Evaluation of Non-Biodegradable Organic Matter and Microbial Community’s Effects on Achievement of Partial Nitrification Coupled with ANAMMOX for Treating Low-Carbon Livestock Wastewater
Abstract
:1. Introduction
2. Materials and Methods
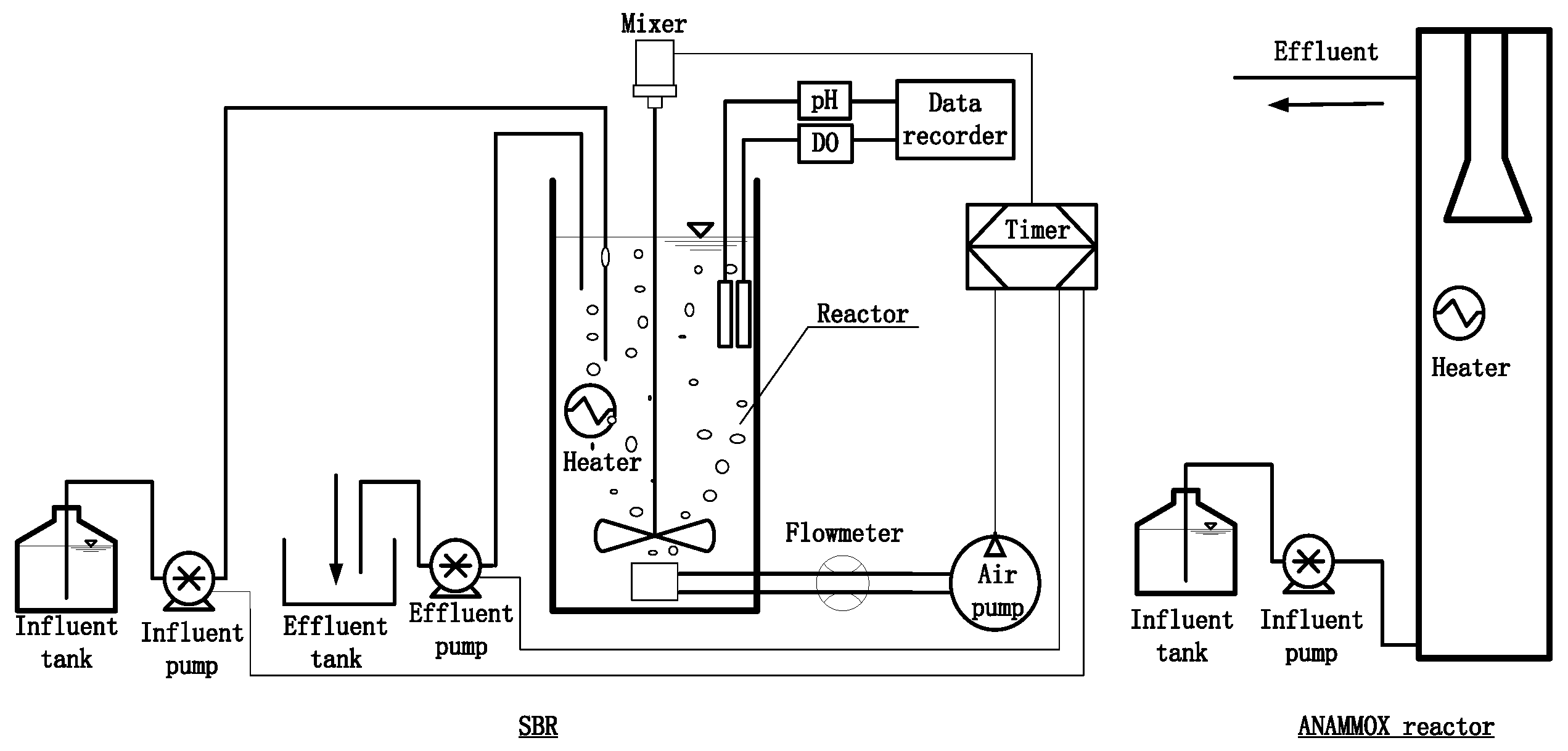
2.1. Experimental Setup
2.2. Analytical Methods
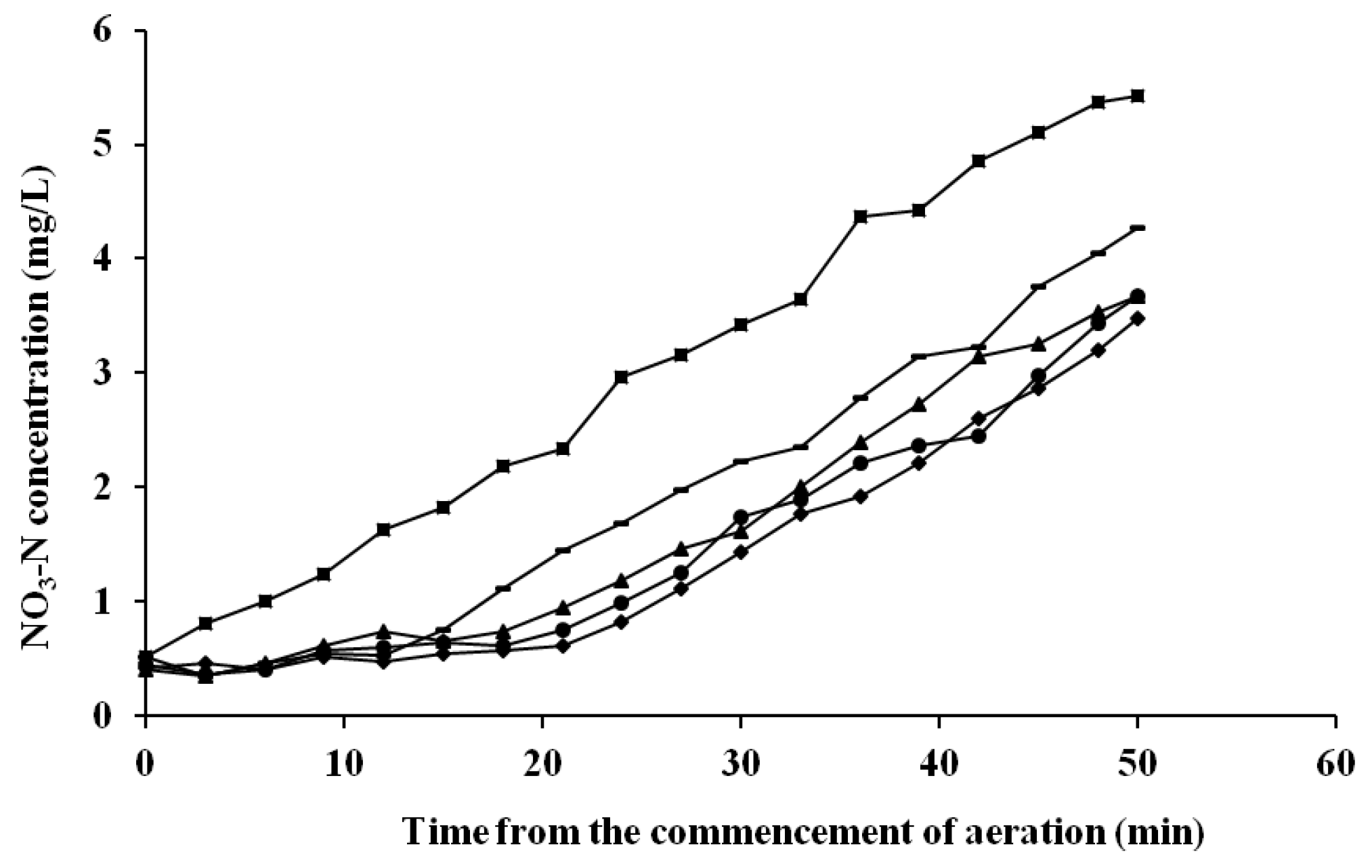
2.3. Batch Experiments
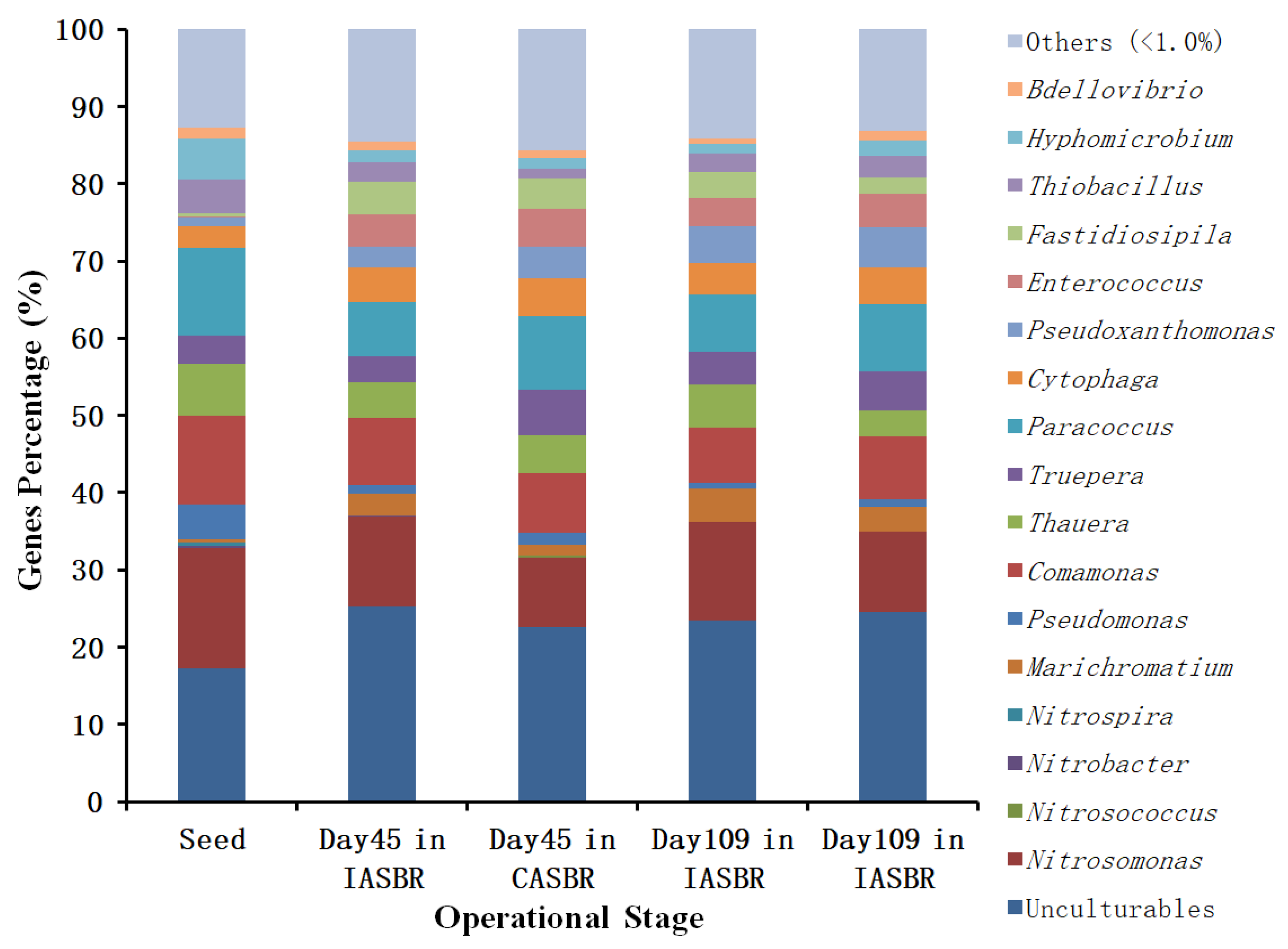
2.4. Microbial Population Analysis by 16S rRNA Gene Sequencing
3. Results and Discussion
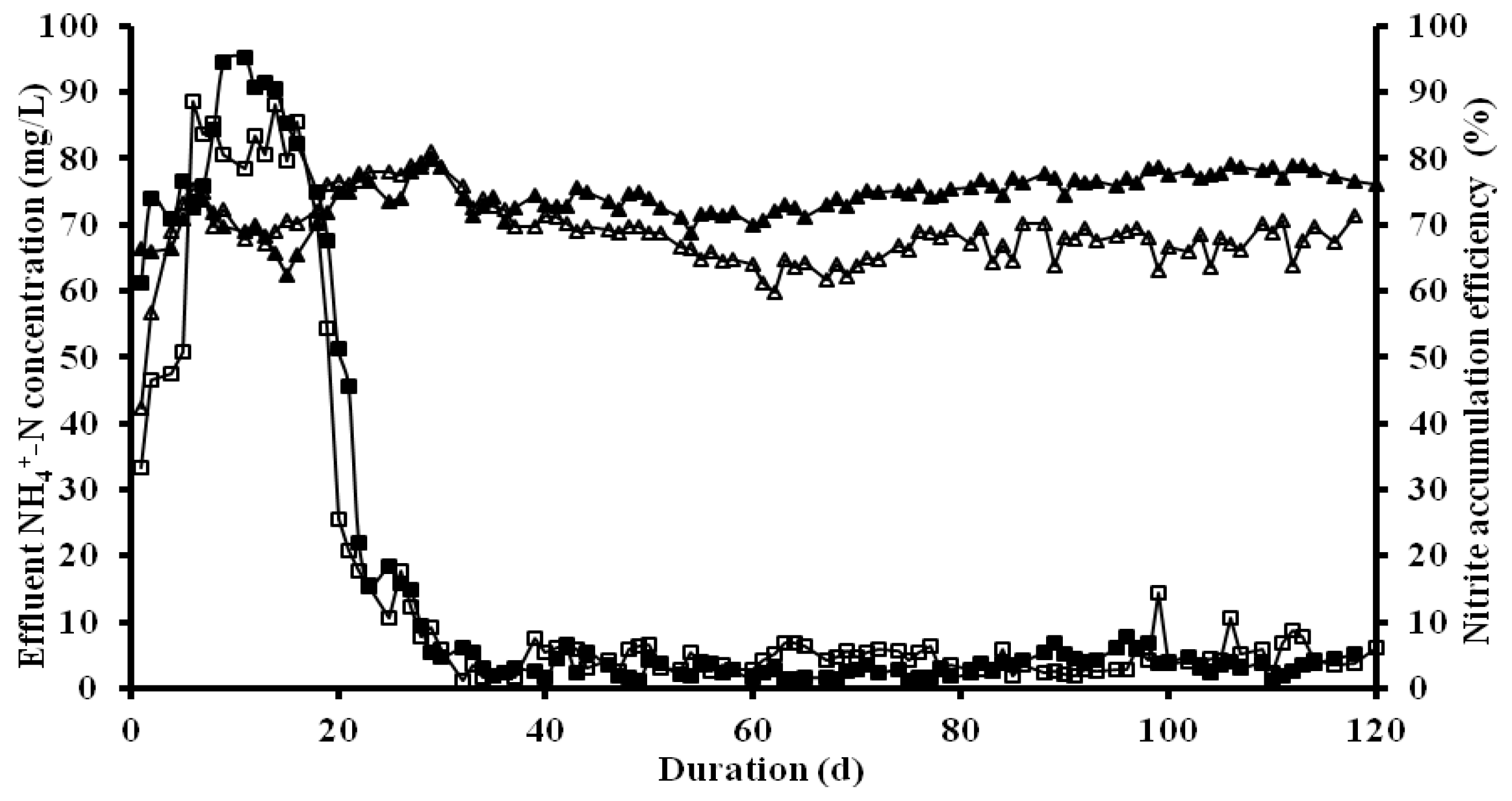
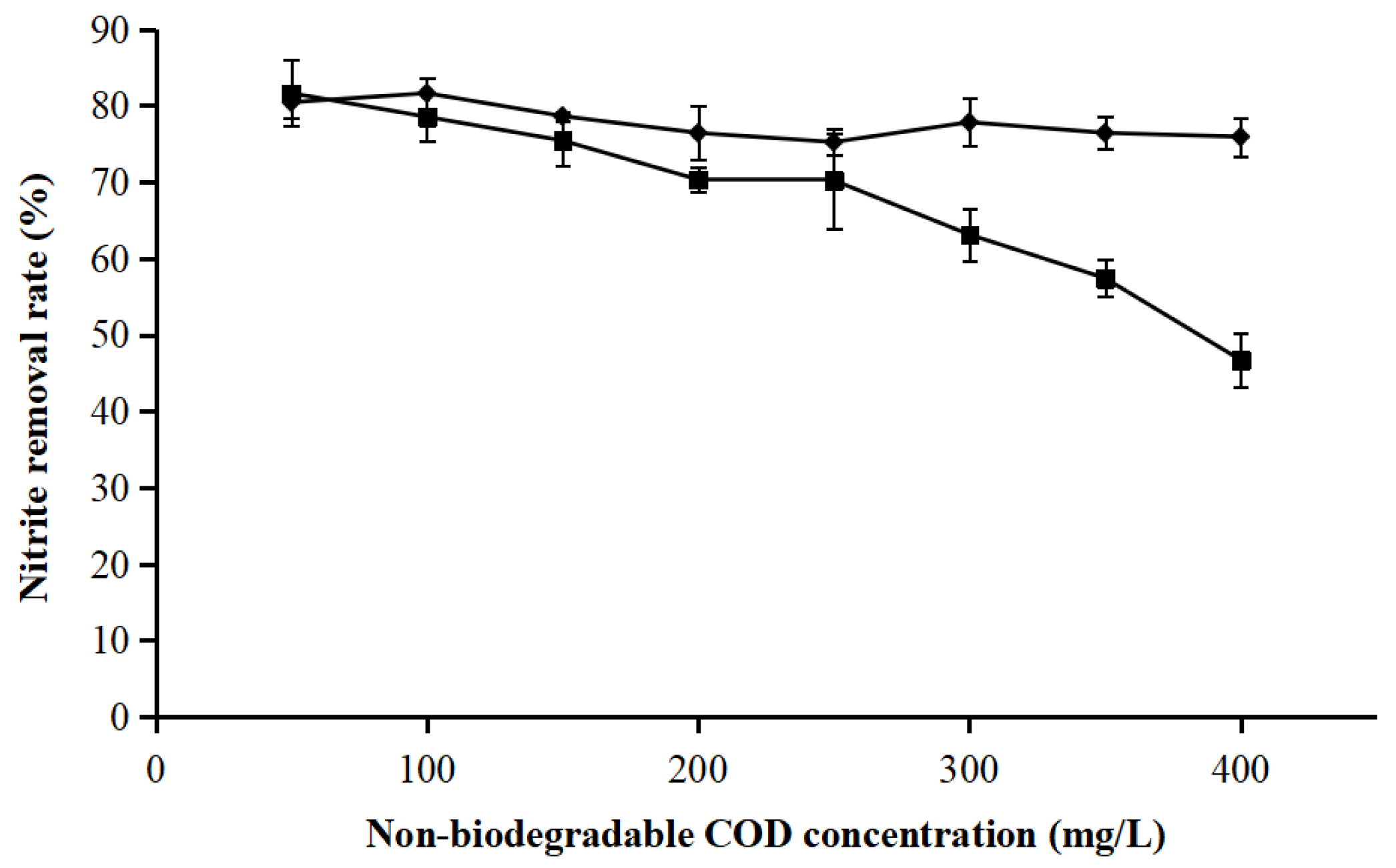
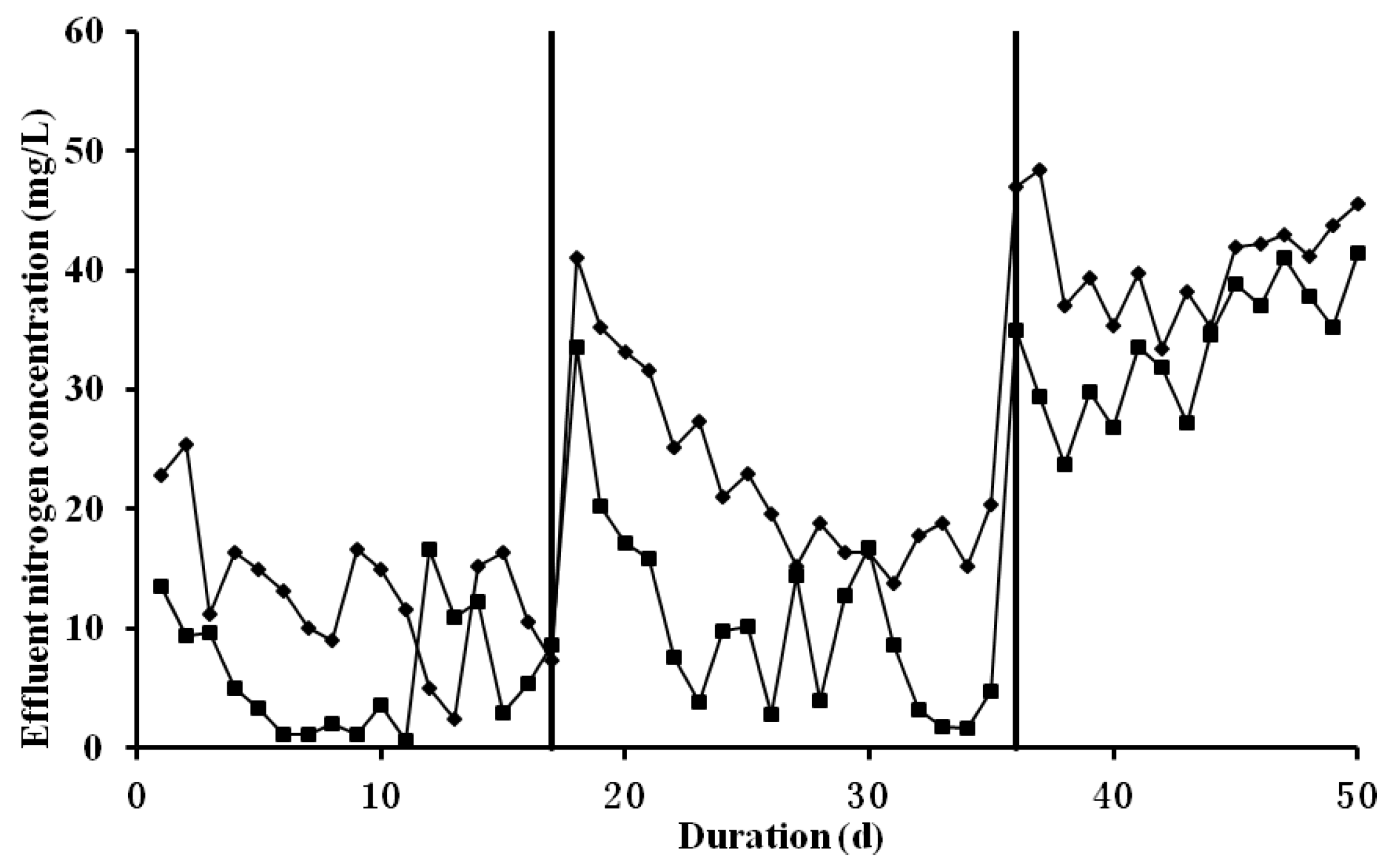
3.1. Overall Performance of Partial Nitrification on Treating Livestock Wastewater
3.2. Cultivation and Performance of ANAMMOX for Treating Partial Nitrification Effluents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, B.; Chen, L. Technologies to recover nitrogen from livestock manure—A review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef]
- Meade, G.; Lalor, S.T.J.; Mc Cabe, T. An evaluation of the combined usage of separated liquid pig manure and inorganic fertiliser in nutrient programmes for winter wheat production. Eur. J. Agron. 2011, 34, 62–70. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.P.; Hu, Z.; Zhan, X. Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour. Technol. 2011, 102, 5728–5733. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Schmidt, J.E.; Angelidaki, I. Innovative process scheme for removal of organic matter, phosphorus and nitrogen from pig manure. Water Res. 2008, 42, 4083–4090. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, F.; Jiang, H.; Ren, S.; Wang, W.; Peng, Y. A continuous-flow combined process based on partial nitrification-Anammox and partial denitrification-Anammox (PN/A + PD/A) for enhanced nitrogen removal from mature landfill leachate. Bioresour. Technol. 2020, 297, 122483. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Miller, M.W.; Holgate, B.; Bunce, R.; Park, H.; Chandran, K.; Wett, B.; Murthy, S.; Bott, C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Rongsayamanont, C.; Limpiyakorn, T.; Khan, E. Effects of inoculum type and bulk dissolved oxygen concentration on achieving partial nitrification by entrapped-cell-based reactors. Bioresour. Technol. 2014, 164, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, J. The characteristics of anaerobic ammonium oxidation (ANAMMOX) by granular sludge from an EGSB reactor. Process Biochem. 2005, 40, 1973–1978. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Peng, Y.; Wang, S.; Zhang, L.; Yang, S.; Li, S. Insight into the impacts of organics on anammox and their potential linking to system performance of sewage partial nitrification-anammox (PN/A): A critical review. Bioresour. Technol. 2020, 300, 122655. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, J. Landfill leachate treatment with a novel process: Anaerobic ammonium oxidation (Anammox) combined with soil infiltration system. J. Hazard. Mater. 2008, 151, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.; Puig, S.; Ganigue, R.; Ruscalleda, M.; Balaguer, M.D.; Colprim, J. Start-up and enrichment of a granular anammox SBR to treat high nitrogen load Wastewaters. J. Chem. Technol. Biotechnol. 2008, 83, 233–241. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Yu, Z.; Morrison, M. Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microb. 2004, 70, 4800–4806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, M.S.; Cormican, P.; Keogh, K.; O’Connor, A.; O’Hara, E.; Palladino, R.A.; Kenny, D.A.; Waters, S.M. Illumina miSeq phylogenetic amplicon sequencing shows a large reduction of an uncharacterised Succinivibrionaceae and an increase of the methanobrevibacter gottschalkii clade in feed restricted cattle. PLoS ONE 2015, 10, e0133234. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Exploring the roles of and interactions among microbes in dry co-digestion of food waste and pig manure using high-throughput 16S rRNA gene amplicon sequencing. Biotechnol. Biofuels 2019, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Ciudad, G.; Gonzalez, R.; Bornhardt, C.; Antileo, C. Modes of operation and pH control as enhancement factors for partial nitrification with oxygen transport limitation. Water Res. 2007, 41, 4621–4629. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, P.; Yu, Y.; Mahmood, Q.; Tang, C. Enrichment of high activity nitrifiers to enhance partial nitrification process. Bioresour. Technol. 2010, 101, 7293–7298. [Google Scholar] [CrossRef]
- Wei, D.; Xue, X.; Yan, L.; Sun, M.; Zhang, G.; Shi, L.; Du, B. Effect of influent ammonium concentration on the shift of full nitritation to partial nitrification in a sequencing batch reactor at ambient temperature. Chem. Eng. J. 2014, 235, 19–26. [Google Scholar] [CrossRef]
- Ferrero, E.M.; de Godos, I.; Rodríguez, E.M.; García-Encina, P.A.; Muñoz, R.; Bécares, E. Molecular characterization of bacterial communities in algal–bacterial photobioreactors treating piggery wastewaters. Ecol. Eng. 2012, 40, 121–130. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Yan, X.; Liu, B.; Zhao, L. Development of group-specific PCR-DGGE fingerprinting for monitoring structural changes of Thauera spp. in an industrial wastewater treatment plant responding to operational perturbations. J. Microbiol. Methods 2008, 75, 231–236. [Google Scholar] [CrossRef]
- Ordaz-Cortés, A.; Thalasso, F.; Salgado-Manjarrez, E.; Garibay-Orijel, C. Treatment of wastewater containing high concentrations of terephthalic acid by Comamonas sp. and Rhodococcus sp.: Kinetic and stoichiometric characterization. Water Environ. J. 2014, 28, 393–400. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, D.; Bai, X.; Zhang, W.; Lu, X. Identification and characterization of a large protein essential for degradation of the crystalline region of cellulose by Cytophaga hutchinsonii. App. Environ. Microb. 2017, 83, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Z.; Bakken, L.R.; Molstad, L.; Frostegard, A.; Bergaust, L.L. Transcriptional and metabolic regulation of denitrification in Paracoccus denitrificans allows low but significant activity of nitrous oxide reductase under oxic conditions. Environ. Microbiol. 2016, 18, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Chen, Z.; Zhao, C.; Yang, S. Nitrogen transformation under different dissolved oxygen levels by the anoxygenic phototrophic bacterium Marichromatium gracile. World J. Microb. Biotechnol. 2017, 33, 113. [Google Scholar] [CrossRef]
- Brockmann, D.; Morgenroth, E. Evaluating operating conditions for outcompeting nitrite oxidizers and maintaining partial nitrification in biofilm systems using biofilm modelling and Monte Carlo filtering. Water Res. 2010, 44, 1995–2009. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Ruan, W.; Ren, H.; Zhao, M. ANAMMOX performance, granulation and microbial response under COD disturbance. J. Chem. Technol. Biotechnol. 2015, 90, 139–148. [Google Scholar] [CrossRef]
- Molinuevo, B.; Garcia, M.C.; Karakashev, D.; Angelidaki, I. ANAMMOX for ammonia removal from pig manure effluents: Effect of organic matter content on process performance. Bioresour. Technol. 2009, 100, 2171–2175. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chen, X.; Xu, X.; Fu, Z.; Zhao, X. Evaluation of Non-Biodegradable Organic Matter and Microbial Community’s Effects on Achievement of Partial Nitrification Coupled with ANAMMOX for Treating Low-Carbon Livestock Wastewater. Appl. Sci. 2022, 12, 3626. https://doi.org/10.3390/app12073626
Zhang M, Chen X, Xu X, Fu Z, Zhao X. Evaluation of Non-Biodegradable Organic Matter and Microbial Community’s Effects on Achievement of Partial Nitrification Coupled with ANAMMOX for Treating Low-Carbon Livestock Wastewater. Applied Sciences. 2022; 12(7):3626. https://doi.org/10.3390/app12073626
Chicago/Turabian StyleZhang, Mingchuan, Xi Chen, Xinyang Xu, Zhongtian Fu, and Xin Zhao. 2022. "Evaluation of Non-Biodegradable Organic Matter and Microbial Community’s Effects on Achievement of Partial Nitrification Coupled with ANAMMOX for Treating Low-Carbon Livestock Wastewater" Applied Sciences 12, no. 7: 3626. https://doi.org/10.3390/app12073626
APA StyleZhang, M., Chen, X., Xu, X., Fu, Z., & Zhao, X. (2022). Evaluation of Non-Biodegradable Organic Matter and Microbial Community’s Effects on Achievement of Partial Nitrification Coupled with ANAMMOX for Treating Low-Carbon Livestock Wastewater. Applied Sciences, 12(7), 3626. https://doi.org/10.3390/app12073626






