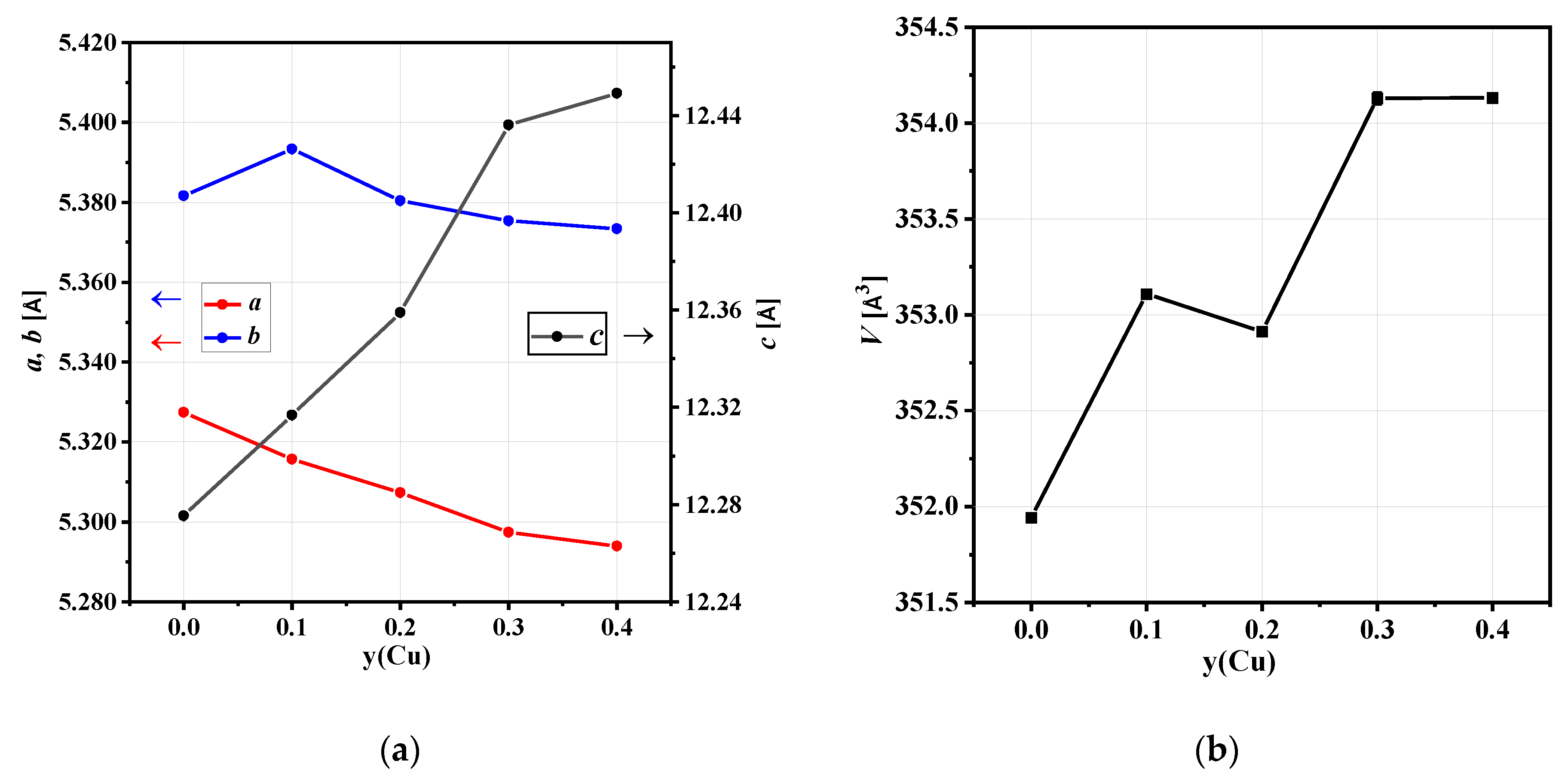
3.1. High-Resolution Neutron Powder Diffraction
3.1.1. NCNCO01
3.1.2. NCNCO03
3.1.3. NCNCO04
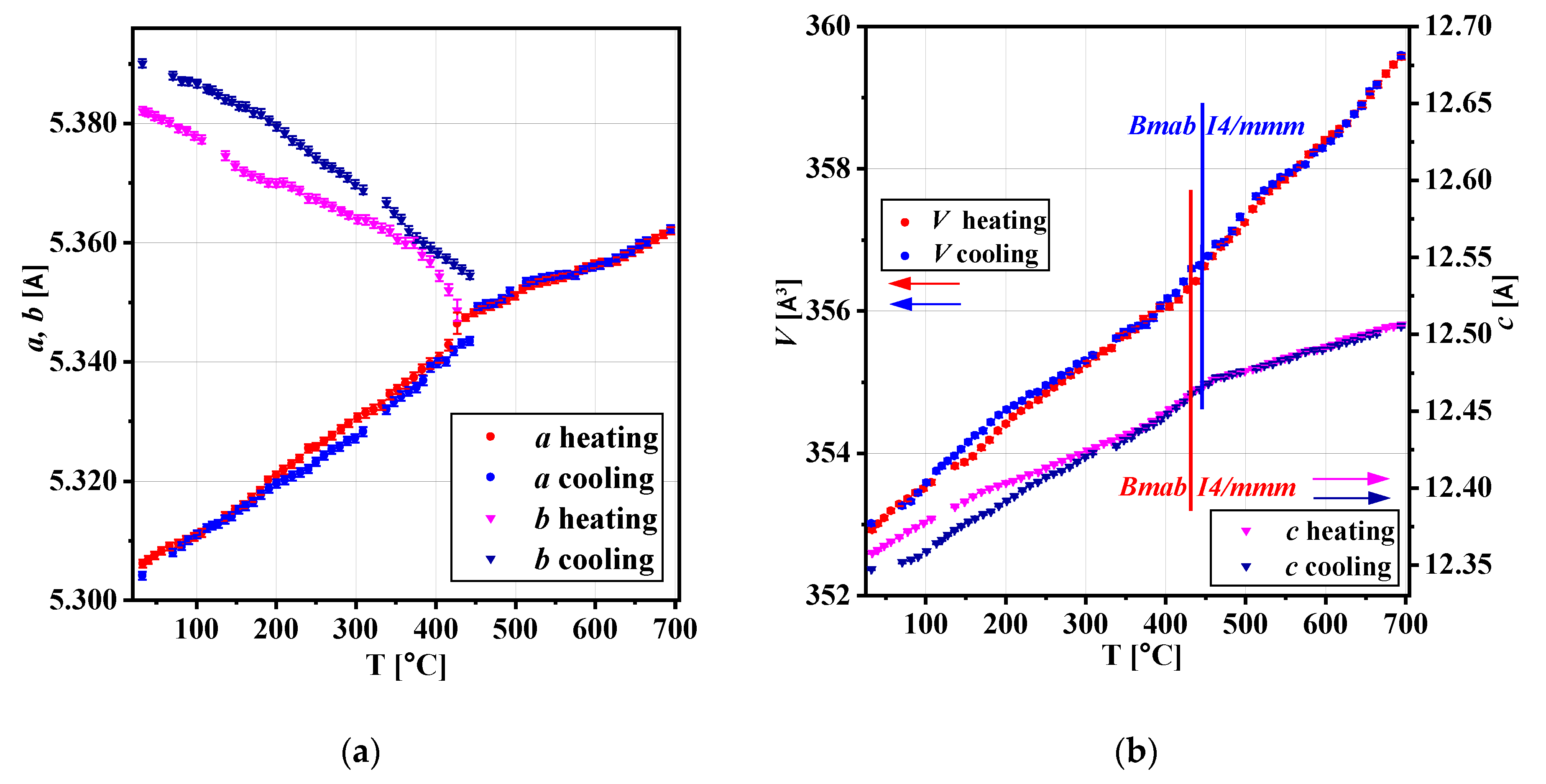
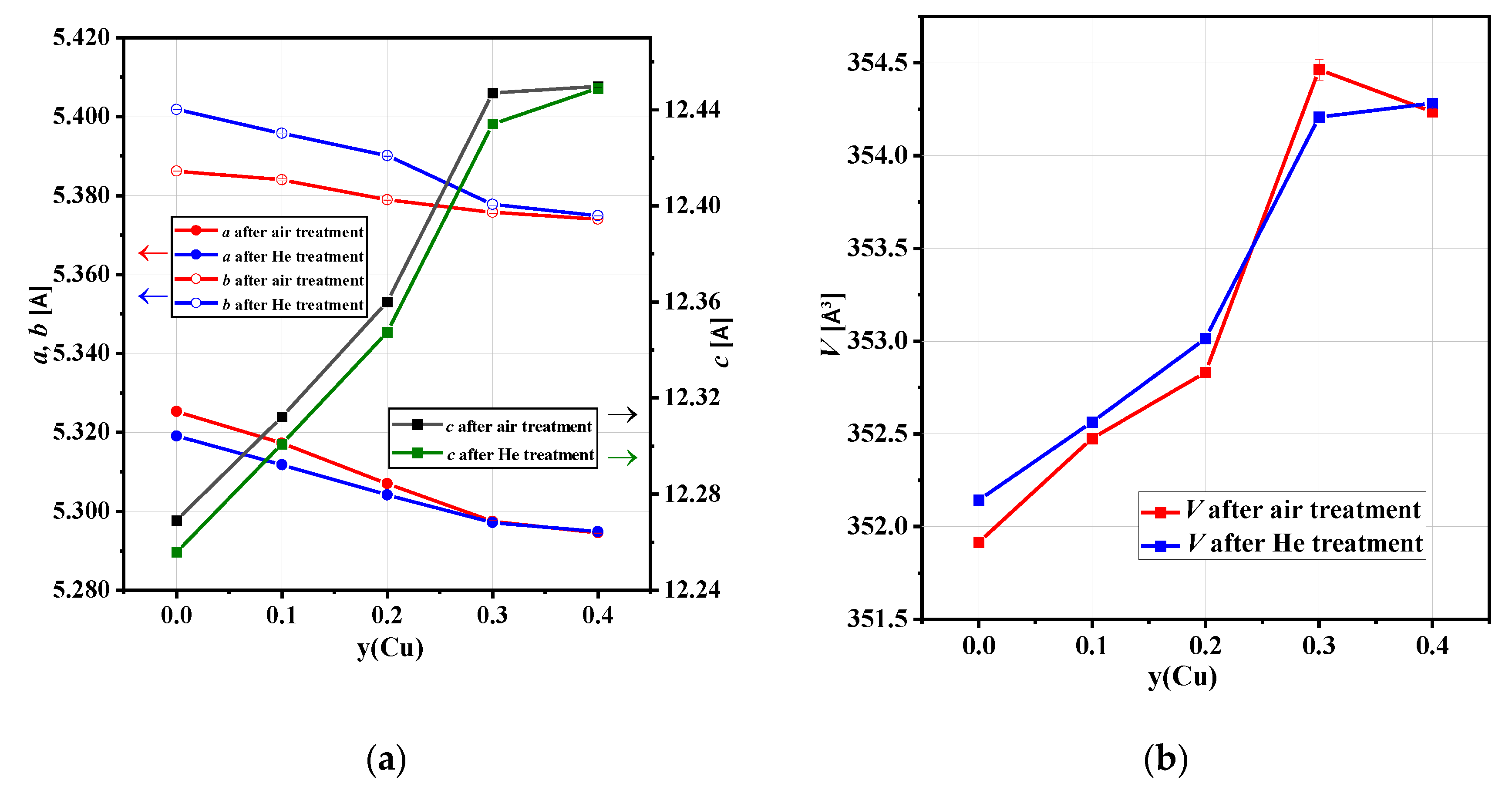
3.2. In-Situ XRD Studies of the Electrode Materials
3.3. XPS Studies of the Electrode Materials
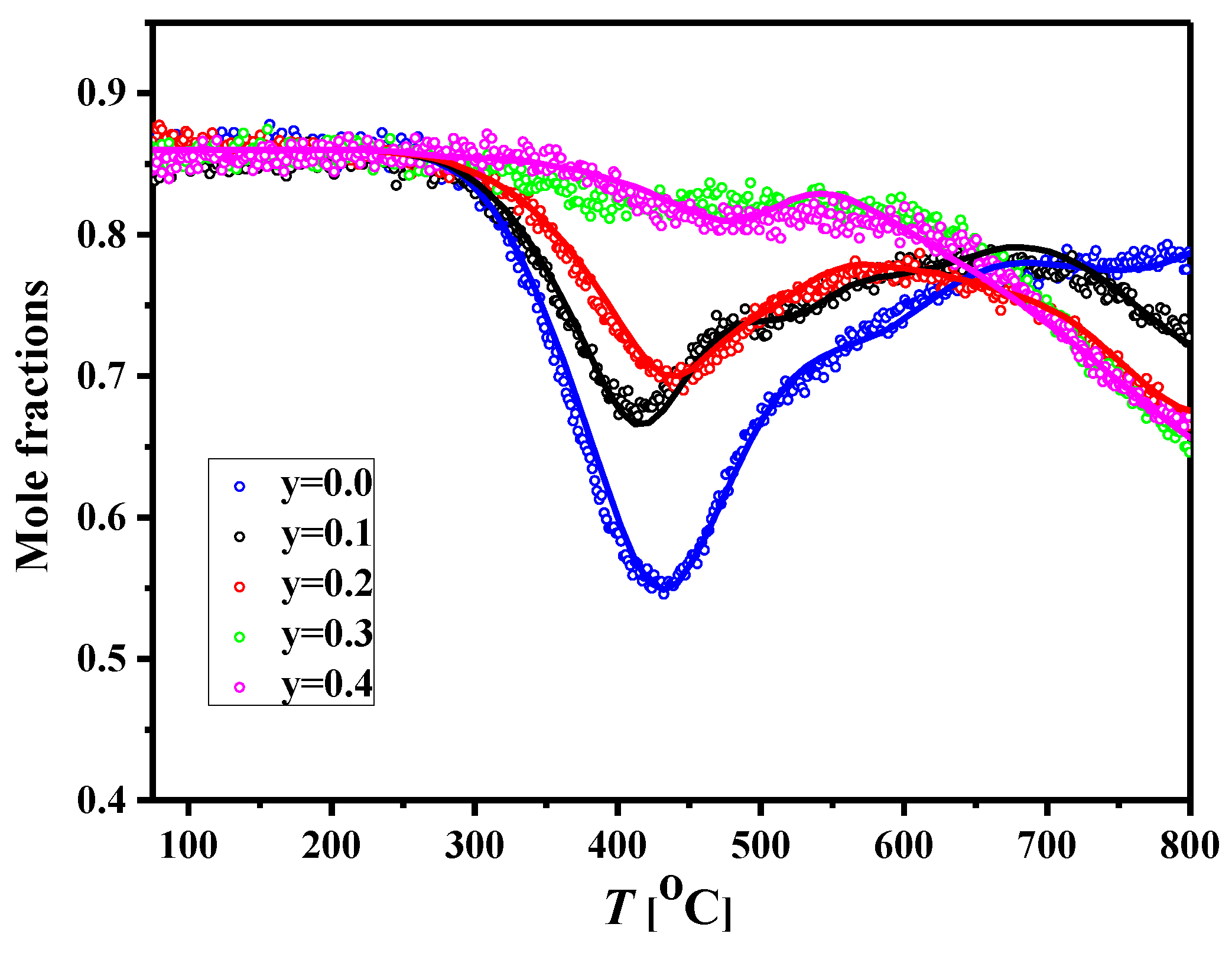
3.4. Oxygen Mobility in the NCNCO Materials
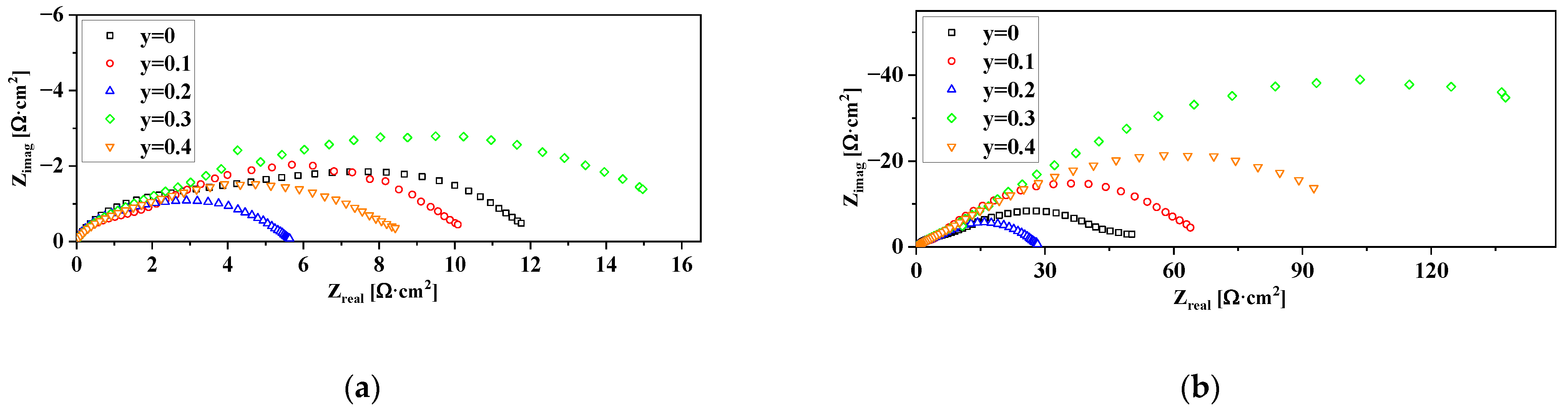
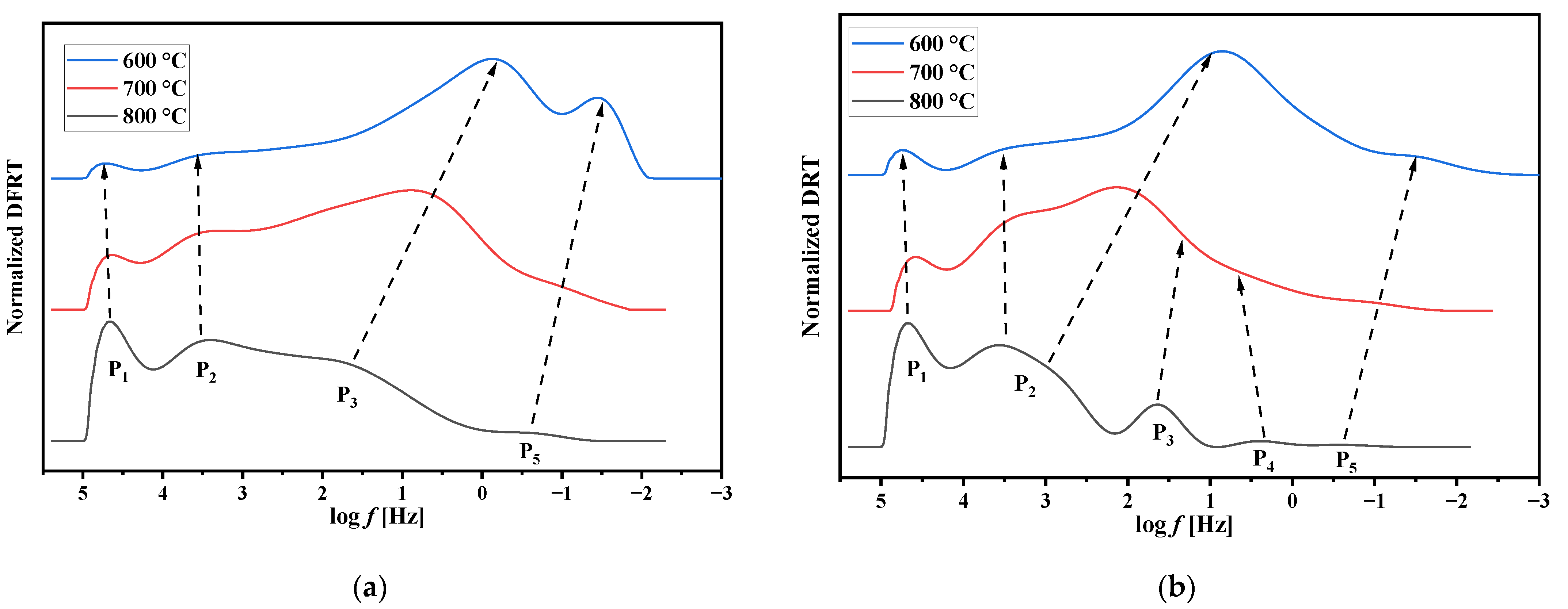
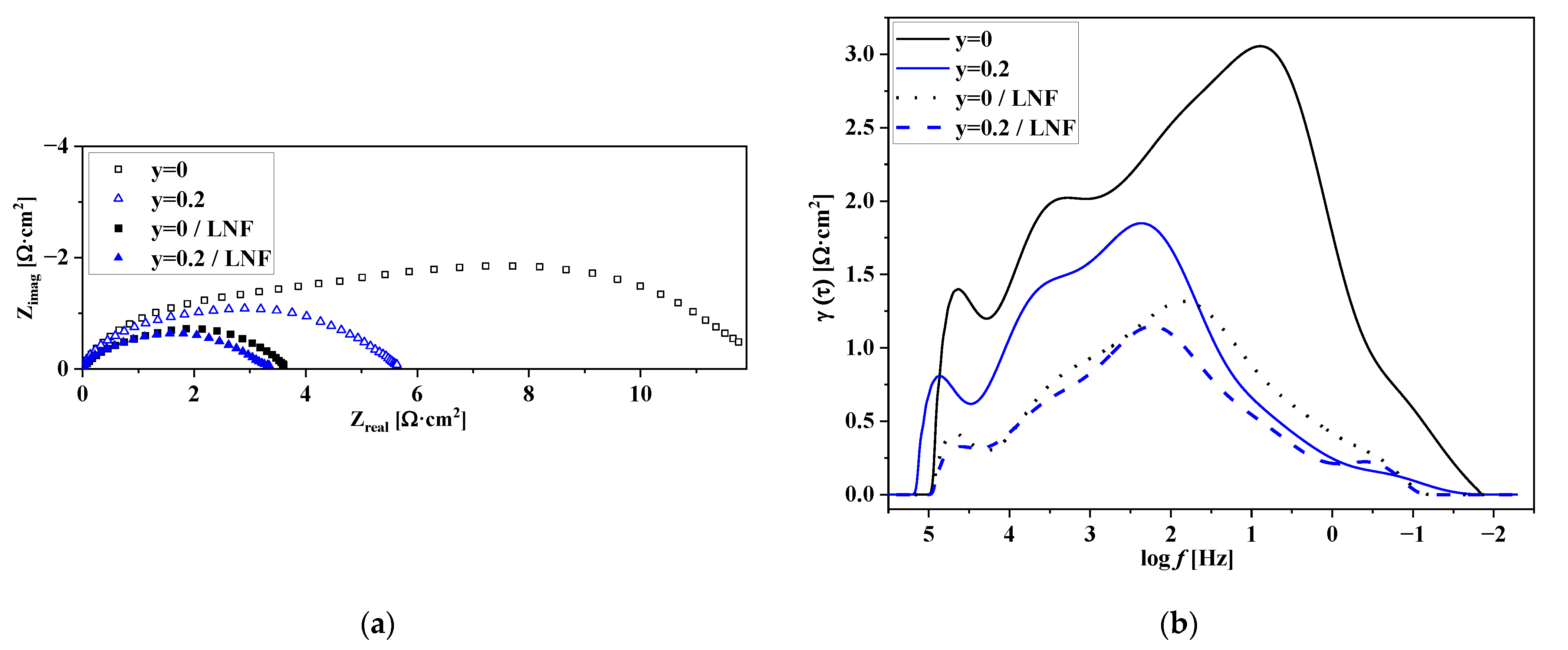
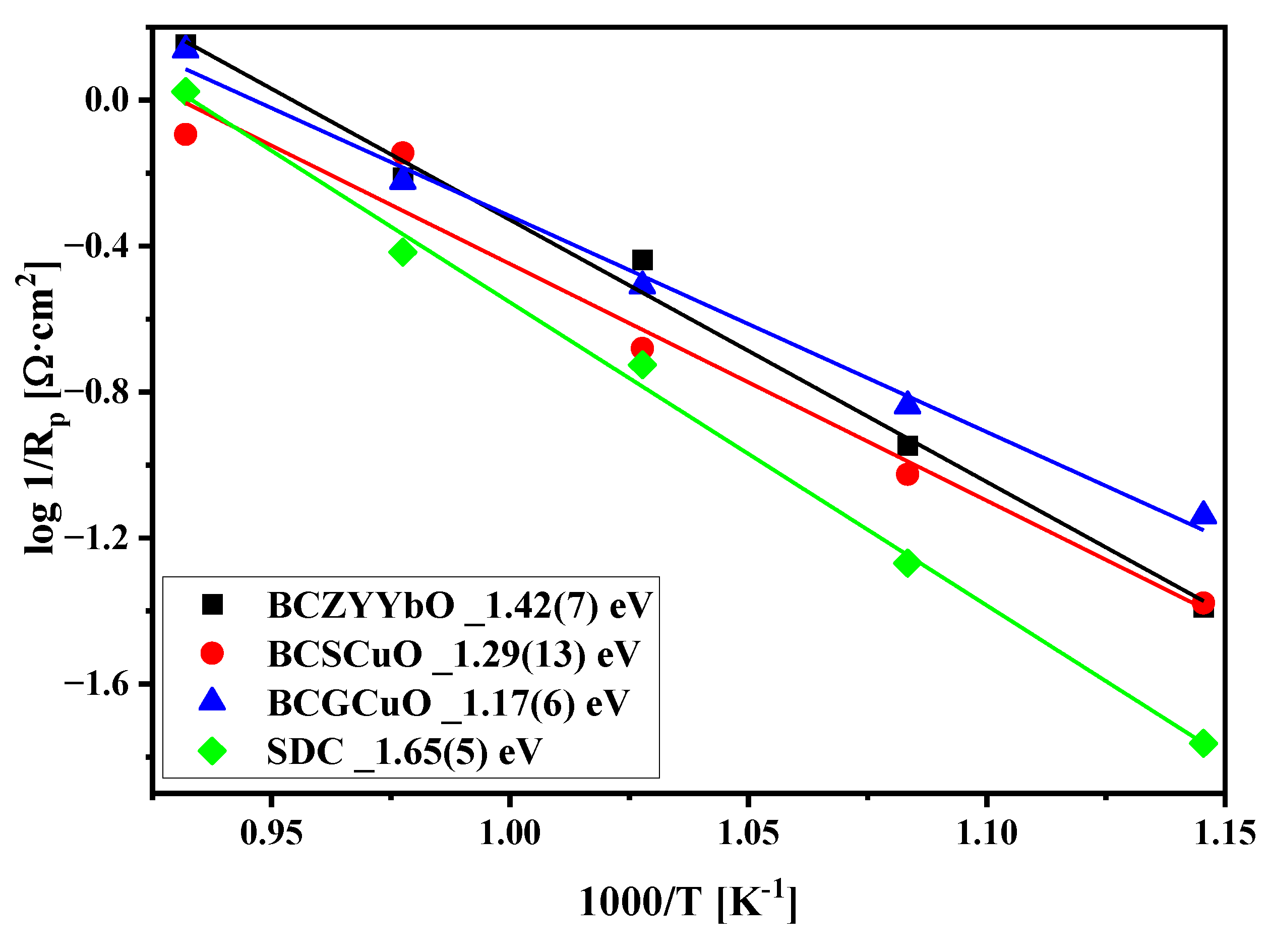
3.5. Electrochemical Performance of the Electrodes with Nd1.6Ca0.4Ni1−yCuyO4+δ Functional Layers. Factors Influencing the Electrode Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunnarsdottir, I.; Davidsdottir, B.; Worrell, E.; Sigurgeirsdottir, S. Sustainable energy development: History of the concept and emerging themes. Renew. Sustain. Energy Rev. 2021, 141, 110770. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable energy in the sustainable development of electrical power sector: A review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen economy and sustainable development goals: Review and policy insight. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alim, M.A.; Alam, T.; Mofijur, M.; Ahmed, S.F.; Perkins, G. A critical review on the development and challenges of concentrated solar power technologies. Sustain. Energy Technol. Assess. 2021, 47, 101434. [Google Scholar] [CrossRef]
- Al-Qadami, E.H.H.; Mustaffa, Z.; Al-Atroush, M.E. Evaluation of the pavement geothermal energy harvesting technologies towards sustainability and renewable energy. Energies 2022, 15, 1201. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Datta, R.; Kumar, M.P.; Davim, J.P.; Pramanik, S. Energy conversion strategies for wind energy system: Electrical, mechanical and material aspects. Materials 2022, 15, 1232. [Google Scholar] [CrossRef]
- Grande, L.; Pedroarena, I.; Korili, S.A.; Gil, A. Hydrothermal liquefaction of biomass as one of the most promising alternatives for the synthesis of advanced liquid biofuels: A review. Materials 2021, 14, 5286. [Google Scholar] [CrossRef] [PubMed]
- Azimov, U.; Okoro, V.; Hernandez, H.H. Recent progress and trends in the development of microbial biofuels from solid waste—A review. Energies 2021, 14, 6011. [Google Scholar] [CrossRef]
- Sasaki, T.; Harada, N.; Kikuchi, T.; Takahashi, K. Review of energy conversion technology using magnetohydrodynamics. IEEJ Trans. Power Energy 2016, 136, 769–772. [Google Scholar] [CrossRef]
- Mamur, H.; Üstüner, M.A.; Bhuiyan, M.R.A. Future perspective and current situation of maximum power point tracking methods in thermoelectric generators. Sustain. Energy Technol. Assess. 2022, 50, 101824. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive review on fuel cell technology for stationary applications as sustainable and efficient poly-generation energy systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Sources 2021, 492, 229630. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Kaleem Ullah, M.; Usman, A. A brief description of high temperature solid oxide fuel cell’s operation, materials, design, fabrication technologies and performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Wang, F.; Lyu, Y.; Chu, D.; Jin, Z.; Zhang, G.; Wang, D. The electrolyte materials for SOFCs of low-intermediate temperature: Review. Mater. Sci. Technol. 2019, 35, 1551–1562. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Shao, Z.; Zhou, W.; Zhu, Z. Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog. Mater. Sci. 2012, 57, 804–874. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, X.; Zhu, T.; Sun, S.; Jia, L.; Li, J. Review on core-shell structured cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 23160–231723. [Google Scholar] [CrossRef]
- Mather, G.C.; Muñoz-Gil, D.; Zamudio-García, J.; Porras-Vázquez, J.M.; Marrero-López, D.; Pérez-Coll, D. Perspectives on cathodes for protonic ceramic fuel cells. Appl. Sci. 2021, 11, 5363. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Tarutina, L.R.; Rudenko, A.O.; Lyagaeva, J.G.; Medvedev, D.A. Ba(Ce,Zr)O3-based electrodes for protonic ceramic electrochemical cells: Towards highly compatible functionality and triple-conducting behavior. Russ. Chem. Rev. 2020, 89, 667–692. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. 2020, 9, 154–195. [Google Scholar] [CrossRef]
- Flura, A.; Dru, S.; Nicollet, C.; Vibhu, V.; Fourcade, S.; Lebraud, E.; Grenier, J.C. Chemical and structural changes in Ln2NiO4+δ (Ln = La, Pr or Nd) lanthanide nickelates as a function of oxygen partial pressure at high temperature. J. Solid State Chem. 2015, 228, 189–198. [Google Scholar] [CrossRef]
- Philippeau, B.; Mauvy, F.; Mazataud, C.; Fourcade, S.; Grenier, J.C. Comparative study of electrochemical properties of mixed conducting Ln2NiO4+δ (Ln = La, Pr and Nd) and La0.6Sr0.4Fe0.8Co0.2O3−δ as SOFC cathodes associated to CeGdO2−δ, La0.8Sr0.2Ga0.8Mg0.2O3−δ and La9Sr1Si6O26.5 electrolytes. Solid State Ion. 2012, 249–250, 17–25. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Bogdanovich, N.M. Structure, transport properties and electrochemical behavior of the layered lanthanide nickelates doped with calcium. Int. J. Hydrog. Energy 2018, 43, 17373–17386. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Pikalova, E.Y.; Kolchugin, A.A.; Fetisov, A.V.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Goncharov, V.B.; Krasnov, A.V.; Skriabin, P.I.; et al. Transport properties of Ca-doped Ln2NiO4 for intermediate temperature solid oxide fuel cells cathodes and catalytic membranes for hydrogen production. Int. J. Hydrog. Energy 2020, 45, 13625–13642. [Google Scholar] [CrossRef]
- Pikalova, E.; Sadykov, V.; Sadovskaya, E.; Eremeev, N.; Kolchugin, A.; Shmakov, A.; Vinokurov, Z.; Mishchenko, D.; Filonova, E.; Belyaev, V. Correlation between structural and transport properties of Ca-doped La nickelates and their electrochemical performance. Crystals 2021, 11, 297. [Google Scholar] [CrossRef]
- Takeda, Y.; Nishijima, M.; Imanishi, N.; Kanno, R. Crystal chemistry and transport properties of Nd2−xAxNiO4 (A = Ca, Sr, Ba, 0 < x < 1.4). J. Solid State Chem. 1991, 96, 72–93. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Filonova, E.; Bogdanovich, N.; Pikalov, S.; Ananyev, M.; Molchanova, N.; Farlenkov, A. Validation of calcium-doped neodymium nickelates as SOFC air electrode materials. Solid State Ion. 2018, 319, 130–140. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Sadykov, V.A.; Filonova, E.A.; Eremeev, N.F.; Sadovskaya, E.M.; Kolchugin, A.A.; Lyagaeva, J.G.; Vedmid, L.B.; Ishchenko, A.V.; Goncharov, V.B. Structure, oxygen transport properties and electrode performance of Ca-substituted Nd2NiO4. Solid State Ion. 2019, 335, 53–60. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.A.; Escudero, M.J.; Daza, L. Evaluation of the La2Ni1−xCuxO4+δ system as SOFC cathode material with 8YSZ and LSGM as electrolytes. Solid State Ionics. 2008, 179, 393–400. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Farlenkov, A.S.; Vylkov, A.I.; Medvedev, D.M. Cu-substituted La2NiO4+δ as oxygen electrodes for protonic ceramic electrochemical cells. Ceram. Intern. 2019, 45, 16105–16112. [Google Scholar] [CrossRef]
- Lee, K.J.; Choe, Y.; Hwang, H. Properties of copper doped neodymium nickelate oxide as cathode material for solid oxide fuel cells. J. Arch. Metall. Mater. 2016, 61, 625–628. [Google Scholar] [CrossRef]
- Wang, C.; Soga, H.; Okiba, T.; Niwa, E.; Hashimoto, T. Construction of structural phase diagram of Nd2Ni1−xCuxO4+δ and effect of crystal structure and phase transition on electrical conduction behaviour. Mat. Res. Bull. 2019, 111, 61–69. [Google Scholar] [CrossRef]
- Sakai, M.; Wang, C.; Okiba, T.; Soga, H.; Niwa, E.; Hashimoto, T. Thermal analysis of structural phase transition behavior of Ln2Ni1−xCuxO4+δ (Ln = Nd, Pr) under various oxygen partial pressures. J. Therm. Anal. Calor. 2019, 135, 2765–2774. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, H.; Swierczek, K.; Du, Z.; Xie, Z. Lattice structure, sintering behavior and electrochemical performance La1.7Ca0.3Ni1−xCuxO4+δ as cathode material for intermediate temperature solid oxide fuel cell. J. Power Sources. 2013, 240, 759–765. [Google Scholar] [CrossRef]
- Huang, X.; Shin, T.X.; Zhou, J.; Irvine, J.T.S. Hierarchically nanoporous La1.7Ca0.3CuO4−δ and La1.7Ca0.3NixCu1−xO4−δ (0.25 ≤ x ≤ 0.75) as potential cathode materials for IT-SOFCs. J. Mater. Chem. 2015, 25, 13468–13475. [Google Scholar] [CrossRef]
- Myung, J.; Huang, X.; Savaniu, C.; Irvine, J. La1.7Ca0.3Ni0.75Cu0.25O4+δ layered perovskite as cathode on La0.9Sr0.1Ga0.8Mg0.2O3 or Ce0.8Gd0.2O2 electrolyte for intermediate temperature solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2016, 712, 269–273. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Zakharov, D.M.; Cherepanov, V.A. Effect of calcium and copper/iron co–doping on defect–induced properties of La2NiO4-based materials. J. Alloys Compd. 2018, 27, 491–501. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Gilev, A.; Medvedev, D.; Pikalova, N.; Guseva, E.; Pikalov, S.; Maignan, A.; Filonova, E. Physico-chemical properties and electrochemical performance of La1.7Ca0.3Ni1−xCuxO4+δ as cathodes for intermediate temperature solid oxide fuel cells. In Proceedings of the International Conference Hypothesis XVI, Muscat, Oman, 15–17 February 2021; p. 66. [Google Scholar]
- Filonova, E.A.; Pikalova, E.Y.; Maksimchuk, T.Y.; Vylkov, A.I.; Pikalov, S.M.; Maignan, A. Crystal structure and functional properties of Nd1.6Ca0.4Ni1−yCuyO4+δ as prospective cathode materials for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 17037–17050. [Google Scholar] [CrossRef]
- FullProf Suite. Crystallographic Tools for Rietveld, Profile Matching and Integrated-Intensity Refinements of X-ray and/or Neutron Data. Available online: https://www.ill.eu/sites/fullprof/ (accessed on 11 March 2022).
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Brouzgou, A.; Bronin, D.; Plaksin, S.V.; Khasanov, A.S.; Tsiakaras, P. Effect of nature of the ceramic component of the composite electrodes based on La1.7Ca(Sr)0.3NiO4+δ on their electrochemical performance. ECS Trans. 2015, 68, 809–815. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Ermakova, L.; Farlenkov, A.; Bronin, D. Methods to increase electrochemical activity of lanthanum nickelate-ferrite electrodes for intermediate and low temperature SOFCs. Int. J. Hydrog. Energy 2021, 46, 35923–35937. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Bamburov, V.G. Ceria-based materials for high-temperature electrochemistry applications. Int. J. Energy Prod. Mgmt. 2016, 1, 272–283. [Google Scholar] [CrossRef][Green Version]
- Kalinina, E.; Shubin, K.; Pikalova, E. Electrophoretic deposition and characterization of the doped BaCeO3 barrier layers on a supporting Ce0.8Sm0.2O1.9 solid-state electrolyte. Membranes 2022, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.; Pikalova, E.; Kolchugin, A.; Pikalova, N.; Farlenkov, A. Comparative study of electrophoretic deposition of doped BaCeO3-based films on La2NiO4+δ and La1.7Ba0.3NiO4+δ cathode substrates. Materials 2019, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Filonova, E.A.; Tokareva, E.S.; Pikalova, N.S.; Vylkov, A.I.; Bogdanovich, N.M.; Pikalova, E.Y. Assessment of prospective cathodes based on (1−x)Ca3Co4O9+δ-xBaCe0.5Zr0.3Y0.1Yb0.1O3−δ composites for protonic ceramic electrochemical cells. J. Solid State Electrochem. 2020, 24, 1509–1521. [Google Scholar] [CrossRef]
- Balagurov, A.; Balagurov, D.; Bobrikov, I.; Bogdzel, A.; Drozdov, V.; Kirilov, A.; Kruglov, V.; Kulikov, S.; Murashkevich, S.; Prikhodko, V. High-resolution neutron Fourier diffractometer at the IBR-2 pulsed reactor: A new concept. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 436, 263–271. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Bobrikov, I.A.; Bokuchava, G.D.; Zhuravlev, V.V.; Simkin, V.G. Correlation Fourier diffractometry: 20 years of experience at the IBR-2 reactor. Phys. Part. Nuclei. 2015, 46, 249–276. [Google Scholar] [CrossRef]
- Piminov, P.A.; Baranov, G.N.; Bogomyagkov, A.V.; Berkaev, D.E.; Borin, V.M.; Dorokhov, V.L.; Karnaev, S.E.; Kiselev, V.A.; Levichev, E.B.; Meshkov, O.I.; et al. Synchrotron radiation research and application at VEPP-4. Phys. Procedia 2016, 84, 19–26. [Google Scholar] [CrossRef]
- Aulchenko, V.M.; Evdokov, O.V.; Kutovenko, V.D.; Pirogov, B.Y.; Sharafutdinov, M.R.; Titov, V.M.; Tolochko, B.P.; Vasiljev, A.V.; Zhogin, I.A.; Zhulanov, V.V. One-Coordinate X-ray Detector OD-3M. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 603, 76–79. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Skriabin, P.I.; Krasnov, A.V.; Bespalko, Y.N.; Pavlova, S.N.; Fedorova, Y.E.; Pikalova, E.Y.; Shlyakhtina, A.V. Oxygen mobility in the materials for solid oxide fuel cells and catalytic membranes (Review). Russ. J. Electrochem. 2019, 55, 701–718. [Google Scholar] [CrossRef]
- SaezPuche, R.; Fernández, F.; Rodríguez Carvajal, J.; Martínez, J.L. Magnetic and X-ray diffraction characterization of stoichiometric Pr2NiO4 and Nd2NiO4 oxides. Solid State Comm. 1989, 72, 273–277. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J.; Fernández-Díaz, M.T.; Martínez, J.L.; Fernández, F.; Saez-Puche, R. Structural phase transitions and three-dimensional magnetic ordering in the Nd2NiO4 Oxide. Europhys. Lett. 1990, 11, 261–268. [Google Scholar] [CrossRef]
- Nakamura, T.; Yashiro, K.; Sato, K.; Mizusaki, J. Oxygen nonstoichiometry and defect equilibrium in La2−xSrxNiO4+δ. Solid State Ion. 2009, 180, 368–376. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Pikalova, E.Y.; Kolchugin, A.A.; Filonova, E.A.; Pikalov, S.M.; Eremeev, N.F.; Ishchenko, A.V.; Lukashevich, A.I.; Bassat, J.M. Transport features in layered nickelates: Correlation between structure, oxygen diffusion, electrical and electrochemical Properties. Ionics 2018, 24, 1181–1193. [Google Scholar] [CrossRef]
- Minervini, L.; Grimes, R.W.; Kilner, J.A.; Sickafus, K.E. Oxygen migration in La2NiO4+δ. J. Mater. Chem. 2000, 10, 2349–2354. [Google Scholar] [CrossRef]
- Nakamura, T.; Oike, R.; Ling, Y.; Tamenori, Y.; Amezawa, K. The determining factor for interstitial oxygen formation in Ruddlesden–Popper Type La2NiO4-based oxides. Phys. Chem. Chem. Phys. 2016, 18, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ling, Y.; Amezawa, K. The effect of interstitial oxygen formation on the crystal lattice deformation in layered perovskite oxides for electrochemical devices. J. Mater. Chem. 2015, 3, 10471–10479. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Okada, K.; Kotani, A. Large-cluster effects on core-level photoemission spectra of quasi-one-dimensional copper compounds. J. Elect. Spectrosc. Relat. Phenom. 1998, 88–91, 255–260. [Google Scholar] [CrossRef]
- Zeng, D.W.; Yung, K.C.; Xie, C.S. UV Nd: YAG laser ablation of copper: Chemical states in both crater and halo studied by XPS. Appl. Surf. Sci. 2003, 217, 170–180. [Google Scholar] [CrossRef]
- Matienzo, J.; Yin, L.I.; Grim, S.O.; Swartz, W.E., Jr. X-ray photoelectron spectroscopy of nickel compounds. Inorg. Chem. 1973, 12, 2762–2769. [Google Scholar] [CrossRef]
- Roberts, M.W.; Smart, R.S.C. The defect structure of nickel oxide surfaces as revealed by photoelectron spectroscopy. J. Chem. Soc. Faraday 1984, 1, 2957–2968. [Google Scholar] [CrossRef]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. The formation and characterisation of Ni3+—An X-ray photoelectron spectroscopic investigation of potassium-doped Ni(110)–O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Payne, B.P.; Grosvenor, A.P.; Biesinger, M.C.; Kobe, B.A.; McIntyre, N.S. Structure and growth of oxides on polycrystalline nickel surfaces. Surf. Interface Anal. 2007, 39, 582–592. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Belyaev, V.D.; Tsvinkinberg, V.A.; Pikalova, E.Y. Oxide ionic transport features in Gd-doped La nickelates. Solid State Ion. 2020, 357, 115462. [Google Scholar] [CrossRef]
- Fedorova, V.; Simonov, M.; Valeev, K.; Bespalko, Y.; Smal, E.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Ishchenko, A.; Sadykov, V. Kinetic regularities of methane dry reforming reaction on nickel-containing modified ceria–zirconia. Energies 2021, 140, 2973. [Google Scholar] [CrossRef]
- Miyoshi, S.; Furuno, T.; Sangoanruang, O.; Matsumoto, H.; Ishihara, T. Mixed conductivity and oxygen permeability of doped Pr2NiO4-based oxides. J. Electrochem. Soc. 2007, 154, B57. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Tripković, Ð.; Tong, X.; Faghihi-Sani, M.A.; Chen, M.; Hendriksen, P.V. Improving oxygen incorporation rate on (La0.6Sr0.4)0.98FeO3−δ via Pr2Ni1−xCuxO surface decoration. J. Power Sources 2020, 457, 228035. [Google Scholar] [CrossRef]
- Yashima, M.; Yamada, H.; Nuansaeng, S.; Ishihara, T. Role of Ga3+ and Cu2+ in the High interstitial oxide-ion diffusivity of Pr2NiO4-based oxides: Design concept of interstitial ion conductors through the higher-valence d10 dopant and Jahn–Teller effect. Chem. Mater. 2012, 24, 4100–4113. [Google Scholar] [CrossRef]
- Yashima, M.; Sirikanda, N.; Ishihara, T. Crystal structure, diffusion path, and oxygen permeability of a Pr2NiO4-based mixed conductor (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ. J. Am. Chem. Soc. 2010, 132, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.B. Limitations of charge-transfer models for mixed-conducting oxygen electrodes. Solid State Ion. 2020, 135, 603–612. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Boukamp, B.A. Distribution (function) of relaxation times, successor to complex nonlinear least squares analysis of electrochemical impedance spectroscopy? J. Phys. Energy 2020, 2, 042001. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the discretization methods on the Distribution of Relaxation Times deconvolution: Implementing radial basis functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Effat, M.B.; Ciucci, F. Bayesian and hierarchical Bayesian based Regularization for deconvolving the Distribution of Relaxation Times from electrochemical impedance spectroscopy data. Electrochim. Acta 2017, 247, 1117–1129. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Oxygen transport phenomena in (La,Sr)2(Ni,Fe)O4 materials. J. Mater. Chem. 2018, 6, 5304–5312. [Google Scholar] [CrossRef]
- Escudero, M.J.; Aguadero, A.; Alonso, J.A.; Daza, L.A. Kinetic study of oxygen reduction reaction on La2NiO4 cathodes by means of impedance spectroscopy. J. Electroanal. Chem. 2007, 611, 107–116. [Google Scholar] [CrossRef]
- Antonova, E.P.; Khodimchuk, A.V.; Usov, G.R.; Tropin, E.S.; Farlenkov, A.S.; Khrustov, A.V.; Ananyev, M.V. EIS analysis of electrode kinetics for La2NiO4+δ cathode in contact with Ce0.8Sm0.2O1.9 electrolyte: From DRT analysis to physical model of the electrochemical process. J. Solid State Electrochem. 2019, 23, 1279–1287. [Google Scholar] [CrossRef]
- Khandale, A.P.; Bansod, M.G.; Bhoga, S.S. Improved electrical and electrochemical performance of co-doped Nd1.8Sr0.2Ni1−xCuxO4+δ. Solid State Ion. 2015, 276, 127–135. [Google Scholar] [CrossRef]
- Shirani-Faradonbeh, H.; Paydar, M. Electrical behavior of the Ruddlesden–Popper phase (Nd0.9La0.1)2Ni0.75Cu0.25O4 (NLNC) and NLNC- x wt% Sm0.2Ce0.8O1.9 (SDC) (x = 10, 30 and 50), as intermediate-temperature solid oxide fuel cells cathode. Ceram. Intern. 2018, 44, 1971–1977. [Google Scholar] [CrossRef]
- Khandale, A.P.; Bhoga, S.S.; Kumar, R.V. Effect of Ni doping on structural, electrical and electrochemical properties of Nd1.8Ce0.2Cu1−xNixO4+δ mixed ionic–electronic conductor. Solid State Ion. 2013, 238, 1–6. [Google Scholar] [CrossRef]
- Montenegro-Hernandez, A.; Mogni, L.V.; Caneiro, A. Validation of Nd2NiO4+δ as oxygen electrode material for intermediate temperature solid oxide cells with LSGM electrolyte. ECS Trans. 2013, 58, 183–190. [Google Scholar] [CrossRef]
- Sun, L.-P.; Li, Q.; Zhao, H.; Huo, L.-H.; Grenier, J.-C. Preparation and electrochemical properties of Sr-doped Nd2NiO4 cathode materials for intermediate-temperature solid oxide fuel cells. J. Power Sources 2008, 183, 43–48. [Google Scholar] [CrossRef]
- Lee, K.J.; Chung, J.H.; Lee, M.J.; Hwang, H.J. Chromium Poisoning of Neodymium Nickelate (Nd2NiO4) Cathodes for Solid Oxide Fuel Cells. J. Korean Ceram. Soc. 2019, 56, 160–166. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Gorshkov, M.Y.; Bainov, I.N.; Vdovin, G.K.; Vylkov, A.I.; Lyagaeva, J.G.; Medvedev, D.A. Barium-doped nickelates Nd2−xBaxNiO4+δ as promising electrode materials for protonic ceramic electrochemical cells. Ceram. Int. 2020, 46, 24355–24364. [Google Scholar] [CrossRef]
- Grenier, J.C.; Mauvy, F.; Lalanne, C.; Bassat, J.-M.; Chauveau, F.; Mougin, J.; Dailly, J.; Marrony, M. A2MO4+δ oxides: Flexible materials for solid oxide cells. ECS Trans. 2009, 25, 2537–2546. [Google Scholar] [CrossRef]
- Antonova, E.P.; Kolchugin, A.A.; Pikalova, E.Y.; Medvedev, D.A.; Bogdanovich, M.N. Development of electrochemically active electrodes for BaCe0.89Gd0.1Cu0.01O3−δ proton conducting electrolyte. Solid State Ion. 2017, 306, 55–61. [Google Scholar] [CrossRef]



| NCNCO01 | As Synthesized | After Treatment in Air | Before Treatment in Vacuum | After Treatment in Vacuum |
|---|---|---|---|---|
| a, Å | 5.31796 (7) | 5.31948 (8) | 5.32090 (7) | 5.32054 (8) |
| b, Å | 5.38898 (8) | 5.38596 (8) | 5.38846 (8) | 5.39752 (8) |
| c, Å | 12.3197 (1) | 12.3235 (2) | 12.3265 (2) | 12.3234 (2) |
| V, Å3 | 353.062 (5) | 353.074 (6) | 353.420 (5) | 353.900 (6) |
| y (Nd/Ca) | −0.0082 (6) | −0.0090 (6) | −0.0091 (6) | −0.0075 (6) |
| z (Nd/Ca) | 0.3614 (1) | 0.3626 (2) | 0.3607 (1) | 0.3608 (2) |
| 1U (Nd/Ca), Å2 | 0.009 (1) | 0.013 (2) | 0.011 (1) | 0.014 (1) |
| U (Ni/Cu), Å2 | 0.011 (1) | 0.006 (2) | 0.0036 (9) | 0.005 (1) |
| z (O1) | −0.0122 (2) | −0.0137 (2) | −0.0122 (2) | −0.0120 (2) |
| U (O1), Å2 | 0.011 (1) | 0.013 (2) | 0.011 (1) | 0.014 (1) |
| y (O2) | 0.0353 (5) | 0.0391 (5) | 0.0365 (5) | 0.0420 (4) |
| z (O2) | 0.1766 (3) | 0.1742 (3) | 0.1743 (3) | 0.1765 (3) |
| U (O2), Å2 | 0.021 (2) | 0.017 (2) | 0.026 (1) | 0.020 (1) |
| 2F (O2) | 1.00 (1) | 0.94 (1) | 0.99 (1) | 1 |
| z (O3) | - | 0.297 (4) | - | - |
| F (O3) | 0 | 0.056 (8) | 0 | 0 |
| wR, %/GoF | 3.71/1.67 | 3.93/1.86 | 3.36/1.73 | 3.34/1.66 |
| 4+δ | 4.00 (2) | 3.98 (3) | 3.98 (2) | 4.00 (2) |
| NCNCO03 | As Synthesized | After Treatment in Air | Before Treatment in Vacuum | After Treatment in Vacuum |
|---|---|---|---|---|
| a, Å | 5.29762 (7) | 5.29898 (7) | 5.30136 (9) | 5.30594 (8) |
| b, Å | 5.37345 (8) | 5.37350 (8) | 5.38033 (9) | 5.38628 (9) |
| c, Å | 12.4607 (1) | 12.4636 (1) | 12.4556 (2) | 12.4479 (2) |
| V, Å3 | 354.711 (5) | 354.890 (6) | 355.271 (7) | 355.753 (7) |
| y (Nd/Ca) | −0.0096 (6) | −0.0071 (6) | −0.0051 (7) | −0.0118 (6) |
| z (Nd/Ca) | 0.3601 (2) | 0.3602 (2) | 0.3610 (2) | 0.3593 (2) |
| 1U (Nd/Ca), Å2 | 0.011 (2) | 0.0112 (2) | 0.008 (2) | 0.0073 (4) |
| U (Ni/Cu), Å2 | 0.009 (2) | 0.0093 (5) | 0.011 (2) | 0.011 |
| z (O1) | −0.0119 (2) | −0.0102 (3) | −0.0132 (3) | −0.0096 (4) |
| U (O1), Å2 | 0.013 (3) | 0.0197 (6) | 0.011 (2) | 0.0189 (7) |
| 2F (O1) | 1 | 1 | 1 | 1 |
| y (O2) | 0.0381 (5) | 0.0423 (4) | 0.0400 (5) | 0.0446 (5) |
| z (O2) | 0.1752 (3) | 0.1776 (4) | 0.1775 (4) | 0.1777 (4) |
| U (O2), Å2 | 0.019 (2) | 0.0188 (8) | 0.021 (2) | 0.0177 (8) |
| F (O2) | 0.96 (1) | 0.924 (9) | 0.95 (1) | 0.96 (1) |
| z (O3) | - | 0.292 (5) | - | - |
| F (O3) | 0 | 0.044 (8) | 0 | 0 |
| wR, %/GoF | 3.73/1.73 | 3.67/1.78 | 4.11/2.00 | 3.74/1.79 |
| 4+δ | 3.93 (2) | 3.94 (2) | 3.90 (2) | 3.92 (2) |
| NCNCO04 | As Synthesized | After the Treatment in Air |
|---|---|---|
| a, Å | 5.2987 (1) | 5.2992 (1) |
| b, Å | 5.3742 (1) | 5.3779 (1) |
| c, Å | 12.4573 (2) | 12.4516 (3) |
| V, Å3 | 354.739 (9) | 354.85 (1) |
| y (Nd/Ca) | −0.0119 (8) | −0.0121 (9) |
| z (Nd/Ca) | 0.3595 (2) | 0.3603 (2) |
| 1U (Nd/Ca). Å2 | 0.0113 (7) | 0.0126 (8) |
| U (Ni/Cu). Å2 | 0.0079 (8) | 0.0098 (9) |
| z (O1) | −0.0123 (3) | −0.0102 (4) |
| U (O1). Å2 | 0.010 (1) | 0.013 (1) |
| 2F (O1) | 0.97 (2) | 0.94 (2) |
| y (O2) | 0.0369 (7) | 0.0433 (7) |
| z (O2) | 0.1736 (4) | 0.1731 (5) |
| U (O2). Å2 | 0.017 (1) | 0.017 (1) |
| F (O2) | 0.92 (1) | 0.92 (2) |
| wR/GoF | 4.72/2.13 | 4.84/2.39 |
| 4+δ | 3.77 (4) | 3.72 (5) |
| Sample | UCP (Å) UCV (Å3) after Treatment in Synthetic Air | UCP (Å) UCV (Å3) after Treatment in He Flow | Orthorhombic Distortion 1 after Synthetic Air/after He | Tpt (°C) Air Flow, Heating/Cooling | Tpt (°C) He Flow, Heating/Cooling | VTEC 2 ×10−6 (K−1) Air Flow, Bmab sp.gr/ I4/mmm sp.gr | VTEC ×10−6 (K−1) He Flow, Bmab sp.gr/ I4/mmm sp.gr |
|---|---|---|---|---|---|---|---|
| NCNCO00 | a = 5.3253 (1) b = 5.3862 (1) c = 12.2690 (2) V =351.916 (7) | a = 5.3191 (1) b = 5.4018 (1) c = 12.2558 (2) V =352.144 (7) | 1.13/1.53 | 430/375 | 385/485 | 9.0/11.7 | 9.3/12.0 |
| NCNCO01 | a = 5.3172 (2) b = 5.3840 (2) c = 12.3121 (5) V =352.47 (2) | a = 5.3118 (1) b = 5.3958 (1) c = 12.3009 (2) V =352.56 (2) | 1.24/1.56 | 410/365 | 365/440 | 7.8/9.1 | 8.2/9.2 |
| NCNCO02 | a = 5.3070 (2) b = 5.3789 (2) c = 12.3600 (4) V =352.83 (1) | a = 5.3042 (2) b = 5.3901 (2) c = 12.3475 (3) V = 353.01 (2) | 1.34/1.59 | 420/400 | 430/445 | 8.7/10.0 | 8.0/10.4 |
| NCNCO03 | a = 5.2974 (3) b = 5.3757 (3) c = 12.4470 (7) V =354.46 (6) | a = 5.2971 (2) b = 5.3778 (2) c = 12.4341 (4) V =354.21 (3) | 1.46/1.50 | 400–500/ 400–500 | 400–500/ 400–500 | n/a | n/a |
| NCNCO04 | a = 5.2946 (1) b = 5.3740 (1) c = 12.4498 (3) V =354.24 (2) | a = 5.2948 (1) b = 5.3749 (1) c = 12.4488 (3) V =354.28 (2) | 1.48/1.49 | 400–500/ 400–500 | 400–500/ 400–500 | n/a | n/a |
| Sample | α | n |
|---|---|---|
| NCNCO04 | 0.185 | 0.111 |
| NCNCO03 | 0.157 | 0.110 |
| NCNCO02 | 0.162 | 0.130 |
| NCNCO01 | 0.069 | ≤0.062 |
| NCNCO00 | 0 | 0 |
| Sample | θfast, (%) | Ea,D, (kJ mol−1) | ||
|---|---|---|---|---|
| NCNO | 3.3(±1.6) × 10−9 | 60 | 1.3 × 10−9 | 120 |
| NCNCO1 | 25 | 4.9 × 10−10 | ||
| NCNCO2 | 20 | 3.3 × 10−10 | ||
| NCNCO3 | 5 | 2.6 × 10−10 | ||
| NCNCO4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimchuk, T.; Filonova, E.; Mishchenko, D.; Eremeev, N.; Sadovskaya, E.; Bobrikov, I.; Fetisov, A.; Pikalova, N.; Kolchugin, A.; Shmakov, A.; et al. High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials. Appl. Sci. 2022, 12, 3747. https://doi.org/10.3390/app12083747
Maksimchuk T, Filonova E, Mishchenko D, Eremeev N, Sadovskaya E, Bobrikov I, Fetisov A, Pikalova N, Kolchugin A, Shmakov A, et al. High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials. Applied Sciences. 2022; 12(8):3747. https://doi.org/10.3390/app12083747
Chicago/Turabian StyleMaksimchuk, Tatiana, Elena Filonova, Denis Mishchenko, Nikita Eremeev, Ekaterina Sadovskaya, Ivan Bobrikov, Andrey Fetisov, Nadezhda Pikalova, Alexander Kolchugin, Alexander Shmakov, and et al. 2022. "High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials" Applied Sciences 12, no. 8: 3747. https://doi.org/10.3390/app12083747
APA StyleMaksimchuk, T., Filonova, E., Mishchenko, D., Eremeev, N., Sadovskaya, E., Bobrikov, I., Fetisov, A., Pikalova, N., Kolchugin, A., Shmakov, A., Sadykov, V., & Pikalova, E. (2022). High-Temperature Behavior, Oxygen Transport Properties, and Electrochemical Performance of Cu-Substituted Nd1.6Ca0.4NiO4+δ Electrode Materials. Applied Sciences, 12(8), 3747. https://doi.org/10.3390/app12083747