Abstract
C-Ring oxidized estrone acetate derivatives as antiproliferative agents were prepared and tested against five cancer cell lines by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Flow cytometry assays to evaluate cell viability and modifications in cell cycle phases and molecular docking research against estrogen receptor α, steroid sulfatase, and 17β-hydroxysteroid dehydrogenase type 1 were performed. 9α-Hydroxy,11β-nitrooxyestrone acetate was the most cytotoxic molecule against hormone-dependent cancer cells. Furthermore, flow cytometry experiments revealed that this 9α-hydroxy,11β-nitrooxy derivative markedly reduced HepaRG cells viability (~92%) after 24 h of treatment. However, 9α-hydroxyestrone acetate led to selective inhibition of HepaRG cells growth, inducing a G0/G1 cycle arrest, and did not originate a proliferation effect on T47-D cancer cells. Docking studies estimated a generally lower affinity of these compounds to estrogen receptor α than predicted for estrone and 17β-estradiol. Therefore, this structural modification can be of interest to develop new anticancer estrane derivatives devoid of estrogenic action.
1. Introduction
Steroids are natural products that play a central physiological role in metabolism, immune and sexual functions [1]. They are also important in several pathological conditions, such as in the maintenance and progression of hormone-dependent cancers [2], mainly through estrogen (ER) and androgen receptors (AR) activation, which are transcription factors that regulate gene expression events that culminate in cell division [3]. Therefore, modifications to the chemical structure of steroidal hormones, such as estrone (E1) or 17β-estradiol (E2), has been considered a relevant strategy to develop new therapeutic agents such as contraceptives such as ethinylestradiol [4] or antitumor agents [5], particularly against hormone-dependent breast cancers, such as fulvestrant [6,7,8,9,10].
These structural modifications have been performed, mainly in A-, B-, and, more frequently, in D-rings. Regarding the C-ring, modifications at this level have been less exploited primarily due to steric reasons. However, the importance of position 11 in estrogens structure for ER binding has been well documented by several research groups (Examples in Figure 1), which makes its C-ring an important point for chemical alterations.
Firstly, it was demonstrated that an enhancement of binding to ER can be achieved with short and nonpolar groups at 11β-position [11]. It was also evidenced that larger hydrophobic substituents in the same position are also tolerated [12]. In fact, E2 derivatives with an undecanamide side chain at 11β-position [13] were devoid of in vitro estrogenic activity (e.g., proliferation of ER+ cells) but maintained high ER binding affinity [14]. Interestingly, 11β-ethyl, 11β-butyl, and 11β-decyl E2 derivatives [15] showed affinity constants for ER binding ranging from 0.4% to 37%. The authors of this work observed that the two 11β-ethyl compounds studied were mainly estrogenic, while the three 11β-butyl and the 11β-decyl derivatives were essentially antiestrogenic. Additional studies also evidenced that the antagonistic activity of these compounds seemed to be more dependent on the size of the 11β-substituent than its nature [16,17]. Moreover, several 11β-modified E2 derivatives [18] were synthesized, and it was shown that nonpolar groups in this position led to an antiestrogen effect. In fact, the estrogen stimulation in endometrial adenocarcinoma Ishikawa cells was inhibited when the 11β-side chain was an ether function with five non-hydrogen atoms. In addition, in in vivo studies using immature rats, the 2-ethoxyethyl derivative inhibited the uterotrophic stimulation of E2, with a useful estrogenic effect in liver and bone. Therefore, this compound was considered a selective estrogen receptor modulator (SERM). Other 11-substituted E2 derivatives (e.g., allyl and benzyl halides) also exhibited significant contraceptive activity [19]. However, 11α-hydroxy-E2 and 11β-hydroxy-E2 [20] had a weak relative binding affinity to ERα.
Concerning both C9 and C11 modifications, for example, the 9α-hydroxymethyl,11-ketone derivative of E1 showed poor affinity to ERα and was devoid of cytotoxic activity [21]. However, some 9α-hydroxy-11β-nitrate esters [22] displayed higher estrogenic potency and postcoital antifertility activity than ethinylestradiol. Interestingly, many 11β-nitrates also showed antitumor activity against xenograft animal models of breast cancer [23]. Unfortunately, these compounds also showed an estrogenic effect, being observed a tumor growth after 15 days of treatment. Recently, 11α-substituted 2-methoxyestradiol derivatives were prepared and showed relevant cytotoxic activity against hepatic HepG2 cells, promoting a G2/M arrest and showing antiestrogen activity [5].
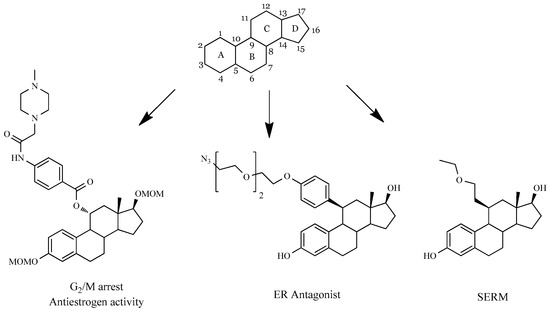
Figure 1.
Some examples of C-ring modifications in estrane series with biological activities [5,17,18]. ER, estrogen receptor; SERM selective estrogen receptor modulator.
In this context, in a previous work, we prepared and evaluated several Δ9,11-E1 derivatives as antiproliferative agents and demonstrated that the presence of this C-ring modification was relevant for their effects on cell proliferation [24]. Therefore, considering our continuous interest in developing antiproliferative steroids [7,25], namely belonging to the estrane series [24,26,27], in this work, we synthesized C-ring oxidized derivatives of E1 acetate and analyzed their cytotoxic effects in breast, prostatic, colon, and hepatic cancer cell lines, as well as in normal fibroblast cells. The cell viability and cell cycle distribution were also studied for the most relevant compounds. An in silico study (molecular docking) was performed for ERα, steroid sulfatase (ST), and 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1).
2. Materials and Methods
2.1. The Chemical Synthesis and Structural Characterization
2,3-Dichloro-5,6-dicyano-p-benzoquinone (DDQ), tetrahydrofuran (THF), 4-(dimethylamino)pyridine (DMAP), potassium peroxymonosulfate (OxoneTM), ammonium cerium(IV) nitrate (CAN), tetraethylammonium chloride (TEAC), E2, 5-fluorouracil (5-FU), and dimethyl sulfoxide (DMSO) were acquired from Sigma-Aldrich (St. Louis, MO, USA). E1 was purchased from Cayman Chemical (Ann Arbor, MI, USA), acetic anhydride (Ac2O) from Fluka, methanol (MeOH) and acetic acid from Fisher Chemical (Waltham, MA, USA), acetone 99% from José Manuel Gomes dos Santos, Lda (Odivelas, Portugal), ethanol (EtOH) 99.9% from Manuel Vieira & Cª (Torres Novas, Portugal), and deuterated DMSO (DMSO-d6) and deuterated chloroform (CDCl3) were acquired from Armar Chemicals (Leipzig, Germany). Thin-layer chromatography (TLC) with an al-backed aluminum/silica gel plate 0.20 mm (Macherey-Nagel 60 F254, Duren, Germany) was used to control all reactions. The CN-15.LC UV chamber (254 nm) was used to visualize TLCs before chemical revelation, which was performed using EtOH/concentrated sulfuric acid (95:5, v:v) and then heating at 120 °C. A rotary vacuum drier from Büchi (R-215) was used to evaporate solvents. The Infrared (IR) spectra were acquired on a Thermoscientific Nicolet iS10 at room temperature in the 4000–400 cm−1 range by averaging 16 scans (spectral resolution of 2 cm−1). 1H and 13C nuclear magnetic resonance (NMR) spectra were acquired in Bruker Avance 400 MHz, and the TOPSPIN 4.07 software (Bruker, Fitchburg, WI, USA) was used. Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane (TMS) or solvent as an internal standard. Coupling constants (J values) are reported in hertz (Hz) and splitting multiplicities are described as s = singlet; brs = broad singlet; d = doublet; dd = double doublet and t = triplet.
2.1.1. Synthesis of 17-Oxoestra-1,3,5(10)-trien-3-yl Acetate (Estrone Acetate, 2)
The synthesis was carried out in accordance with a protocol previously described [28]. Briefly, 60 mg of DMAP and 200 µL of Ac2O were added to a solution of E1 1 (540.7 mg, 2 mmol) in THF (10.8 mL) and the mixture was stirred for 24 h at room temperature (r.t.). Then, the reaction solvent was evaporated and the crude was diluted in CH2Cl2, washed with 10% HCl, saturated solution of NaHCO3 and H2O and dried over anhydrous Na2SO4, filtered and finally the solvent was evaporated to afford compound 2 as a white solid (600 mg, 96%) [28]. IR (υmax, cm−1): 820, 1007, 1204, 1366, 1491, 1605, 1732, 1759, 2876, 2930, 3055; 1H-NMR (400 MHz, CDCl3) δ: 0.89 (s, 3H, C18-CH3), 2.26 (s, 3H, COCH3), 6.79 (d, 1H, J = 2.6 Hz, C4-H), 6.83 (dd, 1H, J1 = 8.6 Hz, J2 = 2.6 Hz, C2-H), 7.27 (d, 1H, J = 8.6 Hz, C1-H); 13C-NMR (100 MHz, CDCl3) δ: 14.0, 21.3, 21.8, 25.9, 26.5, 29.6, 31.7, 36.0, 38.2, 44.3, 48.1, 50.6, 118.9, 121.8, 126.6, 137.6, 138.2, 148.7, 170.0, 220.9.
2.1.2. Synthesis of 9α-Hydroxy-17-oxoestra-1,3,5(10)-trien-3-yl Acetate (4)
The synthesis was carried out in accordance with a protocol previously described [29,30]. Briefly, H2O (3.94 mL), NaHCO3 (1.142 g), acetone (3.16 mL) and TEAC (2 mg) were added to a solution of compound 2 (156.18 mg, 0.5 mmol) in CH2Cl2 (3.56 mL). Then, OxoneTM (2.36 g) was added every 15 min for 2 h followed by stirring on ice for 7 h. After, the crude was diluted in ethyl acetate (EA), washed with 10% Na2SO3 and H2O, dried over anhydrous Na2SO4 and filtered under suction. After solvent evaporation, purification was performed by column chromatography (eluent: EA/petroleum ether (PE) 40–60 °C, 1:4) to yield compound 4 [29,30] (beige solid, 90 mg, 55%). IR (υmax, cm−1): 800, 902, 1013, 1196, 1372, 1448, 1606, 1723, 2832, 2938, 3060, 3351, 3564; 1H-NMR (400 MHz, CDCl3) δ: 0.88 (s, 3H, C18-CH3), 2.26 (s, 3H, COCH3), 6.83 (d, 1H, J = 2.4 Hz, C4-H), 6.89 (dd, 1H, J1 = 8.5 Hz, J2 = 2.4 Hz, C2-H), 7.52 (d, 1H, J = 8.5 Hz, C1-H); 13C-NMR (100 MHz, CDCl3) δ: 13.1, 20.2, 21.3, 21.6, 27.8, 29.6, 32.4, 36.1, 41.3, 43.3, 47.8, 70.2, 119.8, 122.5, 126.7, 138.6, 139.3, 150.2, 169.9, 220.7.
2.1.3. Synthesis of 9α-Hydroxy-11β-nitrooxy-17-oxoestra-1,3,5(10)-trien-3-yl Acetate (5)
The synthesis was carried out in accordance with a protocol previously described [22]. Briefly, a solution of CAN (2.52 g, 4.6 mmol) in 2 mL of H2O was added dropwise to a solution of compound 2 (317.6 mg, 1 mmol) in acetic acid (15 mL). The resulting orange solution was maintained under vigorous stirring for 6 h at r.t. and then was diluted two-fold with H2O and extracted with EA. The organic solution was then washed with saturated NaHCO3 solution, brine, and H2O, dried using anhydrous Na2SO4, and filtered under suction. After solvent evaporation, a purification with column chromatography (eluent: gradient of EA/PE 40–60 °C, 1:4 to 1:2) and recrystallization (MeOH) was performed to afford compound 5 [22] as a light brown solid (128.7 mg, 34%). IR (υmax, cm−1): 853, 970, 1050, 1151, 1207, 1279, 1370, 1493, 1633, 1728, 1750, 2893, 2962, 3447; 1H-NMR (400 MHz, CDCl3) δ: 1.00 (s, 3H, C18-CH3), 2.26 (s, 3H, COCH3), 5.79 (t, 1H, J = 3 Hz, CHONO2), 6.89 (brs, 1H, C4-H), 6.91 (d, J = 8.3 Hz, 1H, C2-H), 7.28 (d, 1H, J = 8.3 Hz, C1-H); 13C-NMR (100 MHz, CDCl3) δ: 15.3, 20.1, 21.3, 21.3, 29.7, 31.3, 35.5, 38.2, 42.2, 46.4, 71.9, 81.4, 120.5, 123.1, 126.3, 134.9, 139.9, 150.6, 169.7, 217.9.
2.1.4. Synthesis of 17-Oxoestra-1,3,5(10),9(11)-trien-3-yl Acetate (6)
The synthesis was carried out in accordance with a protocol previously described [31,32]. Briefly, 37.5 µL of Ac2O and 11.8 mg of DMAP were added to a solution of compound 3 (107.4 mg, 0.4 mmol), prepared as previously described [24,32], in 2.14 mL of THF and the resulting mixture was stirred for 1 h at r.t. Afterward, THF was partially evaporated and the crude was diluted in EA, washed with 10% HCl, saturated NaHCO3 and H2O, dried using anhydrous Na2SO4 and filtered under suction. After solvent evaporation, compound 6 [31] was isolated as a beige solid (110 mg, 89%). IR (υmax, cm−1): 809, 1019, 1062, 1201, 1368, 1449, 1492, 1606, 1647, 1732, 2213, 2925, 3050, 3265; 1H NMR (CDCl3, 400 MHz): δ 0.90 (s, 3H, C18-CH3), 2.26 (s, 3H, COCH3), 6.21 (m, 1H, C11-H), 6.80 (d, 1H, J = 2.3 Hz, C4-H), 6.84 (dd, 1H, J1 = 8.6 Hz, J2 = 2.3 Hz, C2-H), 7.57 (d, 1H, J = 8.7 Hz, C1-H); 13C NMR (CDCl3, 100 MHz): δ 14.5, 21.1, 22.5, 27.6, 29.6, 34.0, 36.2, 37.9, 46.2, 47.8, 119.1, 119.4, 121.8, 125.3, 132.0, 135.2, 137.4, 149.4, 169.7, 221.5.
2.2. Bioactivity Assays
2.2.1. Cell Culture
For this study, MCF-7, LNCaP, NHDF, T47-D, and Caco-2 cells were acquired from American Type Culture Collection (ATCC; Manassas, VA, USA) and HepaRG from Life Technologies—Invitrogen™ (through Alfagene, Carcavelos, Portugal). They grew in 75 cm2 culture flasks at 37 °C in a humidified air incubator under a 5% CO2 atmosphere. High-glucose Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and 1% antibiotic/antimycotic (10,000 units/mL penicillin G, 100 mg/mL streptomycin and 25 μg/mL amphotericin B) (Ab; Sigma-Aldrich, St. Louis, MO, USA) was used to culture MCF-7 cells. DMEM supplemented with 10% FBS, and 1% of the antibiotic mixture of 10,000 units/mL penicillin G and 100 mg/mL of streptomycin (sp; Sigma-Aldrich, St. Louis, MO, USA) was used to culture Caco-2 cells. LNCaP and T47-D cells grew in RPMI 1640 medium with 10% FBS and 1% sp. RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 1% Ab was used to culture fibroblasts (NHDF). Finally, HepaRG cells were cultured in Williams’ E medium supplemented with 10% FBS, 1% sp, 5 µg/mL insulin, and 5 × 10−5 M hydrocortisone hemisuccinate (Sigma-Aldrich, St. Louis, MO, USA).
2.2.2. Stock Solutions
All synthesized compounds and 5-FU (positive control) were dissolved in DMSO at a concentration of 10 mM and stored at 4–8 °C. For use, they were diluted in the corresponding fresh culture medium. The maximum DMSO concentration in the in vitro assays was 1%. Previous experiments revealed that this solvent level has no significant effects on cell proliferation (data not shown).
2.2.3. Antiproliferative Assay
Molecules 1, 2, 4–6 were tested against the previously referred cell lines by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) assay [24]. Briefly, cells suspensions (2 × 104 cells/mL) were seeded in 96-well culture plates followed by 48 h of adherence before exposition to compounds for 72 h. It was used a 30 µM concentration for preliminary assays and 0.1, 1, 10, 25, 50, and 100 µM for concentration-response studies. Negative control was the untreated cells, and 5-FU was used as the positive control. After exposition to compounds and cells washing (100 µL of phosphate buffer saline, PBS), was added 100 µL of the MTT solution (5 mg/mL), prepared in the appropriate serum-free medium. Then, after incubation (4 h at 37 °C) and MTT removal, DMSO was added to dissolve formazan crystals. The absorbance was determined by using a Bio-rad Xmark spectrophotometer (570 nm). Percentages of cell proliferation were expressed relative to the absorbance determined in negative control cells (after background subtraction). Each assay was performed in quadruplicate (n = 4) and independently repeated.
2.2.4. E-Screening Assay
This assay was performed as previously reported [24,33]. Briefly, ER+ breast T47-D cancer cells (2 × 104 cells/mL) were cultured in 96-well culture plates. After 24 h, the complete medium was replaced by the experimental medium (freshly prepared phenol red-free RPMI medium supplemented with 5% of dextran-coated charcoal-treated fetal calf serum (DCC-FCS)) containing the compounds under study (4 and 5) for 6 days (replaced every 3 days). The tested concentrations were 0.1, 0.01, and 0.001 µM. Untreated cells served as a negative control, and cells treated with 17β-estradiol were used as a positive control. The above described MTT assay was used to estimate the percentage of cell proliferation. After background subtraction, cell proliferation values were expressed as a percentage relative to the absorbance determined in negative control cells. Again, each experiment was performed in quadruplicate and independently repeated.
2.2.5. Cell Viability Evaluation
In 6-well plates, HepaRG cells (5 × 104 cells/mL; 48 h of attachment) were treated with experimental molecules (compounds 4 and 5; 50 µM, 24 h). Untreated cells were used as a negative control, and cells treated with 5-FU served as the positive control. Each assay was performed in duplicate and was independently repeated. After the treatment, supernatants were collected, and the trypsinization was performed. Then, the obtained cell suspensions were kept on ice, pelleted, and resuspended in complete culture medium (400 µL). Subsequently, cell suspension (395 µL) was transferred to a FACS tube, and propidium iodide (PI, 5 µL at 1 mg/mL) and EDTA (0.5 µL at 0.123 M) were added. A BD Accuri C6 (San Jose, CA, USA) flow cytometer was used in the channels forward scatter (FSC), side scatter (SSC), and fluorescence channel-3 for PI (FL3) to achieve a minimum of 20,000 events. The Modfit LT software (v. 4.1.7) was used to analyze the results: three regions were created (FSC/FL3 contour plot); R1 (viable cells); R2 (dead cells); and R3 (indeterminate cell population between the other two regions), excluding debris. The percentage of cells in R1 in comparison with the total number of events in R1, R2, and R3 was considered the percentage of viability.
2.2.6. Cell Cycle Distribution Study
In 6-well plates, HepaRG cells (5 × 104 cells/mL; 48 h of attachment) were treated with molecule 4 (50 µM, 24 h). After collecting and washing with PBS, the cells were resuspended with a cold solution of 0.5% bovine serum albumin (BSA; Amresco, Atlanta, GA, USA) in PBS containing EDTA (204 µM) (450 µL) and then fixed with 70% of EtOH and incubated at −20 °C (during 2 days or more). After washing with PBS, fixed cells were resuspended in a PI solution (50 µg/mL; solution in 0.5% BSA in PBS with EDTA) and incubated for 15 min in the dark with Ribonuclease A (0.5 µg/µL; solution in 50% glycerol, 10 mM Tris-HCl, pH 8; Sigma Aldrich, St. Louis, MO, USA). The negative control was untreated cells, and 5-FU was used as a positive control. BD Accuri C6 flow cytometer and Modfit LT software (v. 4.1.7) (Becton Dickinson, San Jose, CA, USA) were used for data acquisition and analysis, respectively. Each experiment was performed in duplicate and independently repeated.
2.2.7. Cell Proliferation Analysis by the Carboxyfluorescein Succinimidyl Ester Assay
This assay was performed as previously described [24]. HepaRG cells (12-well plates, 1 mL/well; 8 × 104 cells/mL) were incubated for 15 min or 30 min with carboxyfluorescein succinimidyl ester (CFSE; 10 µM, BD Horizon, San Jose, CA, USA). After being washed, cells were treated with molecule 4 (50 µM; 48 and 72 h). After incubation, cells were trypsinized, pelleted, and resuspended with fresh medium (300 µL) containing EDTA (5 µL). Negative control was the untreated cells. BD Accuri C6 flow cytometer and BD Accuri Software were used for acquisition and analysis, respectively. The channels FSC, SSC, and fluorescence channel-1 (FL1, for CFSE) were selected. Each experiment was performed in duplicate and independently repeated for 15 min studies, and it was performed in one experiment for 48 and 72 h for 30 min assays.
2.3. Molecular Docking
2.3.1. Preparation of Proteins for Molecular Docking
ERα (PDB ID: 1A52), ST (PDB ID: 1P49), and 17β-HSD1 (PDB ID: 3KLM) crystal structures were obtained from protein data bank (PDB) [34,35,36]. These crystal structures were selected mainly due to the fact that these enzymes were complexed with endogenous molecules such as E2 and DHT, which are also structurally similar to the tested compounds, and also considering their high resolution.
Software Chimera (v. 1.10.1) was used to delete the coordinates of all non-standard residues, including the co-crystalized ligand. Using AutoDockTools (v. 1.5.6) software, nonpolar hydrogens were merged and Kollman and Gasteiger partial charges were added. Then, prepared structures were converted from the PDB format to PDBQT for docking studies.
2.3.2. Preparation of Ligands
ChemDraw and Chem3D (v. 12.0) were used to build all ligands. In Chem3D were performed the energy minimization and geometry optimization, and the final structures were saved as a PDB file format. The energy minimization was performed in the range from −20 to −40 kcal·mol−1. AutoDockTools software converted PDB to PDBQT format.
2.3.3. Grid Parameters
AutoDock vina and AutoDockTools were used to calculate the grid parameters, which were based on the coordinates of the adequate co-crystalized ligand: E2, N-acetyl-D-glucosamine, and 5α-dihydrotestosterone (DHT). The grid box (its size was 20 × 20 × 20 with 1.0 Å of spacing) was centered on the ligand with the following coordinates: for ERα, the coordinates were x = 107.27, y = 13.94, z = 96.38; for ST were x = 62.033, y = −12.215, z = 52.512; and for 17β-HSD1 were x = 11.643, y = 9.297, z = −11.887.
2.3.4. Docking Simulations
AutoDock vina executable was used to perform molecular docking [37]. The parameter exhaustiveness used was 15. Discovery Studio Visualizer program from BIOVIA and PyMOL software were used to analyze and visualize the results.
2.3.5. Validation of the Molecular Docking Performance
To validate docking process, the root-mean-square distance (RMSD) value should be less than 2.0 Å [38]. In this study, re-docking ERα with E2, ST with N-acetyl-D-glucosamine, and 17β-HSD1 with DHT were performed for method validation. As expected, low RMSD values (<2) were obtained for all the cases, which validated the docking process.
2.4. Statistical Analysis
IC50 estimation was performed by sigmoidal fitting analysis (95% confidence level). Results were expressed as mean ± standard deviation (SD), and statistical significance was analyzed through the t-Student test (two groups) and one-way ANOVA (three groups) followed by Bonferroni post-hoc tests. Results were considered statistically significant when p < 0.05.
3. Results
3.1. Chemistry
Scheme 1 represents the performed synthesis of the three C-ring oxidized E1 acetate analogs explored in the present study as previously described [32,39,40,41]. The acetylation of compound E1 (96% yield) was successfully achieved using Ac2O and DMAP [39]. Compound 4 was synthesized at an acceptable yield through the 9α-hydroxylation procedure involving the in situ formation of dimethyldioxirane as described in the literature [40]. The oxidation of E1 acetate using CAN [41] allowed the preparation of 9α-hydroxy-11β-(nitrooxy)-17-oxoestra-1,3,5(10)-trien-3-yl acetate (compound 5). The preparation of steroid 6 was firstly attempted by means of a DDQ-mediated dehydrogenation of estrone acetate 2 [32]. However, in addition to incomplete substrate consumption, a mixture of inseparable products was obtained. Therefore, we decided to start the synthesis of compound 6 by the successful transformation of estrone E1 into Δ9,11-estrone 3, again by using DDQ, as previously described [24,32]. Then, the acetylation of compound 3 allowed the preparation of compound 6 with an 89% yield. Starting from this 3-acetylated compound, two additional C-ring oxidized E1 derivatives were also tried to prepare. For this, by application of allylic oxidation reaction conditions, previously described by some of us [42], we attempted to synthesize 12-oxo-Δ9,11-estrone acetate. However, low reactivity was observed, accompanied by the formation of a complex mixture of products (TLC control). In addition, by using m-chloroperoxybenzoic acid [43], we could prepare 9α,11α-estrone acetate. However, in the purification step to isolate the pure 9α,11α-diastereoisomer, we found that this compound was unstable and was quickly transformed into several products (TLC control). This fact was also previously reported [44]. Therefore, this compound was not included in the present study.
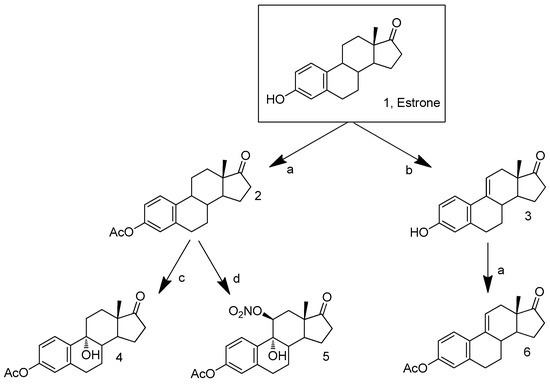
Scheme 1.
Synthetic route to prepare the C-ring oxidized estrone acetate derivatives 4, 5 and 6. Reagents and conditions: (a) acetic anhydride, DMAP, THF, rt; (b) DDQ, MeOH, reflux; (c) OxoneTM, acetone, CH2Cl2, H2O, NaHCO3, TBAHS, 15 °C; (d) CAN, H2O, acetic acid, rt.
Spectral analysis (IR, 1H- and 13C-NMR; spectra presented in Supplementary Information) for all prepared compounds are in agreement with the literature [22,28,30,31]. The existence of the acetate group in compounds 2 and 6 was evidenced by a signal near 2.26 ppm (1H-NMR). The presence of the 9α-hydroxyl functional group of compound 4 was detected by the appearance of OH signals in the IR spectrum as well as by a signal at 70.21 ppm in the 13C-NMR spectra when compared with spectral data for compound 2. In addition, the signal of C11 proton (compound 6) at 6.21 ppm indicated the presence of the Δ9,11 double bond. Moreover, the triplet at 5.76 ppm corresponds to the typical signal of the 11α-hydrogen of the 9α-hydroxy-11β-nitrooxy derivative 5 [22].
3.2. Cell Proliferation
The antiproliferative activity was studied on MCF-7, T47-D, LNCaP, HepaRG, and Caco-2 cancer cells and on normal fibroblasts (NHDF) by the MTT assay [45]. Figure 2 summarizes the results of the initial screening at 30 µM for all compounds. IC50 was calculated when a reduction in cell proliferation was higher than 50% (Table 1). The most cytotoxic compound was molecule 5, with the lowest determined IC50 values for all tested cells. Interestingly, higher selectivity index (SI) was determined for compound 5 (Table 2) in hormone-dependent (MCF-7, T47-D, and LNCaP) cancer cells. A SI value higher than 2 indicates a high selectivity for cancer cells [46]. In addition, compounds 4 and 5 were tested on T47-D (ER+) cells to evaluate their potential estrogenic capability (Figure 3) [33,47] in comparison with E2, which led to an increase in proliferation of T47-D cells in all concentrations studied. Unfortunately, compound 5 also led to cell proliferation at 0.1 µM (140%) when compared with the negative control. On the other hand, and interestingly, compound 4 did not exhibit proliferative action.
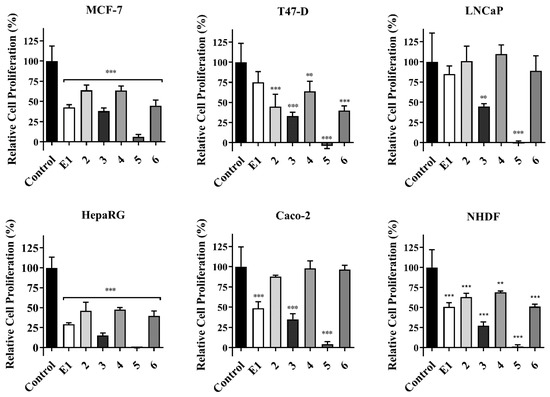
Figure 2.
Relative cell proliferation of MCF-7, T47-D, LNCaP, HepaRG, and Caco-2 cancer cells and normal fibroblasts (NHDF) exposed to the tested compounds for 72 h at 30 µM (MTT assay). Data are expressed as a percentage of cell proliferation relative to the negative control, are presented as mean ± SD, and are representative of at least two independent experiments. ** p < 0.01; *** p < 0.001 vs. control (Student t-test).

Table 1.
IC50 (µM) of compounds 2, 4–6 and 5-fluorouracil (5-FU) against MCF-7, T47-D, LNCaP, HepaRG, and Caco-2 cancer cells and normal fibroblasts (NHDF) a.

Table 2.
Selectivity index (SI) a of compounds 1, 5 and 5-fluorouracil (5-FU).
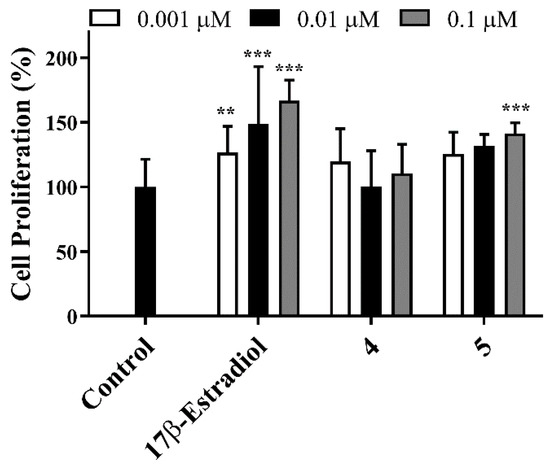
Figure 3.
E-screening assay of compounds 4 and 5 in T-47D cells. Each bar represents the mean ± SD (two independent experiments). ** p < 0.01 vs. control; *** p < 0.001 vs. control (one-way ANOVA post-hoc Bonferroni test).
3.3. Flow Cytometry Experiments
HepaRG cell survival was evaluated by flow cytometry (PI staining) for the compounds with the highest interest (Figure 4). The reduction in cell viability for compound 4 was not statistically significant at 24 h. However, compound 5 led to a drastic reduction in cell viability (92%). Additionally, in Figure 5 are presented images of HepaRG cells after the treatment with this compound.
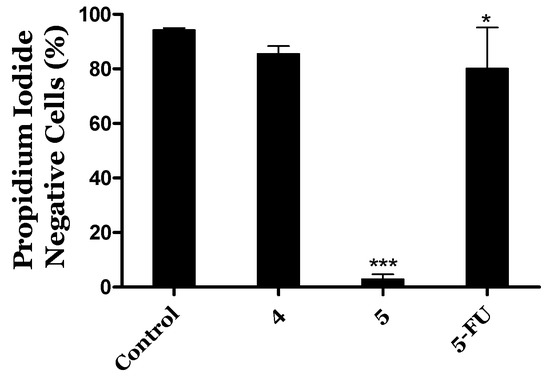
Figure 4.
Percentage of viable HepaRG cells treated with steroids 4 and 5 (50 µM, for 24 h) by flow cytometric assay with propidium iodide (PI) staining. Untreated cells were used as the control. The percentage of cells in R1 (live cells, PI negative) as compared to the total number of events in R1, R2 (dead cells), and R3 (undetermined cells) was considered the percentage of viability. Each bar represents the mean ± SD (originated from two independent experiments). * p < 0.05; *** p < 0.001 vs. control (two-way ANOVA post-hoc Bonferroni test).
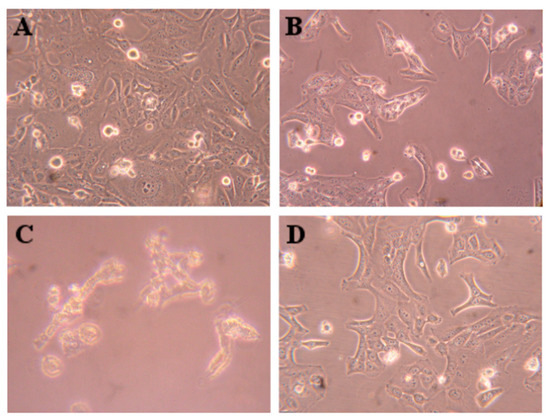
Figure 5.
Microscopic visualization of HepaRG cells (A), control treated with compound 4 (B), compound 5 (C), and 5-fluorouracil (D) at 50 µM for 24 h. Amplification of 100×.
Despite the interesting IC50 and SI values determined for compound 5 (Table 1 and Table 2) and the drastic reduction in cell viability, this compound had a potential estrogenic effect (Figure 3), which is not interesting for the development of anticancer drugs. Therefore, it was decided to explore the effects of compound 4 in cell cycle progression by flow cytometry as well as by the carboxyfluorescein succinimidyl ester assay. By means of a cell cycle distribution study, it was evidenced that steroid 4 (50 µM) induced a G0/G1 cell cycle arrest at 24 h post treatment (Figure 6), with an increase in cell percentage in G0/G1 and a reduction in the percentage of cells in S phase. Using an adapted carboxyfluorescein succinimidyl ester assay, the cell proliferation after 48 and 72 h was also studied [48]. Interestingly, compound 4 led to a higher intensity signal than control cells due to a lower number of cell replications (Figure 7). Furthermore, from 48 to 72 h, control cell median fluorescence diminished (evidence of cell division), but the fluorescence of treated cells remained unchanged, suggesting that no cell division occurred during this time period.
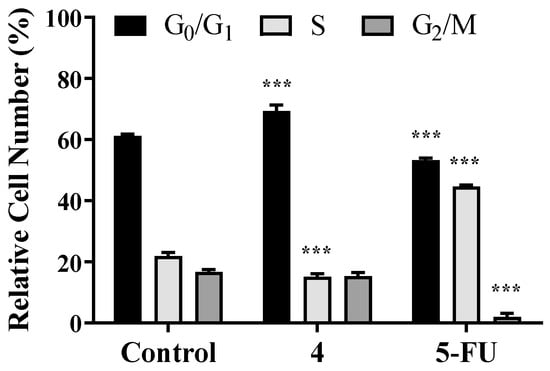
Figure 6.
HepaRG cycle distribution after treatment with compound 4 (at 50 µM) for 24 h. 5-Fluorouracil ((5-FU), 50 µM) was used as positive control and untreated cells as a negative control. Each bar represents the mean ± SD (originating from two independent experiments). *** p < 0.001 vs. control (two-way ANOVA post-hoc Bonferroni test).
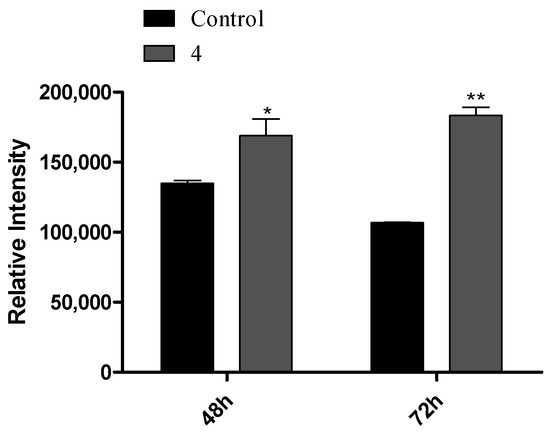
Figure 7.
HepaRG relative fluorescence intensity after treatment with compound 4 (50 µM) for 48 and 72 h, obtained by the carboxyfluorescein succinimidyl ester assay. Each bar represents the median with a range of two samples. * p < 0.05; ** p < 0.01 vs. control (two-way ANOVA post-hoc Bonferroni test).
3.4. Molecular Docking
The knowledge of the target and respective binding site of the molecule under study is essential in drug development [49]. Three steroidal targets have been studied by molecular docking: ERα, ST, and 17β-HSD type 1. ERα is involved in the control of many physiological processes, such as cell proliferation [50]. ST and 17β-HSD type 1 are also relevant for the regulation of cell replication by adjusting steroid hormone levels [51]. In fact, ST makes the conversion of 3-sulfated steroids into their hydroxylated analogs, including the transformation of E1 sulfate into E1. In addition, 17β-HSD type 1 reduces the 17-carbonyl group of androstane and estrane molecules to more potent 17β-hydroxysteroids [52]. Therefore, their deregulation is involved in cancer development, particularly in hormone-dependent breast cancers.
Molecular docking studies were made against ERα, ST, and 17β-HSD1 by means of AutoDock vina executable. The results obtained for compounds 2, 4–6 are summarized in Table 3. For all the tested compounds, binding energy values were similar for ERα and higher than those determined for E2, which suggests a weak affinity to this target. In addition, by analyzing the interactions between these compounds and the macromolecule, marked differences were observed when compared with the interactions performed by E2. However, regarding the results for compound 5, which presented the best affinity value, it is possible to verify some common interactions between this compound and the reference compound (E2). In fact, although compound 5 does not establish the essential conventional hydrogen bonds with His524 and Glu353 residues, there are some interactions in common, such as Van der Waals interactions with Leu387, Met388, and Leu391 residues and alkyl and pi-alkyl interactions with Phe404, as it is shown in Figure 8. In addition, despite the absence of interaction with Glu353, this compound establishes a Van der Waals interaction with Hist524, a weaker interaction when compared with the conventional hydrogen bond. Concerning the 17β-HSD1 enzyme, the lowest energy was obtained with compounds 2 and 6 (Figure 9). Although the energy values were similar to those previously obtained with the ligand DHT, these compounds do not perform the essential interaction for inhibitory activity, which is the hydrogen bond with His221 [53]. However, both compounds seem to interact with Leu149, Pro187, and Val143 residues through alkyl and pi-alkyl interactions, similarly to DHT. In addition, compounds 2 and 6 establish a conventional hydrogen bond with Ser222, although the biological significance of this interaction is unclear. Furthermore, their binding mode is very similar, and both structures are practically overlapping within the macromolecule. Concerning ST enzyme, in addition to the weak affinity energies obtained, no relevant interactions with the amino acid involved in the binding site (Leu74, Arg98, Thr99, Val101, Leu103, Leu167, Val177, Phe178, Thr180, Gly181, Thr484, and Phe488) [54] were observed.

Table 3.
Predicted affinity energies of compounds E1, 2, 4–6 calculated from molecular docking against known protein targets of steroidal molecules (ERα, ST, and 17β-HSD1) a.
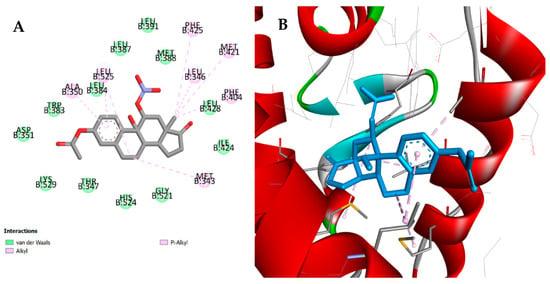
Figure 8.
Predicted interactions and binding mode of the best ranked and synthetized compound 5, with ERα in 2D (panel A) and 3D (panel B). (A) Van der Waals interactions are displayed in green, and alkyl and pi-alkyl interactions in soft pink. (B) Binding mode of compound 5 in active site of ERα. Van der Waals interactions are displayed in green, and alkyl and pi-alkyl interactions in soft pink.
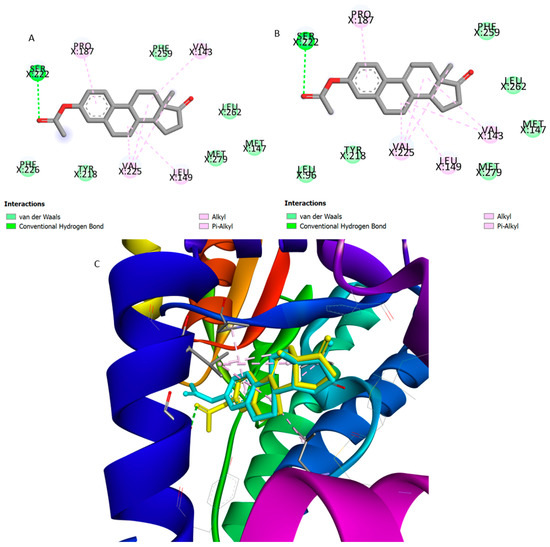
Figure 9.
Predicted interactions of the best ranked and synthetized compounds 2 and 6, with 17β-HSD1 in 2D (panel A and B) and 3D (panel B). (A) Van der Waals interactions are displayed in light green, conventional hydrogen bonds in green, and alkyl and pi-alkyl in pink. Both compounds, as co-crystalized ligand DHT, present alkyl and pi-alkyl interactions with Pro187, Val143, and Leu149. (B) Van der Waals interactions are displayed in light green, conventional hydrogen bonds in green, and alkyl and pi-alkyl in pink. (C) 3D representation of overlapping of compound 2 (in turquoise) and 6 (in yellow) in the macromolecule binding pocket.
4. Discussion
The chemical synthesis was performed under relatively mild reaction conditions. The use of CAN as an oxidant originated compound 5 in 34% yield [41]. In fact, and interestingly, in A-ring aromatic steroid derivatives, CAN leads to hydroxylation of the C9 benzyl atom and nitration of the C11 homobenzyl position [55]. The mechanism possibly involves the dehydration at C9-C11 followed by a nucleophilic addition at C11 and the formation of a radical at C9, which reacts with cerium (IV) to form a carbon cation, which can react with a second nucleophile, giving rise to hydroxyl group [22]. Steroid 6 was synthesized (89% yield) by the successful transformation of estrone E1 into Δ9,11-estrone 3 by DDQ, followed by its acetylation [39]. Dehydrogenation using DDQ is widely used to obtain aromatic and α,β-unsaturated carbonyls. The mechanism includes the hydride transfer to the quinone oxygen with the formation of a carbocation in the substrate, followed by the transference of a proton to the phenolate ion and the concomitant formation of a double bond [56]. Isolated or in situ formed dioxiranes have been used as oxidants because they are selective and can allow mild reaction conditions [57]. In this context, the hydroxylation of E1 acetate by dimethyldioxirane enabled the selective oxyfunctionalization at C9α [40], affording steroid 4 (55% yield). The reaction involves the in situ formation of dimethyldioxirane by the reaction of OxoneTM with acetone and subsequent oxidative attack involving the tertiary carbon.
Concerning the cell viability studies and structure-activity relationship data, the introduction of a 9α hydroxyl group to E1 acetate (compound 4) did not enhance the antiproliferative activity in hormone-dependent cancer cells when compared with E1 acetate (compound 2). However, this hydroxylation led to a higher antiproliferative activity against HepaRG cells (IC50 = 32.04 µM) than observed with E1 acetate (compound 2) and Δ9,11-E1 acetate (compound 6). Interestingly, the existence of the 11β-nitrooxy group, in addition to the 9α-hydroxyl (compound 5), markedly increased the antiproliferative effect against all cell types studied (Table 1). These are relevant results because in literature, in vitro studies for compounds 4 and 5 are not known to our knowledge. However, it was already evidenced that 11-nitrates of 9α,11β-dihydroxyestratrienes containing various substituents at positions 3 and 17 showed interesting antitumor activity in mongrel rats bearing a model of alveolar breast cancer [23]. In addition, stimulation of the tumor growth was observed after 15 days of treatment, probably due to the estrogenic effect inherent to steroidal molecules [23]. In addition, the 11β-nitrate of 17α-ethynylestradiol-3,17-diacetate also exhibited antiestrogen activity in an assay with the uterus of immature animals [58]. Our results for compound 5 showed an antiproliferative effect more pronounced in hormone-dependent cells (IC50 = 5.87 µM for MCF-7 cells; IC50 = 7.40 µM for T47-D cells; IC50 = 5.30 µM for LNCaP cells). However, in the E-screening assay, compound 5 also revealed estrogenic activity in T47-D cells, which seems to be in concordance with the literature [22,58]. In this context and as an example, Zhang et al. [18] synthesized 11β-estradiol carboxylates, esters, and ethers and showed that when 11β-chain had four or five non-hydrogen atoms, the estrogenic effect diminished, and a SERM (selective estrogen receptor modulator) was obtained. In addition, other structure-activity relationship studies showed that large hydrophobic substituents at 11β side chains originated antiestrogenic compounds and that different chemical groups in this chain promoted different affinities to ER [16,17]. Thus, the potential estrogenic activity associated with compound 5 can be explained by the small size of the 11β-nitrooxy group. On the other hand, in our study, compound 4, which only has a 9α-hydroxyl group, did not show a T47-D proliferation. Interestingly, Alsayari and co-workers [21] demonstrated that 3-methoxyestra-1,3,5(10),9(11)-tetraen-17-one was an estrogenic compound with affinity to ERα and ERβ. Otherwise, the 9-hydroxymethyl-11-keto analog showed low cytotoxicity against MCF-7 cells and also was an estrogenic compound. Thus, the size of the group at C9 seems to be important for estrogenic and cytotoxic activities. Concerning the cell cycle effects of these compounds, some studies showed that several steroids [59,60] as well as non-steroids [61,62,63] lead to cell cycle arrest. In this context and as an example, 11α-substituted 2-methoxyestradiol derivatives led to a cell cycle arrest at G2/M and demonstrated an important antiestrogenic effect [5]. In addition, as an example of non-steroidal compounds interfering in the cell cycle, 2-benzyl-1-(3,4,5-trimethoxyphenyl)-1H-naphtho [1,2-e][1,3]oxazin-3(2H)-one also induced G2/M phase arrest on A2780 cancer cell line and in silico studies showed a relevant molecular interaction with tubulin [63]. Remarkably, in our study, compound 4 induced a G0/G1 phase arrest, probably because it interferes with many proteins of the cell cycle such as cyclin-dependent kinases and others [64,65], which are important for the cellular regulation of DNA replication. However, additional studies are required to clarify the biological mechanisms. The estrogenicity absence of compound 4 associated with an arrest in G0/G1 showed that this steroid may be a suitable starting point to develop potentially improved anticancer drugs. In addition, the CFSE assay showed that compound 4 promoted a low number of cell divisions of HepaRG cancer cells, being less proliferative. This result is in agreement with the cell cycle analysis results. Finally, Δ9,11-E1 acetate (compound 6) showed weaker antiproliferative activity in all studied cell lines when compared with analog 3, which has a 3-hydroxyl group [24].
Molecular docking studied the interactions between these C-ring oxidized E1 acetate analogs and proteins that interact with them [66,67]. Few studies were reported, including docking with C-ring modified steroids [21,68]. Concerning ERα results, weaker binding energies for compound 6 were observed, compared with E2 and with compound 3, according to our previous report [24]. In addition, compounds 4 and 5 also had weaker binding energies to ERα than E1 and E2. These results were expected because nonpolar groups are preferable in C9 and C11 positions for the interaction with ERα [11,69]. Thus, the cytotoxicity originated by compound 5 can be associated with another mechanism of action than the interaction with ERα. In this context, another possible reason is the generation of nitric oxide, which has a cytotoxic effect [70]. As some compounds structurally similar to the described in this work were reported as 17β-HSD1 [53] and ST [54,71] inhibitors, we also included these two proteins in our molecular docking study. Concerning the 17β-HSD1 enzyme, for compounds 2 and 6, although the binding energies were similar to DHT, there is a lack of the important conventional hydrogen bonds present in the binding mode of DHT, as described above. In fact, typical steroidal 17β-HSD1 E1/E2 inhibitors had modifications in D-ring at C16 and C17 positions of the steroid nucleus [72,73]. However, Deluca et al. [74] showed that an estratriene derivative with a C9 modification and fluorine-substitution in position 17 had relevant 17β-HSD1 inhibitory activity. So, modifications only in C9 are not enough to inhibit this enzyme. Taking into account the ST enzyme, these experimental compounds should not markedly bind and interact with this protein with energy lower than the control, N-acetyl-D-glucosamine. Among ST inhibitors based on the E1 skeleton, previous reports showed that 17α-benzyl-derivatives, 17β-arylsulfonamides, 17-diisopropylcarbamoyl-3-O-sulfamates, 2-methoxy-3-O-sulfamates, and 2-methoxy-3,17β-bissulfamates are potent ST inhibitors [60]. However, our results suggested that C-ring modifications tested in the present work were not useful to improve the binding energy to the ST enzyme. Globally, docking results for the prepared compounds with these three targets revealed similar or lower binding energies than the co-crystalized ligands, as well as the absence of some relevant binding points. Therefore, despite that, with these docking results, it is not possible to exclude that these compounds can act by interacting with the three explored proteins. Other targets should be considered in further docking studies. These include not only other proteins influencing hormonal biosynthesis and effects but also proteins and other biomolecules affecting the cell cycle, considering that compound 4 leads to an arrest at G0/G1 phase. Therefore, further in silico and experimental studies would be necessary to further elucidate the activity of these compounds.
5. Conclusions
C-ring modifications in the E1 acetate scaffold originated molecules with higher cytotoxic activities than E1 acetate. Of these, the 9α-hydroxy,11β-nitrooxy derivative 5 showed to be the most cytotoxic molecule against hormone-dependent cancer cells. In addition, the introduction of 11β-nitrooxy group originated a drastic reduction in HepaRG cell viability and increased the proliferation of T47-D cells in E-screening assay. Therefore, similarly to previous studies, it was difficult to dissociate the estrogenic effect from the cytotoxic activity. Importantly, 9α-hydroxyestrone acetate 4 promoted a selective cytotoxic effect on HepaRG cells, induced an arrest at G0/G1 phase, and, interestingly, did not promote T47-D proliferation. This last finding is of major importance since it has been described that usually, estrogen derivatives could stimulate cell proliferation through the interaction with their receptors and consequently stimulate tumor growth. Overall, this preliminary study demonstrated that this structural modification can be of interest to develop new anticancer estrane derivatives without estrogenic activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12073579/s1. Figure S1. IR spectrum of compound 2; Figure S2. 1H-NMR spectrum of compound 2 in CDCl3; Figure S3. 13C-NMR spectrum of compound 2 in CDCl3; Figure S4. IR spectrum of compound 3; Figure S5. 1H-NMR spectrum of compound 3 in DMSO-d6; Figure S6. 13C-NMR spectrum of compound 3 in DMSO-d6; Figure S7. IR spectrum of compound 4; Figure S8. 1H-NMR spectrum of compound 4 in CDCl3; Figure S9. 13C-NMR spectrum of compound 4 in CDCl3; Figure S10. IR spectrum of compound 5; Figure S11. 1H-NMR spectrum of compound 5 in CDCl3; Figure S12. 13C-NMR spectrum of compound 5 in CDCl3; Figure S13. IR spectrum of compound 6; Figure S14. 1H-NMR spectrum of compound 6 in CDCl3; Figure S15. 13C-NMR spectrum of compound 6 in CDCl3.
Author Contributions
Data curation, A.O.S., S.S. and G.A.; Formal analysis, C.C., M.M., V.B., A.O.S. and S.S.; Funding acquisition, A.F., S.S. and G.A.; Investigation, C.C., M.M., P.P., V.B., A.O.S. and S.S.; Methodology, C.C., M.M., A.O.S. and S.S.; Project administration, A.F. and S.S.; Resources, A.O.S., A.F. and G.A.; Software, V.B. and S.S.; Supervision, A.F., S.S. and G.A.; Validation, A.O.S., S.S. and G.A.; Writing—Original draft, C.C., V.B. and S.S.; Writing—Review and editing, C.C., M.M., A.O.S., S.S. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support provided by FEDER funds through the POCI-COMPETE 2020—Operational Programme Competitiveness and Internationalisation in Axis I—Strengthening research, technological development, and innovation (project POCI-01-0145-FEDER-007491) and National Funds by FCT—Foundation for Science and Technology (Project UID/Multi/00709/2013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support provided by FEDER funds through the POCI-COMPETE 2020—Operational Program Competitiveness and Internationalization in Axis I (project POCI-01-0145-FEDER-007491) and National Funds by FCT—Foundation for Science and Technology (project UID/Multi/00709/2019). The authors also would like to thank the support provided by FEDER funds through the “Programa Operacional do Centro” (CENTRO-01-0145-FEDER-000013).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Soronen, P.; Laiti, M.; Törn, S.; Härkönen, P.; Patrikainen, L.; Li, Y.; Pulkka, A.; Kurkela, R.; Herrala, A.; Kaija, H.; et al. Sex steroid hormone metabolism and prostate cancer. Steroid Biochem. Mol. Biol. 2004, 92, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Capper, C.P.; Rae, J.M.; Auchus, R.J. The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm. Cancer 2016, 7, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Sutton, E.L. Oral contraception. Med. Clin. N. Am. 2015, 99, 479–503. [Google Scholar] [CrossRef] [PubMed]
- Lao, K.; Wang, Y.; Chen, M.; Zhang, J.; You, Q. Design, synthesis and biological evaluation of novel 2-methoxyestradiol analogs as dual selective estrogen receptor modulators (SERMs) and antiangiogenic agents. Eur. J. Med. Chem. 2017, 139, 390–400. [Google Scholar] [CrossRef]
- Lee, C.I.; Goodwin, A.; Wilcken, N. Fulvestrant for hormone-sensitive metastatic breast cancer (review). Cochrane Database Syst. Rev. 2017, 3, CD011093. [Google Scholar]
- Salvador, J.A.R.; Carvalho, J.F.S.; Neves, M.A.C.; Silvestre, S.M.; Leitão, A.J.; Silva, M.M.C.; Sá e Melo, M.L. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef]
- de Almeida Chuffa, L.G.; Lupi-Júnior, L.A.; Costa, A.B.; de Arruda Amorim, J.P.; Seiva, F.R.F. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Groner, A.C.; Brown, M. Role of steroid receptor and coregulator mutations in hormone-dependent cancers. J. Clin. Investig. 2017, 127, 1126–1135. [Google Scholar] [CrossRef]
- Napolitano, E.; Fiaschi, R.; Carlson, S.K.E.; Katzenellenbogen, J.A. 11β-Substituted Estradiol Derivatives. 2. Potential Carbon-11- and Iodine-Labeled Probes for the Estrogen Receptor. J. Med. Chem. 1995, 38, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Anstead, G.M.; Carlson, K.E.; Katzenellenbogen, J.A. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 1997, 62, 268–303. [Google Scholar] [CrossRef]
- Claussner, A.; Nédélec, L.; Nique, F.; Philibert, D.; Teutsch, G.; Velde, P. Van 11β-Amidoalkyl Estradiols, A New Series of Pure Antiestrogens. J. Steroid Biochem. Mol. Biol. 1992, 41, 609–614. [Google Scholar] [CrossRef]
- Poirier, D.; Mérand, Y.; Labrie, C.; Labrie, F. D-ring alkylamide derivatives of estradiol: Effect on ER-binding affinity and antiestrogenic activity. Bioorg. Med. Chem. Lett. 1996, 6, 2537–2542. [Google Scholar] [CrossRef]
- Lobaccaro, C.; Pons, J.F.; Duchesne, M.J.; Auzou, G.; Pons, M.; Nique, F.; Teutsch, G.; Borgna, J.L. Steroidal affinity labels of the estrogen receptor. 3. Estradiol 11β-n-alkyl derivatives bearing a terminal electrophilic group: Antiestrogenic and cytotoxic properties. J. Med. Chem. 1997, 40, 2217–2227. [Google Scholar] [CrossRef]
- Aliau, S.; Delettre, G.; Mattras, H.; El Garrouj, D.; Nique, F.; Teutsch, G.; Borgna, J.-L. Steroidal affinity labels of the estrogen receptor α. 4. Electrophilic 11β-Aryl derivatives of estradiol. J. Med. Chem. 2000, 43, 613–628. [Google Scholar] [CrossRef]
- Hanson, R.N.; Hua, E.; Adam Hendricks, J.; Labaree, D.; Hochberg, R.B. Synthesis and evaluation of 11β-(4-Substituted phenyl) estradiol analogs: Transition from estrogen receptor agonists to antagonists. Bioorg. Med. Chem. 2012, 20, 3768–3780. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Labaree, D.C.; Hochberg, R.B. Nonpolar and short side chain groups at C-11β of estradiol result in antiestrogens. J. Med. Chem. 2005, 48, 1428–1447. [Google Scholar] [CrossRef]
- Dwivedy, I.; Gupta, A.; Grover, A.; Srivastava, V.; Singh, M.M.; Ray, S. Synthesis and in vivo evaluation of 11-substituted estradiol derivatives as anti-implantation agents. Bioorg. Med. Chem. Lett. 2008, 18, 4102–4105. [Google Scholar] [CrossRef]
- Wang, P.; McInnes, C.; Zhu, B.T. Structural Characterization of the Binding Interactions of Various Endogenous Estrogen Metabolites with Human Estrogen Receptor α and β Subtypes: A Molecular Modeling Study. PLoS ONE 2013, 8, e74615. [Google Scholar] [CrossRef][Green Version]
- Alsayari, A.; Kopel, L.; Ahmed, M.S.; Pay, A.; Carlson, T.; Halaweish, F.T. Design, synthesis, and biological evaluation of steroidal analogs as estrogenic/anti-estrogenic agents. Steroids 2017, 118, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.H.; Crowe, D.F.; Avery, M.A.; Chong, W.K.M.; Tanabe, M. 11β-Nitrate Estrane Analogues: Potent Estrogens. J. Med. Chem. 1989, 32, 2306–2310. [Google Scholar] [CrossRef] [PubMed]
- Rzheznikov, V.M.; Golubovskaya, L.E.; Minailova, O.N.; Osetrova, I.P.; Smirnova, Z.S. Steroidal Nitrates: Synthesis and Antitumor Activity of of 9α,11β-Dihydroxyestra-1,3,5(10)-triene 11-nitrates. Pharm. Chem. J. 2003, 37, 10–12. [Google Scholar] [CrossRef]
- Canário, C.; Matias, M.; Brito, V.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. Δ9,11-Estrone derivatives as potential antiproliferative agents: Synthesis, in vitro biological evaluation and docking studies. C. R. Chim. 2020, 23, 201–217. [Google Scholar] [CrossRef]
- Brito, V.; Santos, A.O.; Almeida, P.; Silvestre, S. Novel 4-azaandrostenes as prostate cancer cell growth inhibitors: Synthesis, antiproliferative effects, and molecular docking studies. C. R. Chim. 2019, 22, 73–83. [Google Scholar] [CrossRef]
- Canário, C.; Silvestre, S.; Falcão, A.; Alves, G. Steroidal Oximes: Useful Compounds with Antitumor Activities. Curr. Med. Chem. 2018, 25, 660–686. [Google Scholar] [CrossRef] [PubMed]
- Canário, C.; Matias, M.; Brito, V.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. New Estrone Oxime Derivatives: Synthesis, Cytotoxic Evaluation and Docking Studies. Molecules 2021, 26, 2687. [Google Scholar] [CrossRef]
- Simeón, J.L.L.; Morales, J.E.T.; Navarro, F.A.V.; Manchado, F.C.; Montoto, L.G.P. Actividad catalítica del acetato de vanadilo en la acetilación de alcoholes secundarios. Rev. CENIC Cienc. Químicas 2004, 35, 141–145. [Google Scholar]
- D’Accolti, L.; Fusco, C.; Lampignano, G.; Capitelli, F.; Curci, R. Oxidation of natural targets by dioxiranes. Part 6: On the direct regio- and site-selective oxyfunctionalization of estrone and of 5α-androstane steroid derivatives. Tetrahedron Lett. 2008, 49, 5614–5617. [Google Scholar] [CrossRef]
- Quinkert, G.; Weber, W.-D.; Schwartz, U. Process for Synthesizing Estrone or Estrone Derivatives. U.S. Patent 4,357,278, 2 November 1982. [Google Scholar]
- Bovicelli, P.; Lupattelli, P.; Mincione, E.; Prencipe, T.; Curci, R. Oxidation of Natural Targets by Dioxiranes. Oxyfunctionalization of Steroids. J. Org. Chem. 1992, 57, 2182–2184. [Google Scholar] [CrossRef]
- Stéphan, E.; Zen, R.; Authier, L.; Jaouen, G. Improved synthesis of a protected 11-oxoestrone. Steroids 1995, 60, 809–811. [Google Scholar] [CrossRef]
- Ayan, D.; Maltais, R.; Roy, J.; Poirier, D. A new nonestrogenic steroidal inhibitor of 17β-hydroxysteroid dehydrogenase type I blocks the estrogen-dependent breast cancer tumor growth induced by estrone. Mol. Cancer Ther. 2012, 11, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, D.M.; Wang, Y.; Williams, S.P.; Sigler, P.B. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5998–6003. [Google Scholar] [CrossRef]
- Hernandez-Guzman, F.G.; Higashiyama, T.; Pangborn, W.; Osawa, Y.; Ghosh, D. Structure of human estrone sulfatase suggests functional roles of membrane association. J. Biol. Chem. 2003, 278, 22989–22997. [Google Scholar] [CrossRef] [PubMed]
- Aka, J.A.; Mazumdar, M.; Chen, C.-Q.; Poirier, D.; Lin, S.-X. 17β-Hydroxysteroid Dehydrogenase Type 1 Stimulates Breast Cancer By Dihydrotestosterone Inactivation in Addition To Estradiol Production. Mol. Endocrinol. 2010, 24, 832–845. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Carugo, O. How root-mean-square distance (r.m.s.d.) values depend on the resolution of protein structures that are compared. J. Appl. Crystallogr. 2003, 36, 125–128. [Google Scholar] [CrossRef]
- Murugan, R.; Scriven, E.F.V. Applications of dialkylaminopyridine (DMAP) catalysts in organic synthesis. Aldrichima Acta 2003, 36, 21–27. [Google Scholar] [CrossRef]
- Schwarz, S.; Schumacher, M.; Nanninga, A.; Weber, G.; Thieme, I.; Undeutsch, B.; Elger, W. 17β-Hydroxy-11α-(3’-sulfanylpropyl)oxy-estra-1,3,5(10)-trien-3-yl sulfamate—A novel hapten structure: Toward the development of a specific enzyme immunoassay (EIA) for estra-1,3,5(10)-triene-3-yl sulfamates. Steroids 1999, 64, 460–471. [Google Scholar] [CrossRef]
- Sykes, P.J.; Rutherford, F.J.; Laing, S.B.; Phillipps, G.H.; Turnbull, J.P. Oxidation of ring a-aromatic steroids to 9,11β-diol 11-nitrates with ceric ammonium nitrate. Tetrahedron Lett. 1971, 12, 3393–3396. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Silvestre, S.M. Bismuth-catalyzed allylic oxidation using t-butyl hydroperoxide. Tetrahedron Lett. 2005, 46, 2581–2584. [Google Scholar] [CrossRef]
- Liang, C.D.; Baran, J.S. Synthesis and Conformational Stabilities of 11-Oxo-9α- and 9β-Estradiol 3-Benzyl Ether. Tetrahedron 1976, 32, 2067–2069. [Google Scholar] [CrossRef]
- Gao, H. Approaches to partial syntheses of 11-oxo steroids. A brief review. Org. Prep. Proced. Int. 1997, 29, 499–539. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Dévéhat, F.L.-L.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Cortés-Benítez, F.; Roy, J.; Maltais, R.; Poirier, D. Impact of androstane A- and D-ring inversion on 17β-hydroxysteroid dehydrogenase type 3 inhibitory activity, androgenic effect and metabolic stability. Bioorg. Med. Chem. 2017, 25, 2065–2073. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, L.; Hernández-Linares, M.G.; Escobar, M.L.; López-Muñoz, H.; Zenteno, E.; Fernández-Herrera, M.A.; Guerrero-Luna, G.; Carrasco-Carballo, A.; Sandoval-Ramírez, J. Antiproliferative, Cytotoxic and Apoptotic Activity of Steroidal Oximes in Cervicouterine Cell Lines. Molecules 2016, 21, 1533. [Google Scholar] [CrossRef]
- Makar, S.; Saha, T.; Swetha, R.; Gutti, G.; Kumar, A.; Singh, S.K. Rational Approaches of Drug Design for the Development of Selective Estrogen Receptor Modulators (SERMs), Implicated in Breast Cancer. Bioorg. Chem. 2020, 94, 103380. [Google Scholar] [CrossRef]
- Miki, Y.; Iwabuchi, E.; Ono, K.; Sasano, H.; Ito, K. Exploring Protein-Protein Interaction in the Study of Hormone-Dependent Cancers. Int. J. Mol. Sci. 2018, 19, 3173. [Google Scholar] [CrossRef]
- Cornel, K.M.C.; Krakstad, C.; Delvoux, B.; Xanthoulea, S.; Jori, B.; Bongers, M.Y.; Konings, G.F.J.; Kooreman, L.F.S.; Kruitwagen, R.F.; Salvesen, H.B.; et al. High mRNA levels of 17β-hydroxysteroid dehydrogenase type 1 correlate with poor prognosis in endometrial cancer. Mol. Cell. Endocrinol. 2017, 442, 51–57. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Day, J.M.; Tutill, H.J.; Purohit, A.; Reed, M.J. Design and validation of specific inhibitors of 17β-hydroxysteroid dehydrogenases for therapeutic application in breast and prostate cancer, and in endometriosis. Endocr. Relat. Cancer 2008, 15, 665–692. [Google Scholar] [CrossRef] [PubMed]
- Daśko, M.; Demkowicz, S.; Biernacki, K.; Ciupak, O.; Kozak, W.; Masłyk, M.; Rachon, J. Recent progress in the development of steroid sulphatase inhibitors–examples of the novel and most promising compounds from the last decade. J. Enzym. Inhib. Med. Chem. 2020, 35, 1163–1184. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, L.E.; Rzheznikov, V.M. Oxidation of estra-1,3,5(10)-triene-3,11α,17β-triol triacetate with ceric ammonium nitrate. Russ. J. Org. Chem. 2007, 43, 1730–1732. [Google Scholar] [CrossRef]
- Batista, V.S.; Crabtree, R.H.; Konezny, S.J.; Luca, O.R.; Praetorius, J.M. Oxidative functionalization of benzylic C-H bonds by DDQ. New J. Chem. 2012, 36, 1141–1144. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Silvestre, S.; Moreira, V.M. Recent Developments in Oxidative Processes in Steroid Chemistry. Curr. Org. Chem. 2012, 16, 1243–1276. [Google Scholar] [CrossRef]
- Golubovskaya, L.E.; Ivanenko, T.I.; Rzheznikov, V.M. Steroidal nitrates. Part III. Synthesis and antiestrogen activity of the 11α-nitroxy analog of ethynylestradiol. Pharm. Chem. J. 2009, 43, 560–562. [Google Scholar] [CrossRef]
- Berényi, Á.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Mernyák, E.; Frank, É.; Schneider, G.; Zupkó, I. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Shavva, A.G. Estrone Sulfatase Inhibitors as new anticancer agents. In Chemistry and Biological Activity of Steroids; IntechOpen: London, UK, 2016; pp. 1–26. [Google Scholar]
- Bader, A.; Bkhaitan, M.M.; Abdalla, A.N.; Abdallah, Q.M.A.; Ali, H.I.; Sabbah, D.A.; Albadawi, G.; Abushaikha, G.M. Design and Synthesis of 4-O-Podophyllotoxin Sulfamate Derivatives as Potential Cytotoxic Agents. Evid.-Based Complement. Altern. Med. 2021, 2021, 6672807. [Google Scholar] [CrossRef]
- Shen, G.; Wang, C.; Luo, Y.; Wang, J.; Wang, R.; Xu, W.; Zhang, Y.; Zhang, Y.; Zhang, D.; Jin, C. 2-(6-Hydroxyhexylthio)-5,8-dimethoxy-1,4-naphthoquinone Induces Apoptosis through ROS-Mediated MAPK, STAT3, and NF- κ B Signalling Pathways in Lung Cancer A549 Cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 7375862. [Google Scholar] [CrossRef]
- Mirzaei, S.; Qayumov, M.; Gangi, F.; Behravan, J.; Ghodsi, R. Synthesis and biological evaluation of oxazinonaphthalene-3-one derivatives as potential anticancer agents and tubulin inhibitors. Iran. J. Basic Med. Sci. 2020, 23, 1388–1395. [Google Scholar] [PubMed]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and cdks in development and cancer: A perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Romagosa, C.; Simonetti, S.; López-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Cajal, S.R.Y. P16Ink4a overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Lespérance, M.; Barbeau, X.; Roy, J.; Maltais, R.; Lagüe, P.; Poirier, D. Chemical synthesis of C3-oxiranyl/oxiranylmethyl-estrane derivatives targeted by molecular modeling and tested as potential inhibitors of 17β-hydroxysteroid dehydrogenase type 1. Steroids 2018, 140, 104–113. [Google Scholar] [CrossRef]
- Woo, L.W.L.; Leblond, B.; Purohit, A.; Potter, B.V.L. Synthesis and evaluation of analogues of estrone-3-O-sulfamate as potent steroid sulfatase inhibitors. Bioorg. Med. Chem. 2012, 20, 2506–2519. [Google Scholar]
- El-Kady, D.S.; Abd Rabou, A.A.; Tantawy, M.A.; Abdel-Rahman, A.A.H.; Abdel-Megeed, A.A.S.; AbdElhalim, M.M.; Elmegeed, G.A. Synthesis and Evaluation of Novel Cholestanoheterocyclic Steroids as Anticancer Agents. Appl. Biochem. Biotechnol. 2019, 188, 635–662. [Google Scholar] [CrossRef]
- Palomino, E. Chemical modulation of activity in steroidal estrogens. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 387–398. [Google Scholar] [CrossRef]
- Kerwin, J.F.; Lancaster, J.R.; Feldman, P.L. Nitric Oxide: A New Paradigm for Second Messengers. J. Med. Chem. 1995, 38, 4343–4362. [Google Scholar] [CrossRef]
- Nussbaumer, P.; Billich, A. Steroid sulfatase inhibitors. Med. Res. Rev. 2004, 24, 529–576. [Google Scholar] [CrossRef]
- Maltais, R.; Ayan, D.; Trottier, A.; Barbeau, X.; Lagüe, P.; Bouchard, J.E.; Poirier, D. Discovery of a non-estrogenic irreversible inhibitor of 17β-Hydroxysteroid dehydrogenase type 1 from 3-substituted-16β-(m-carbamoylbenzyl)-estradiol derivatives. J. Med. Chem. 2014, 57, 204–222. [Google Scholar] [CrossRef]
- Maltais, R.; Trottier, A.; Barbeau, X.; Lagüe, P.; Perreault, M.; Thériault, J.; Lin, S.; Poirier, D. Impact of structural modifications at positions 13, 16 and 17 of 16β-(m-carbamoylbenzyl)-estradiol on 17β-hydroxysteroid dehydrogenase type 1 inhibition and estrogenic activity. J. Steroid Biochem. Mol. Biol. 2016, 161, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Deluca, D.; Möller, G.; Rosinus, A.; Elger, W.; Hillisch, A.; Adamski, J. Inhibitory effects of fluorine-substituted estrogens on the activity of 17β-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2006, 248, 218–224. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).