Analysis of the Refraction Effect in Ultrasound Breast Tomography
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
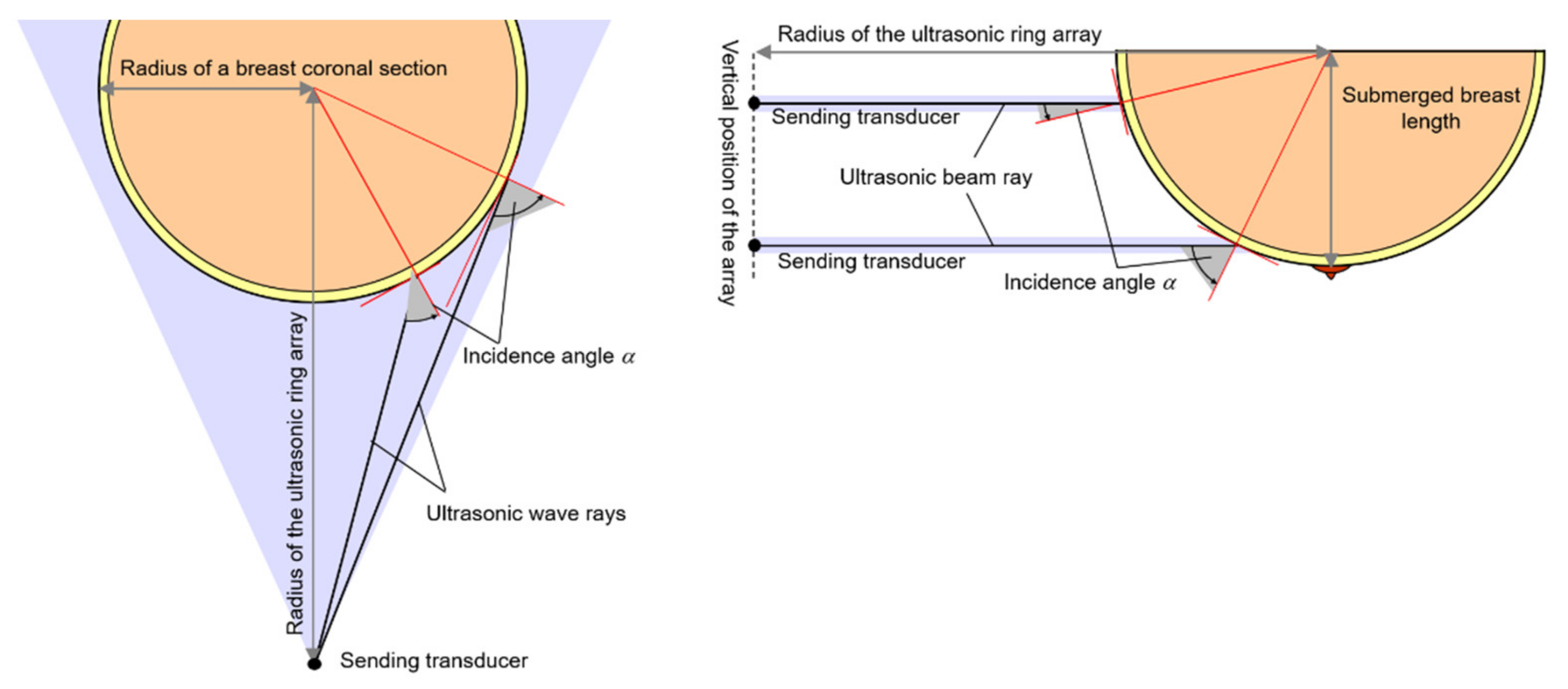
2.1. Refraction in Transmission Method
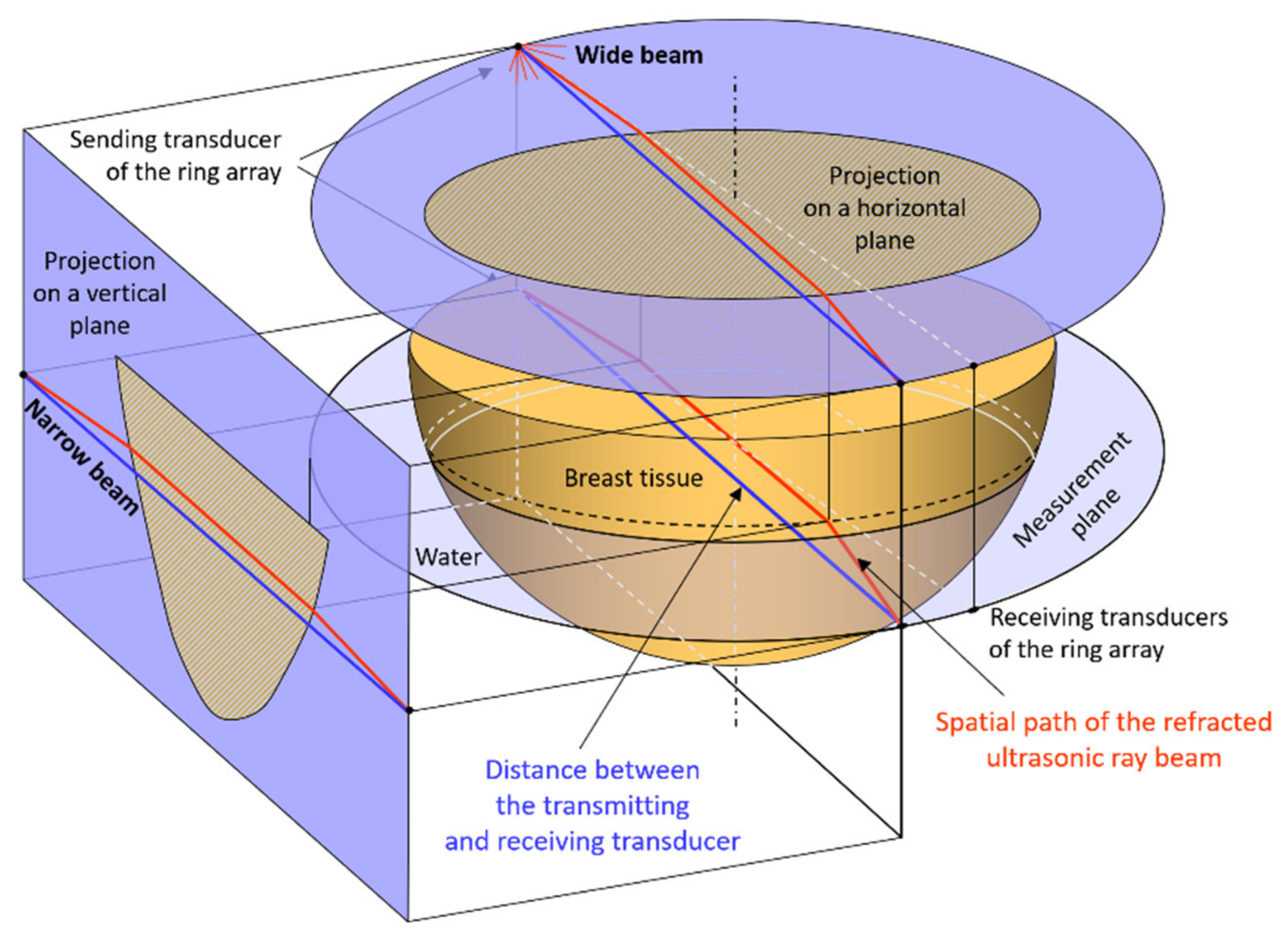
2.2. Refraction in Ultrasound Transmission Tomography Scanning
2.3. Acoustic Parameters of Water, Fat, and Glandular Tissue of the Breast
2.4. Method and Measuring System
3. Results
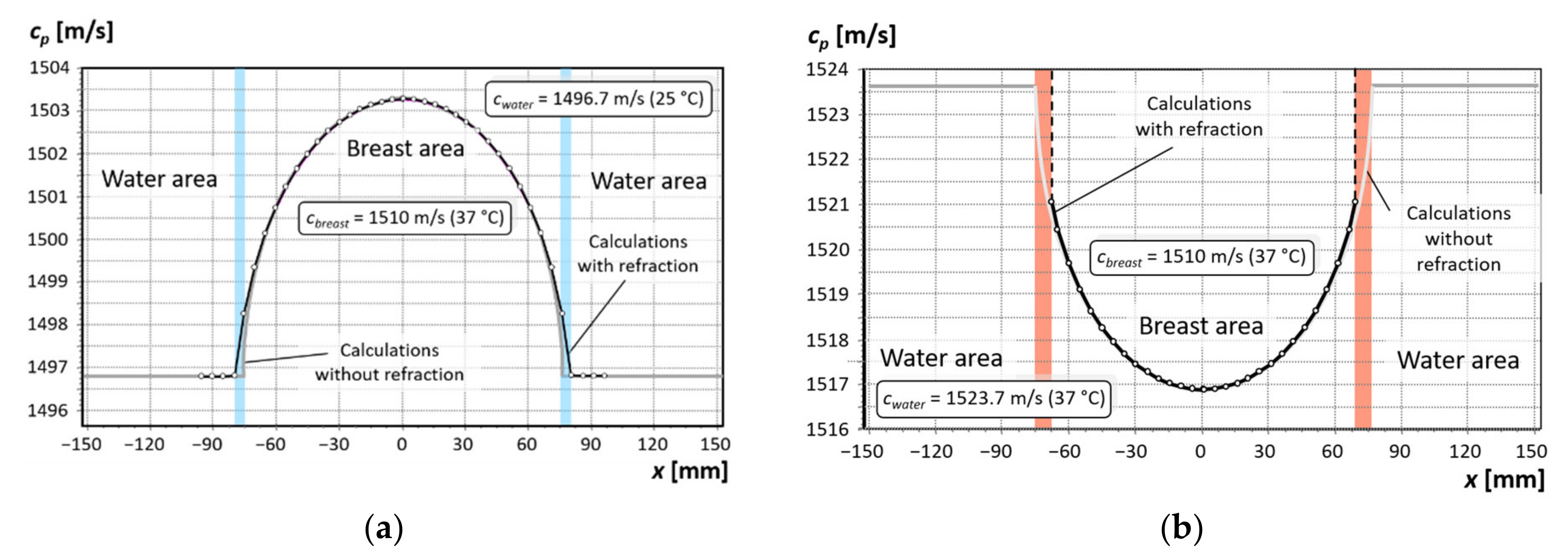
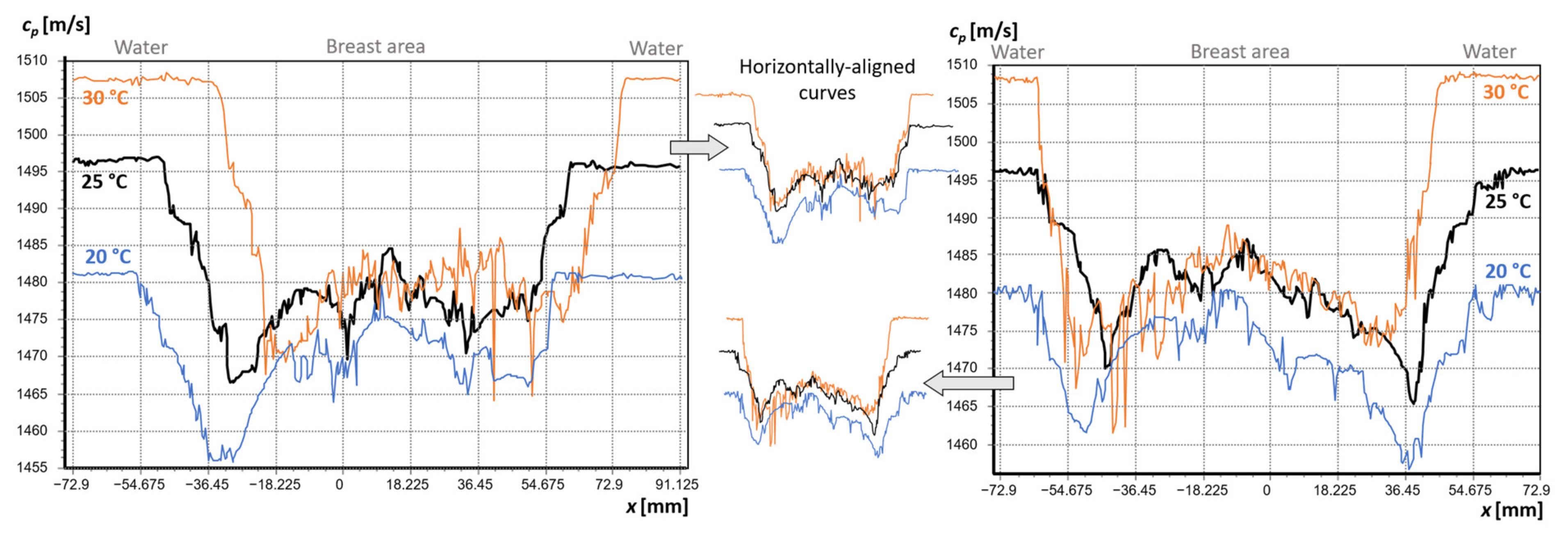
3.1. Analysis of the Effect of Water Temperature on the Refraction of the Beam Path
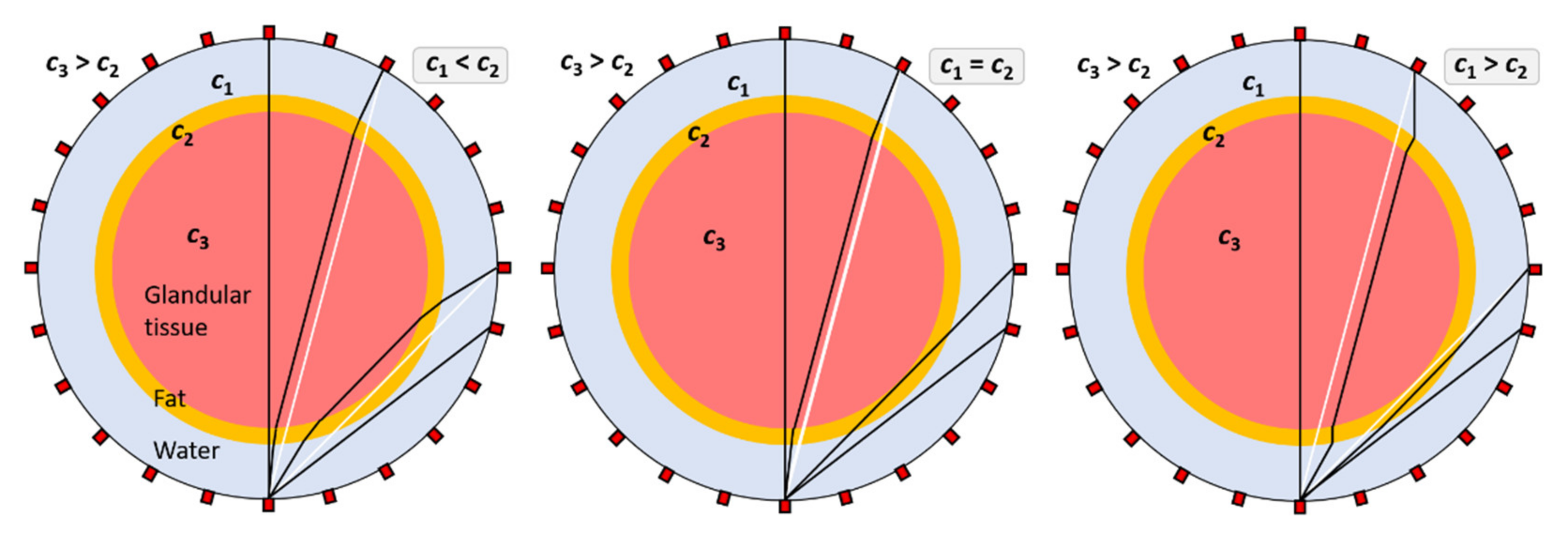
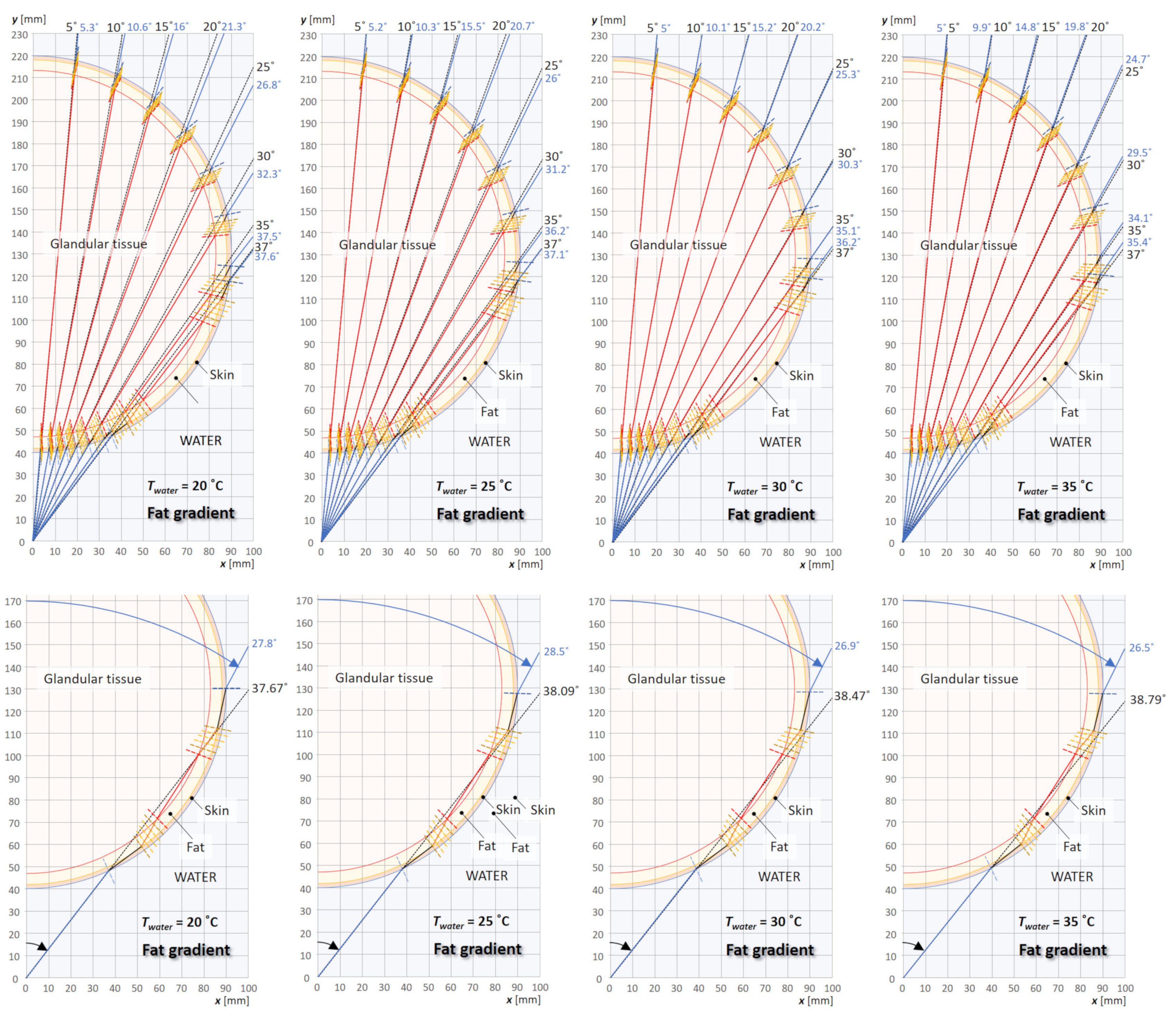
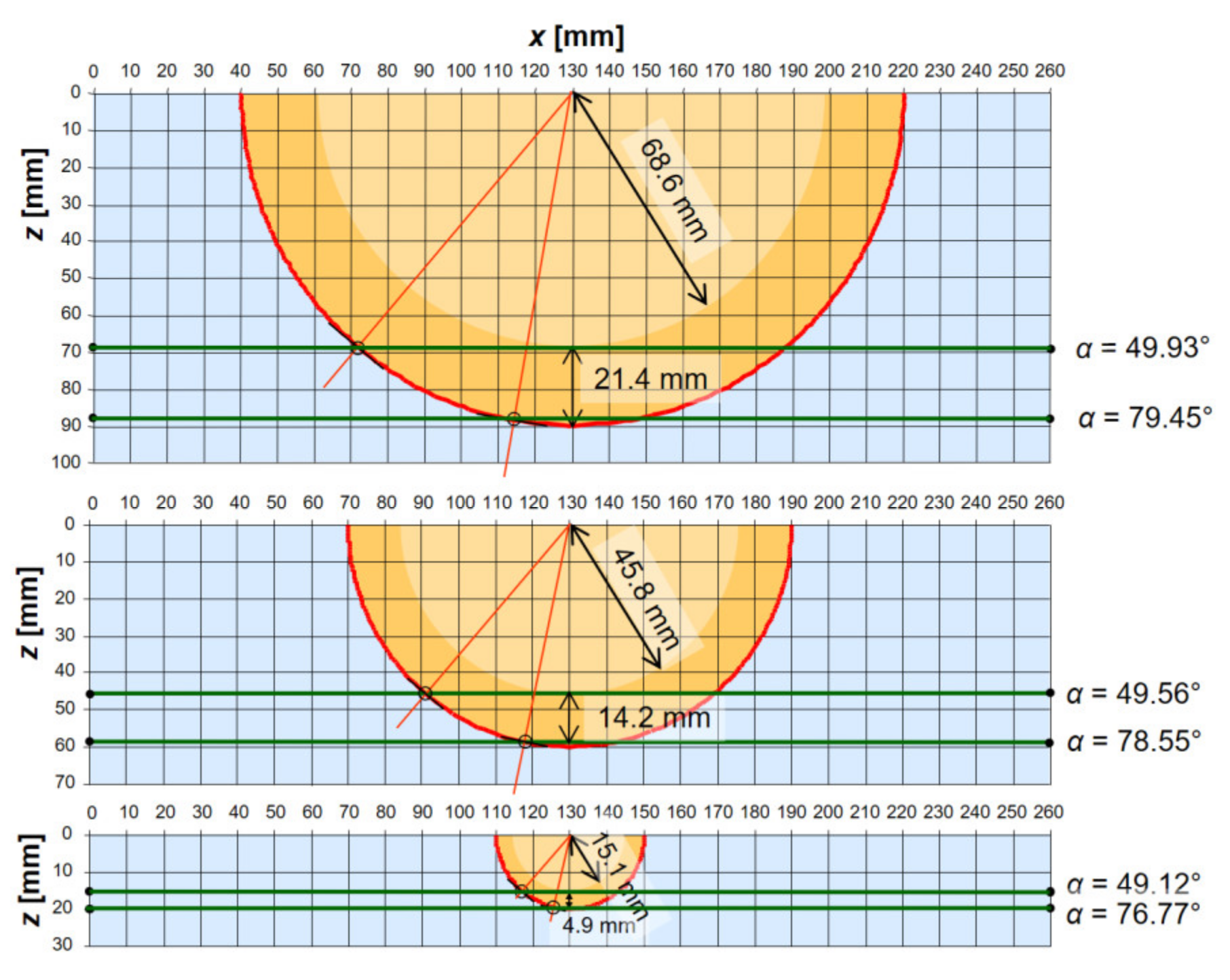
3.2. Analysis of Beam Ray Incident Angles on a Cross-Section of a Breast in Water
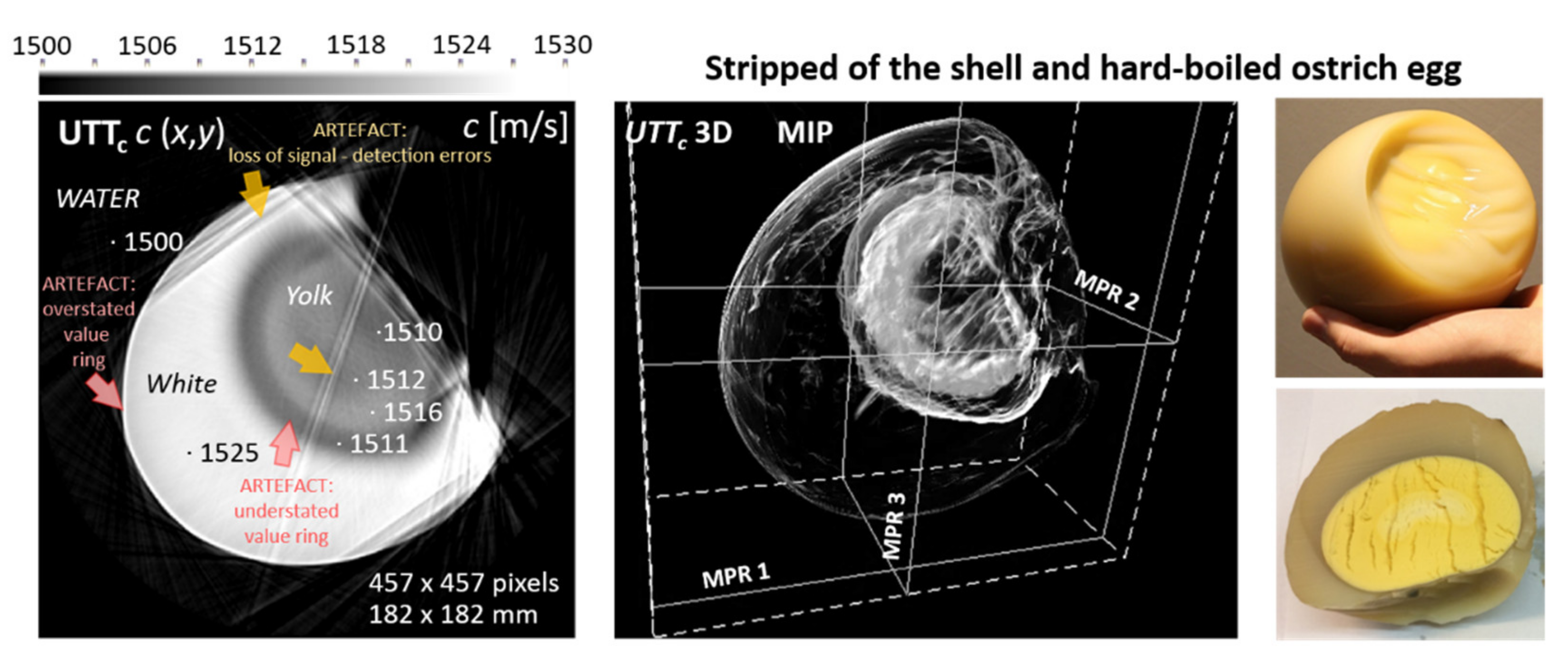
3.3. In Vivo Breast Measurements
4. Discussion
5. Practical Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 21 March 2021).
- Zhang, B.-N.; Cao, X.-C.; Chen, J.-Y.; Chen, J.; Fu, L.; Hu, X.-C.; Jiang, Z.-F.; Li, H.-Y.; Liao, N.; Liu, D.-G.; et al. Guidelines on the diagnosis and treatment of breast cancer. Gland Surg. 2012, 1, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Berg, C.D.; Visvanathan, K.; Robson, M. Estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers. J. Natl. Cancer Inst. 2009, 101, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, C.K.; Johnson, C.J.; Mongomery, P.G.; Maxwell, J.R. Premedication to reduce discomfort during screening mammography. Radiology 2008, 248, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Saarenmaa, I.; Salminen, T.; Geiger, U.; Heikkinen, P.; Hyvärinen, S.; Isola, J.; Kataja, V.; Kokko, M.-L.; Kokko, R.; Kumpulainen, E.; et al. The effect of age and density of the breast on the sensitivity of breast cancer diagnostic by mammography and ultrasonography. Breast Cancer Res. Treat. 2001, 67, 117–123. [Google Scholar] [CrossRef]
- Kopans, D.B. Negative mammographic and US findings do not help exclude breast cancer. Radiology 2002, 222, 857–858. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Zhu, W.; Hubbard, R.; Geller, B.; Dittus, K.; Braithwaite, D.; Wernli, K.J.; Miglioretti, D.L.; O’Meara, E.S. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern. Med. 2013, 173, 807–816. [Google Scholar] [CrossRef]
- Gøtzsche, P.C. Mammography screening is harmful and should be abandoned. J. R. Soc. Med. 2015, 108, 341–345. [Google Scholar] [CrossRef]
- Biller-Andorno, N.; Jüni, P. Abolishing Mammography Screening Programs? A View from the Swiss Medical Board. N. Engl. J. Med. 2014, 370, 1965–1967. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R.J.; Greenberg, K.L.; Stackhouse, R.M.; Geisel, J.L.; Butler, R.S.; Philpotts, L.E. Screening US in patients with mammographically dense breasts: Initial experience with Connecticute Public Acte 09-41. Radiology 2012, 265, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zonderland, H.M.; Coerkamp, E.G.; Hermans, J.; van de Vijver, M.; Van Voorthuisen, A.E. Diagnosis of breast cancer: Contribution of US as an adjunct to mammography. Radiology 1999, 213, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, V.; Ferrari, A.; Ghirardi, M.; Bergonzini, R.; Bellarosa, S.; Angelini, O.; Bani, C.; Ciatto, S. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. Radiol. Med. 2006, 111, 440–448. [Google Scholar] [CrossRef]
- Warren, R.M.L.; Pointon, L.; Thompson, D.; Hoff, R.; Gilbert, F.J.; Padhani, A.; Easton, D.; Lakhani, S.R.; Leach, M.O. Reading protocol for dynamic contrast-enhanced MR images of the breast: Sensitivity and specificity analysis. Radiology 2005, 236, 779–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duric, N. Ultrasound Tomography: A Breast Imaging Modality Whose Time Has Come. Med. Phys. 2015, 42, 3699. [Google Scholar] [CrossRef]
- Malik, B.; Terry, R.; Wiskin, J.; Lenox, M. Quantitative transmission ultrasound tomography: Imaging and performance characteristics. Med. Phys. 2018, 45, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Gemmeke, H.; Hopp, T.; Zapf, M.; Caiser, C.; Ruiter, N.V. 3D Ultrasound Computer Tomography: Hardware Setup, Reconstruction Methods and First Clinical Results. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2017, 873, 59–65. [Google Scholar] [CrossRef]
- Opieliński, K.J.; Pruchnicki, P.; Szymanowski, P.; Szepieniec, W.K.; Szweda, H.; Świś, E.; Jóźwik, M.; Tenderenda, M.; Bułkowski, M. Multimodal ultrasound computer-assisted tomography: An approach to the recognition of breast lesions. Comput. Med. Imaging Graph. 2018, 65, 102–114. [Google Scholar] [CrossRef]
- Milewski, T.; Michalak, M.; Wiktorowicz, A.; Opieliński, K.; Pruchnicki, P.; Bułkowski, M.; Gielecki, J.; Jóźwik, M. Hybrid Ultrasound Tomography Scanner—A Novel Instrument Designed to Examine Breast as a Breast Cancer Screening Method. Biomed. J. Sci. Tech. Res. 2019, 14, 10822–10826. [Google Scholar] [CrossRef]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomographic Imaging; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2001; p. 327. [Google Scholar] [CrossRef]
- Opieliński, K.J. Full Angle Ultrasound Spatial Compound Imaging. In Proceedings of the 7th Forum Acusticum, Kraków, Poland, 7–12 September 2014; European Acoustics Association: Madrid, Spain, 2014. [Google Scholar]
- Borowski, J.; Opieliński, K.J. Model of Multipath Propagation of Ultrasonic Pulses in Soft Tissue Using Divergent Beam Tomography Method. Vib. Phys. Syst. 2019, 30, 1–8. [Google Scholar]
- Glassner, A.S. An Introduction to Ray Tracing; Academic Press: Palo Alto, CA, USA, 1989. [Google Scholar]
- Andersen, A.H. A ray tracing approach to restoration and resolution enhancement in experimental ultrasound tomography. Ultrason. Imaging 1990, 12, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Xiaolei, Q.; Takashi, A.; Hirofumi, N.; Haruka, I.; Satoshi, T.; Shu, T.; Shin-Ichiro, U.; Ichiro, S.; Yoichiro, M. Bent Ray Ultrasound Tomography Reconstruction Using Virtual Receivers for Reducing Time Cost. In Medical Imaging 2015: Ultrasonic Imaging and Tomography, Proceedings of SPIE Medical Imaging 9419, Orlando, Florida, USA, 21–26 February 2015; Johan, G., Bosch, N.D., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2015; Volume 94190F. [Google Scholar] [CrossRef]
- Podvin, P.; Lecomte, I. Finite difference computation of traveltimes in very contrasted velocity models: A massively parallel approach and its associated tools. Geophys. J. Int. 1991, 105, 271–284. [Google Scholar] [CrossRef]
- Hormati, A.; Jovanović, I. Robust Ultrasound Travel-Time Tomography Using the Bent Ray Model. In Medical Imaging 2010: Ultrasonic Imaging, Tomography, and Therapy, Proceedings of SPIE Medical Imaging 7629, San Diego, CA, USA, 13 February 2010; Stephen, J.D., McAleavey, A., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2010; Volume 76290I. [Google Scholar] [CrossRef]
- Labyed, Y.; Lianjie, H. Toward Real-Time Bent-Ray Breast Ultrasound Tomography Using GPUs. In Medical Imaging 2014: Ultrasonic Imaging and Tomography, Proceedings of SPIE Medical Imaging 9040, San Diego, CA, USA, 18–20 February 2014; Johan, G., Bosh, N.D., Doyley, M.M., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 90401N. [Google Scholar] [CrossRef]
- Yu, Y.; Yi, S.; Yue, Z.; Yang, X.; Jing, J.; Naizhang, F. A Modified Ray Tracing Method for Ultrasound Computed Tomography in Breast Imaging. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Tarantola, A. Inverse Problem Theory and Methods for Model Parameter Estimation; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2005. [Google Scholar] [CrossRef]
- Sandhu, G.; Li, C.; Roy, O.; Schmidt, S.; Duric, N. High-resolution quantitative whole-breast ultrasound: In vivo application using frequency-domain waveform tomography. In Medical Imaging 2015: Ultrasonic Imaging and Tomography, Proceedings of SPIE Medical Imaging 9419, Orlando, Florida, USA, DC, 21–26 Feb 2015; Johan, G., Bosch, N.D., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2015; Volume 94190D. [Google Scholar] [CrossRef]
- Taskin, U. Full-Waveform Inversion for Breast Ultrasound. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2021. [Google Scholar]
- Javaherian, A.; Lucka, F.; Cox, B.T. Refraction-corrected ray-based inversion for three-dimensional ultrasound tomography of the breast. Inverse Probl. 2020, 36, 41. [Google Scholar] [CrossRef]
- Andersen, A.H.; Kak, A.C. Digital ray tracing in two-dimensional refractive fields. J. Acoust. Soc. Am. 1982, 72, 1593–1606. [Google Scholar] [CrossRef]
- Andersen, A.H. Ray linking for computed tomography by rebinning of projection data. J. Acoust. Soc. Am. 1987, 81, 1190–1192. [Google Scholar] [CrossRef]
- Langan, R.T.; Lerche, L.; Cutler, R.J. Traycing of rays through a heterogeneous media: An accurate and efficient procedure. Geophysics 1985, 50, 1456–1465. [Google Scholar] [CrossRef]
- Lytle, R.J.; Dines, K.A. Iterative Ray Tracing between Boreholes for Underground Image Reconstruction. IEEE Trans. Geosci. Remote Sens. 1980, GE-18, 234–240. [Google Scholar] [CrossRef]
- Duck, F.A. Physical Properties of Tissue—A Comprehensive Reference Book; Academic Press: London, UK, 1990. [Google Scholar]
- Kossof, G.; Fry, E.K.; Jellins, J. Average velocity of ultrasound in the human female breast. J. Acoust. Soc. Am. 1973, 53, 1730–1736. [Google Scholar] [CrossRef]
- Opieliński, K.; Gudra, T. Main error sources in ultrasound transmission tomography imaging. In Proceedings of the 16th International Congress on Acoustics and 135th Meeting Acoustical Society of America, Seattle, DC, USA, 20–26 June 1998; American Institute of Physics: College Park, MD, USA, 1998; Volume 2, pp. 407–408. [Google Scholar]
- Opieliński, K.; Gudra, T. Ultrasound transmission tomography image distortions caused by the refraction effect. Ultrasonics 2000, 38, 424–429. [Google Scholar] [CrossRef]
- Opieliński, K.J. Ultrasonic parameters of hen’s egg. Mol. Quantum Acoust. 2007, 28, 203–216. [Google Scholar]
- Lewitt, R.M. Reconstruction algorithms: Transform methods. Proc. IEEE 1983, 71, 390–408. [Google Scholar] [CrossRef]
- Pope, T.L., Jr.; Read, M.E.; Medsker, T.; Buschi, A.J.; Brenbridge, A.N. Breast skin thickness: Normal range and causes of thickening shown on film-screen mammography. J. Can. Assoc. Radiol. 1984, 35, 365–368. [Google Scholar] [PubMed]
- The Foundation for Research on Information Technologies in Society (IT’IS). Available online: https://itis.swiss/virtual-population/tissue-properties/database/acoustic-properties/ (accessed on 10 January 2022).
- Radiopaedia. Available online: https://radiopaedia.org (accessed on 14 November 2019).
- Sato, Y.; Shiraga, N.; Nakajima, S.; Tamura, S.; Kikinis, R. Local Maximum Intensity Projection (LMIP): A New Rendering Method for Vascular Visualization. J. Comput. Assist. Tomogr. 1988, 22, 912–917. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J. 2013 ACR BI-RADS Atlas: Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: College Park, MD, USA, 2013; p. 689. [Google Scholar]










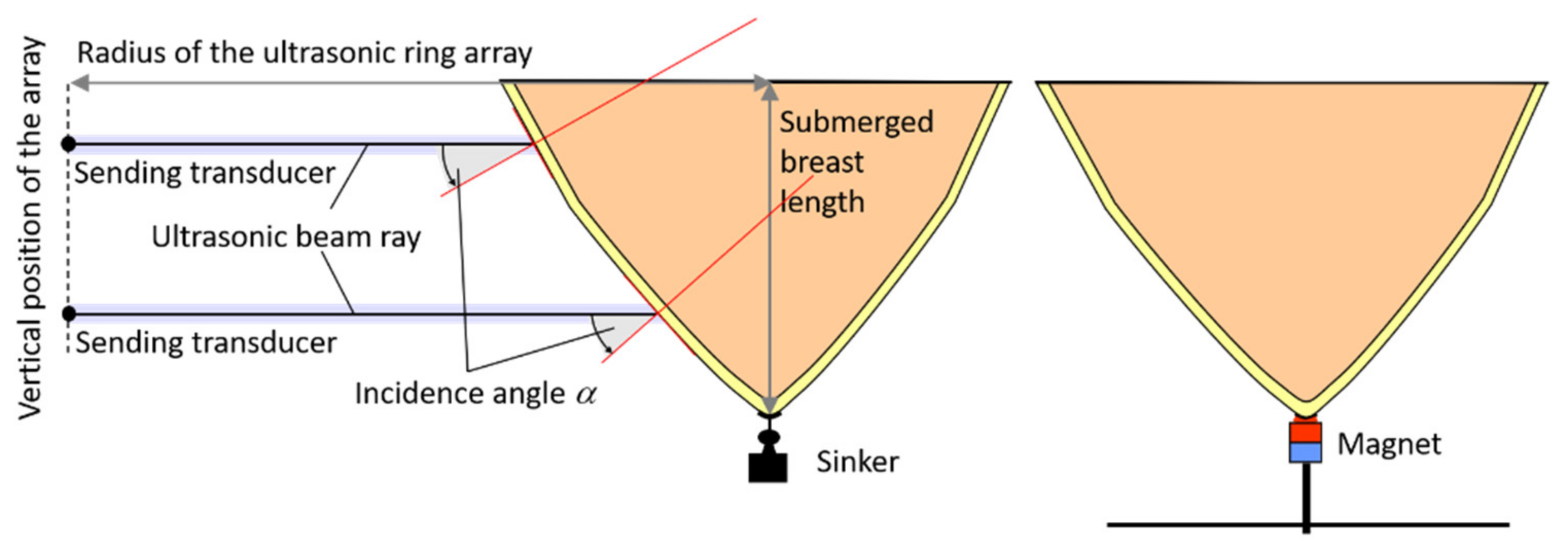
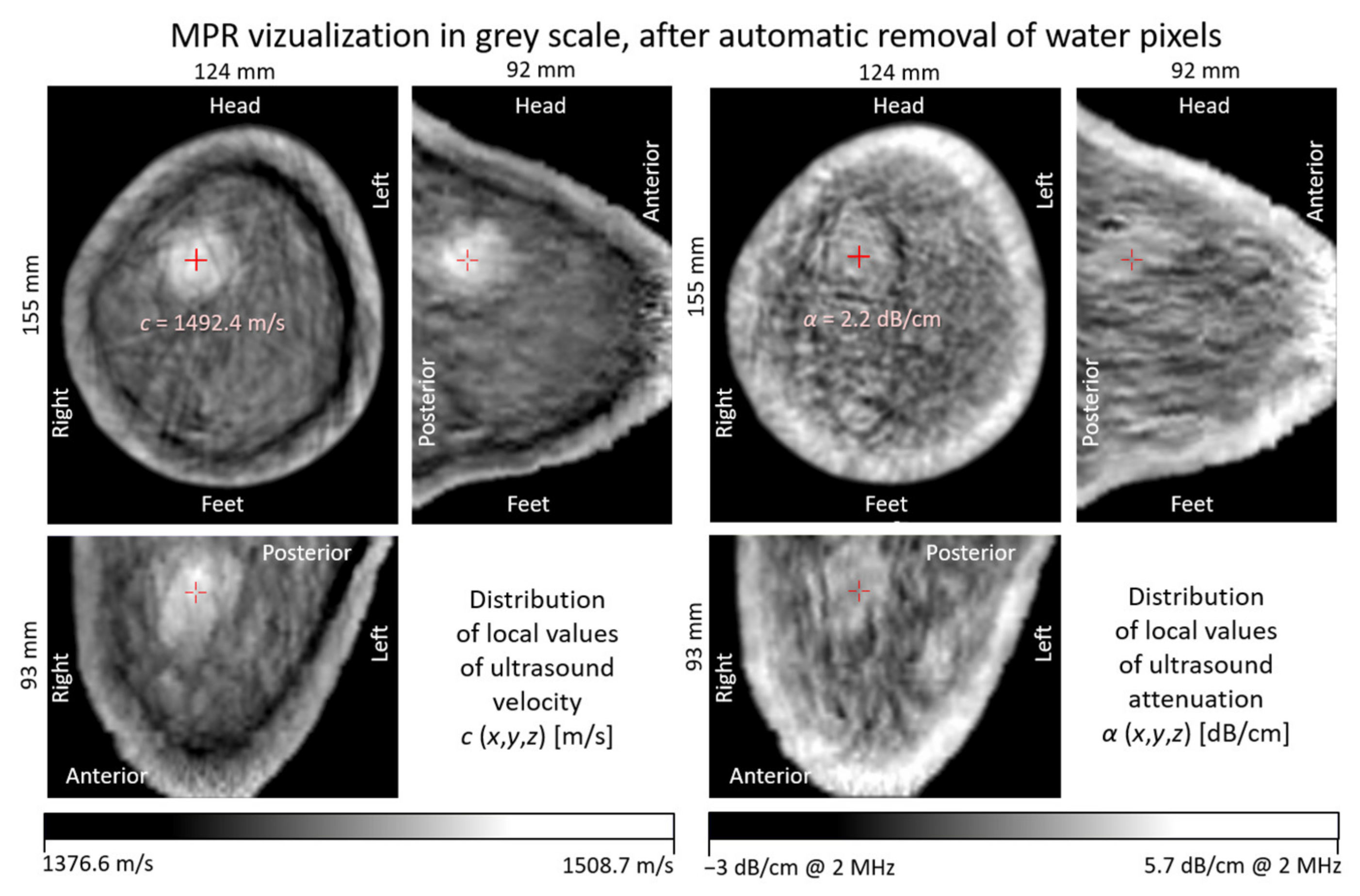
| Water Temperature [°C] | Skin @ cFat = 1470 m/s cBreast = 1515 m/s | Fat @ cSkin = 1624 m/s cBreast = 1515 m/s | Breast @ cSkin = 1624 m/s cFat = 1470 m/s | |||
|---|---|---|---|---|---|---|
| ∆opt [°] | ∆opt [°] | ∆opt [°] | ||||
| 20 | 1537 | 3.75 | 1412 | 3.98 | 1430 | −6.57 |
| 1720 | 1.95 | 1490 | 2.74 | 1564 | 9.30 | |
| 25 | 1537 | 2.33 | 1412 | 2.56 | 1430 | −7.65 |
| 1720 | 0.69 | 1490 | 1.39 | 1564 | 7.71 | |
| 30 | 1537 | 1.15 | 1412 | 1.38 | 1430 | −8.58 |
| 1720 | −0.38 | 1490 | 0.26 | 1564 | 6.38 | |
| 35 | 1537 | 0.16 | 1412 | 0.38 | 1430 | −9.36 |
| 1720 | −1.28 | 1490 | −0.69 | 1564 | 5.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opieliński, K.J.; Bułkowski, M.; Gabryel, A.; Wiktorowicz, A. Analysis of the Refraction Effect in Ultrasound Breast Tomography. Appl. Sci. 2022, 12, 3578. https://doi.org/10.3390/app12073578
Opieliński KJ, Bułkowski M, Gabryel A, Wiktorowicz A. Analysis of the Refraction Effect in Ultrasound Breast Tomography. Applied Sciences. 2022; 12(7):3578. https://doi.org/10.3390/app12073578
Chicago/Turabian StyleOpieliński, Krzysztof J., Mariusz Bułkowski, Andrzej Gabryel, and Andrzej Wiktorowicz. 2022. "Analysis of the Refraction Effect in Ultrasound Breast Tomography" Applied Sciences 12, no. 7: 3578. https://doi.org/10.3390/app12073578
APA StyleOpieliński, K. J., Bułkowski, M., Gabryel, A., & Wiktorowicz, A. (2022). Analysis of the Refraction Effect in Ultrasound Breast Tomography. Applied Sciences, 12(7), 3578. https://doi.org/10.3390/app12073578







