Application of Infrared Thermography in E-Bike Battery Pack Detail Analysis—Case Study
Abstract
:1. Introduction
2. Electric Bicycles
2.1. A Brief History of Bicycles
- 1817 Appearance of first bicycle, the Vélocipede, by Karl Freiherr von Drais in Germany [12].
- 1861 Relocation of pedals to the front wheel in “Michaulina” by Pierre Michaux in France [15].
- 1869 Introduction of solid rubber tires [16].
- 1864 Appearance of the chain by James Slater [17].
- 1870 Invention of the tangentially spoked wheel by James Starley [17].
- 1874 Women’s bicycle with a side seat and one pedal by James Starley [17].
- 1885 A modern kind of bicycle (Rover) designed by John Starley Kemp [18].
- 1895 Appearance of the first electric bicycle by Ogden Bolton Jr. [21].
- 1896 Cycling became a sport at the first Olympic games in the Athens in 1896 [16].
- 1975 Panasonic made the E-bike driven by 24 V lead-acid car batteries [15].
- 1989 Sanyo introduced NiCd batteries [22].
- 1993 Yamaha presented PAS, an electrically power assisted bicycle [23].
- 2002 EU introduced and set on e-mobility legislation 2002/24/EC [24].
- 2003 NiMH chemistry prevailed in batteries for electrical driving systems [23].
- 2004 Li-ion became a standard battery choice [23].
- 2005 onward, a demand for PEDELECs grows constantly [25].
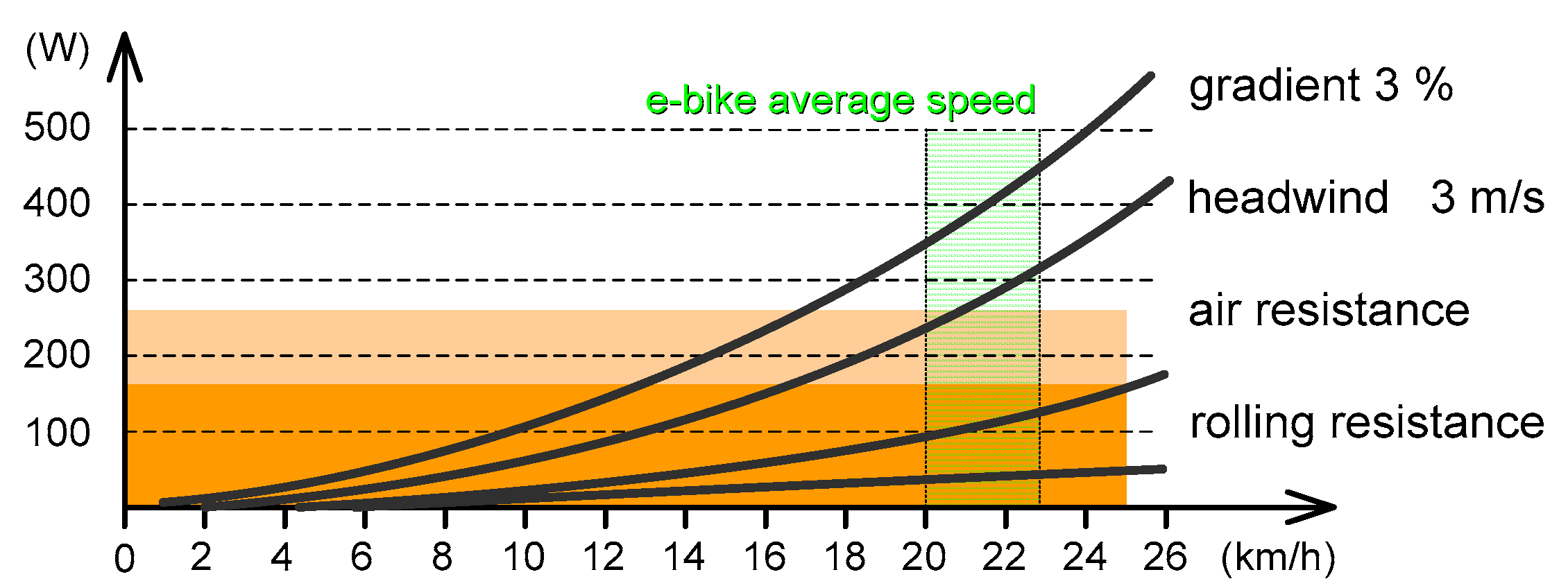
2.2. Technical and Regulatory Aspects of Electric Cycling
- W mechanical power at the wheel, [W] (which is slightly less than what the rider produces due to losses and coupling efficiency);
- Ka drag factor, [kg/m] (usually in interval of 0.1 to 0.3; for a small recumbent rider 0.1; large upright rider 0.3; typical values 0.2–0.25);
- V bike velocity, [m/s];
- s road slope, [%], (e.g., 5% = 0.05);
- Cr rolling resistance coefficient [1] (0.02 for racing tires; 0.08 for heavily grooved MTB; and 0.03 typically for road bikes);
- Vw heading wind velocity, [m/s];
- mr driver mass, [kg];
- mb bike mass, [kg];
- g gravitational constant, [m/s2] (taken an average value of 9.81 m/s2).
3. Analysis and Diagnostics of the Battery Pack
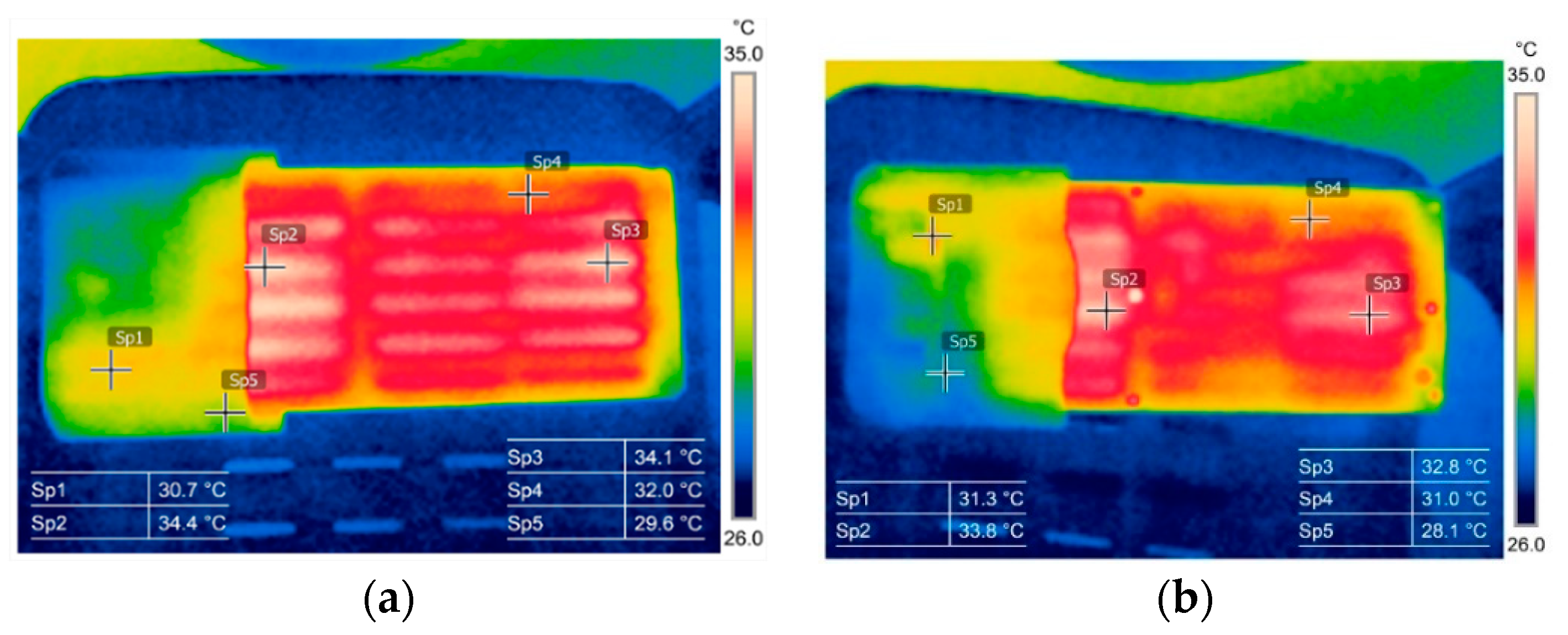
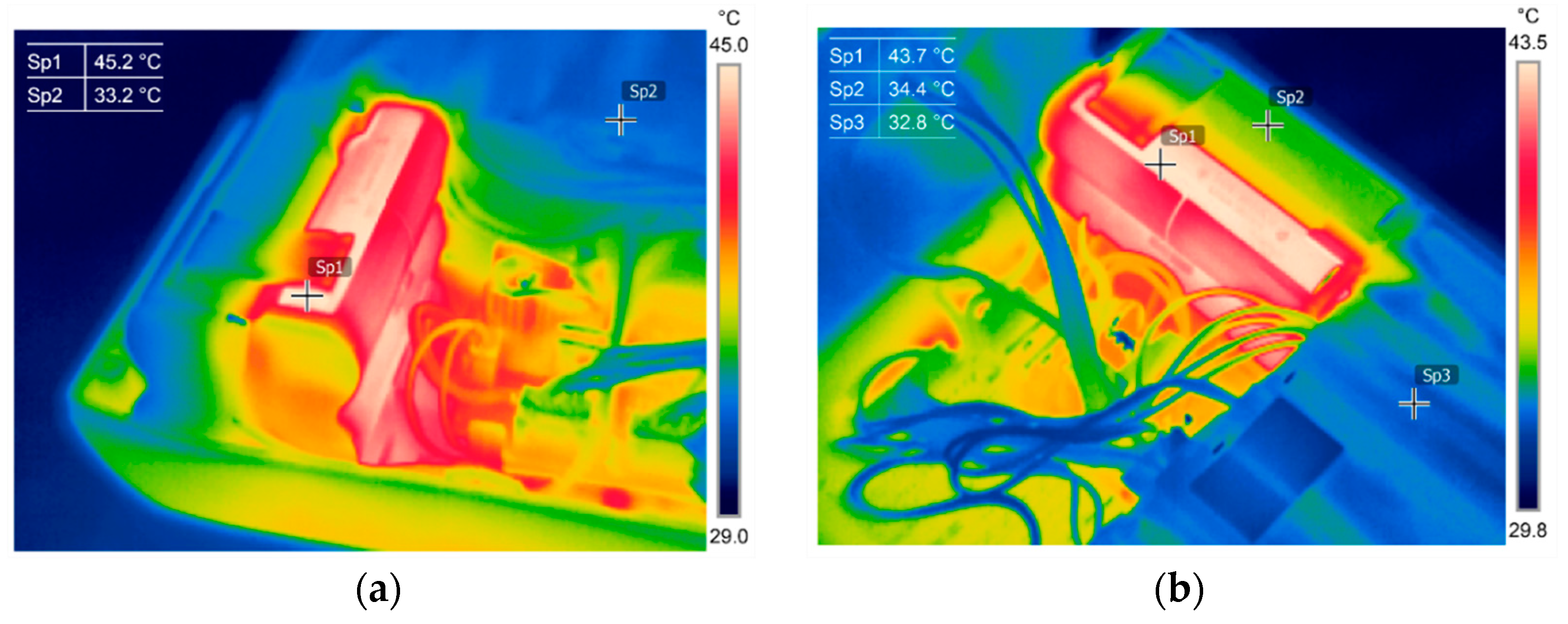
3.1. Thermography in the Battery State Assessment and Analysis
3.2. Battery Cells Electric Parameters and Functional Analysis
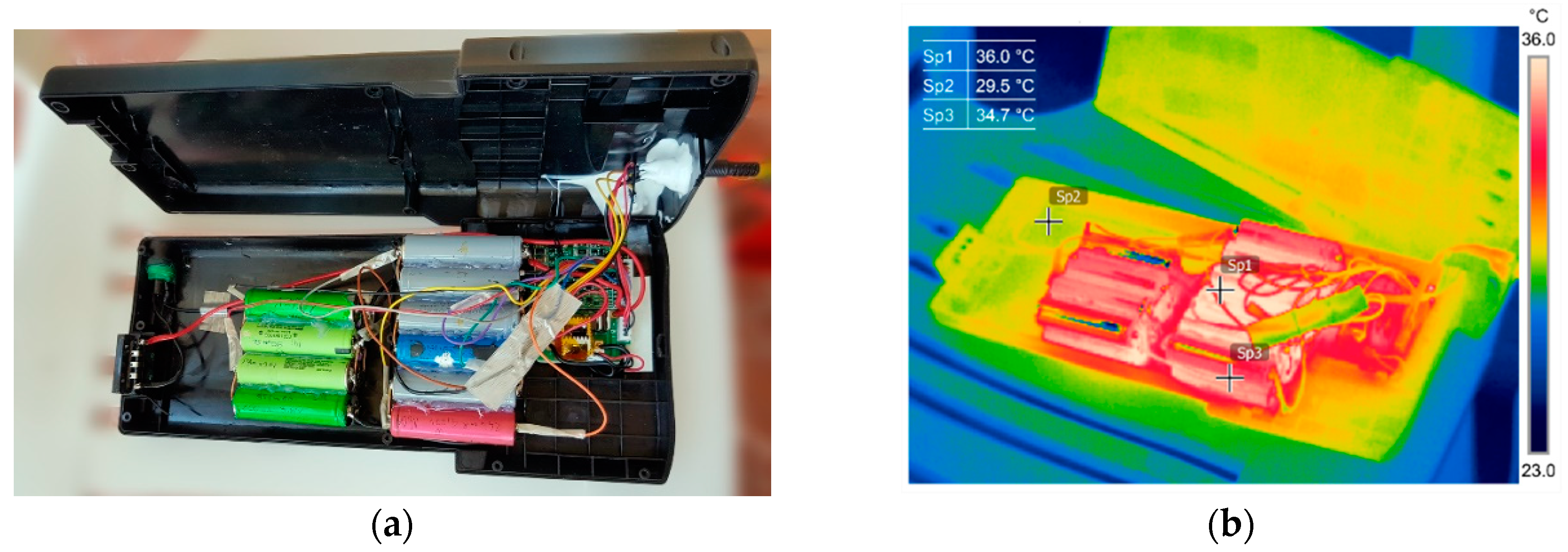
4. Battery Pack Revitalization, Results, and Discussion
4.1. Revival and Optimization of Battery Assembly
4.2. Functional Assessment and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glavaš, H.; Karakašić, M.; Petrović, I.; Vidaković, D. PEDELEC Li-Ion Battery Pack Lifetime. In Proceedings of the VIII International Conference Industrial Engineering and Environmental Protection 2018 (IIZS 2018), University of Novi Sad Technical Faculty “Mihajlo Pupin”, Zrenjanin, Serbia, 11–12 October 2018; pp. 27–33, ISBN 978-86-7672-309-6. [Google Scholar]
- Kim, J.; Mallarapu, A.; Finegan, D.P.; Santhanagopalan, S. Modeling cell venting and gas-phase reactions in 18650 lithium-ion batteries during thermal runaway. J. Power Sources 2021, 489, 229496. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Jeevarajan, J.A.; Mukherjee, P.P. Degradation-Safety Analytics in Lithium-Ion Cells: Part I. Aging under Charge/Discharge Cycling. J. Electrochem. Soc. 2020, 167, 160510. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Bruen, T.; Widanage, W.D.; Gama-Valdez, M.A.; Marco, J. A Study of Cell-to-Cell Interactions and Degradation in Parallel Strings: Implications for the Battery Management System. J. Power Source 2016, 329, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Berg, P.; Spielbauer, M.; Tillinger, M.; Merkel, M.; Schoenfuss, M.; Bohlen, O.; Jossen, A. Durability of lithium-ion 18650 cells under random vibration load with respect to the inner cell design. J. Energy Storage 2020, 31, 101499. [Google Scholar] [CrossRef]
- Glavaš, H.; Barić, T.; Keser, T. Pedelec—Pedal Electric Cycle. In Proceedings of the 35th Conference on Transportation Systems with International Participation Automation in Transportation 2015, Zagreb, Croatia, London, UK, 3–8 November 2015. [Google Scholar]
- Kabir, M.M.; Demirocak, D.E. Degradation mechanisms in Li-ion batteries: A state-of-the-art review. Int. J. Energy Res. 2017, 41, 1963–1986. [Google Scholar] [CrossRef]
- EuroVelo. The European Cycle Route Network. Available online: http://www.eurovelo.com/en/eurovelos (accessed on 30 April 2021).
- Weiss, M.; Dekker, P.; Moro, A.; Scholz, H.; Patel, M.K. On the electrification of road transportation—A review of the environmental, economic, and social performance of electric two-wheelers. Transp. Res. Part D Transp. Environ. 2015, 41, 348–366. [Google Scholar] [CrossRef] [PubMed]
- GoPedelec! (A Manual). 2015. Available online: www.gopedalec.eu (accessed on 24 July 2015).
- Hoffmann, U. Das Fahrradbuch, Kauf, Technik, Wartung, Reparaturen E-Bikes und Pedelecs, Stiftung Warentest; Stiftung Warentest: Berlin, Germany, 2012. [Google Scholar]
- Leaserad at Go Pedelec. Available online: http://www.datei.de/public/extraenergy/GoPedelec_Handbuch-DE-V1_0-b.pdf (accessed on 30 April 2021).
- Kirkpatrick MacMillan (1812–1878) Inventor of the Pedal Bicycle. 2021. Available online: http://gaukartifact.com/2013/04/05/kirkpatrick-macmillan-1812-1878-inventor-of-the-pedal-bicycle (accessed on 28 February 2021).
- Wilson, D.G. Bicycling Science, 3rd ed.; MIT Press Cambridge: London, UK, 2004. [Google Scholar]
- Remusat, T. Pierre Michaux and the History of the Motorized Bike, HISTORIA; Librairie Jules Tallandier: Paris, France, 2001; pp. 40–41. [Google Scholar]
- Herlihy, D.V. Bicycle: The History; Yale University Press: New Haven, CT, USA, 2006; ISBN 0-300-10418-9. [Google Scholar]
- Renold: A Company History. 2021. Available online: https://www.renold.com/company/history (accessed on 30 April 2021).
- Hadland, T.; Lessing, H.E. Bicycle Design, An Illustrated History; Massachusetts Institute of Technology: Cambridge, MA, USA, 2014; ISBN 978-0-262-02675-8. [Google Scholar]
- Kyle, C.R.; Weaver, M.D. Aerodynamics of human-powered vehicles. Proc. Inst. Mech. Eng. Part A J. Power Energy 2004, 218, 141–154. [Google Scholar] [CrossRef]
- Clayton, N. The Birth of the Bicycle; Amberley Publishing: Stroud, UK, 2016; ISBN 1445648822. [Google Scholar]
- Bolton, O., Jr. Electrical Bicycle. U.S. Patent No. 552,271, 31 December 1895. Available online: https://patents.google.com/patent/US552271?oq=Ogden+Bolton+Jr (accessed on 21 February 2021).
- Ron/Spinningmagnets. Electric Bike History, Patents from the 1800’s. 2013. Available online: https://www.electricbike.com/e-bike-patents-from-the-1800s/ (accessed on 30 April 2021).
- Power Assisted Bicycle, Two Decades of Electrically Power Assisted Bicycles; Yamaha Motor Co., Ltd.: Iwata, Japan, 2013.
- European Commission. Directive 2002/24/EC of the European Parliament and of the Council of 18 March 2002 Relating to the Type-Approval of Two or Three-Wheel Motor Vehicles. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002L0024 (accessed on 30 April 2021).
- Neupert, H. Light-Electric-Vehicles Global Market Trends and Standardization; China Cycle Show: Shanghai, China, 2014. [Google Scholar]
- Desmond, K. Gustave Trouve: French Electrical Genius (1839–1902); McFarland & Company: Jefferson, NC, USA, 2015; ISBN 0786497092. [Google Scholar]
- Schnepf, J. U.S. Patent US627066A, 1898. Available online: https://patents.google.com/patent/US627066 (accessed on 30 April 2021).
- eCycleElectric. Available online: http://www.ecycleelectric.com/susanne-bruesch (accessed on 30 April 2021).
- CROW. Design Manual for Bicycle Traffic; CROW: Ede, The Netherlands, 2016; ISBN 9789066286597. [Google Scholar]
- Glavaš, H.; Józsa, L.; Barić, T. Infrared Thermography in Energy Audit of Electrical Installations. Teh. Vjesn. 2016, 23, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Baehr, H.D.; Stephan, K. Wärme-und Stoffübertragung; Springer GmbH Deutschland: Wien, Austria, 2019; ISBN 978-3-662-58441-5. [Google Scholar]
- Zhang, G.; Tian, H.; Ge, S.; Marple, D.; Sun, F.; Wang, C.Y. Visualization of self-heating of an all-climate battery by infrared thermography. J. Power Sources 2018, 376, 111–116. [Google Scholar] [CrossRef]
- Panasonic. CGR18650CG Datasheet; Panasonic: Osaka, Japan, 2018. [Google Scholar]
- Ye, M.; Song, X.; Xiong, R.; Sun, F. A Novel Dynamic Performance Analysis and Evaluation Model of Series-Parallel Connected Battery Pack for Electric Vehicles. IEEE Access 2019, 7, 14256–14265. [Google Scholar] [CrossRef]
- Baronti, F.; Di Rienzo, R.; Papazafiropulos, N.; Roncella, R.; Saletti, R. Investigation of series-parallel connections of multi-module batteries for electrified vehicles. In Proceedings of the IEEE International Electric Vehicle Conference (IEVC), Florence, Italy, 17–19 December 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Chen, H.; Shen, J. A degradation-based sorting method for lithium-ion battery reuse. PLoS ONE 2017, 12, e0185922. [Google Scholar] [CrossRef]
- Masomtob, M.; Sukondhasingha, R.; Becker, J.; Sauer, D.U. Parametric Study of Spot Welding between Li-ion Battery Cells and Sheet Metal Connectors. In Proceedings of the Engineering Journal, 7th Thai Society of Mechanical Engineers-International Conference on Mechanical Engineering, (TSME-ICoME 2016), Chiang Mai, Thailand, 13–16 December 2016; Volume 21. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, C. Thermal runaway mechanism of lithium-ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Ouyang, M.; Ren, D.; Lu, L.; Li, J.; Feng, X.; Han, X.; Liu, G. Overcharge-induced capacity fading analysis for large format lithium-ion batteries with LiyNi1/3Co1/3Mn1/3O2+LiyMn2O4 composite cathode. J. Power Sources 2015, 279, 626–635. [Google Scholar] [CrossRef]
- Salinas, F.; Krüger, L.; Neupert, S.; Kowal, J. A second life for li-ion cells rescued from notebook batteries. J. Energy Storage 2019, 24, 100747. [Google Scholar] [CrossRef]
- Pop, V.; Bergveld, J.H.G.; Op het Veld, P.P.L.; Regtien, D.D.; Notten, P.H.L. Modeling Battery Behavior for Accurate State-of-Charge Indication. J. Electrochem. Soc. 2006, 153, A2013–A2022. [Google Scholar] [CrossRef] [Green Version]
- Bolsinger, C.; Zorn, M.; Birke, K.P. Electrical contact resistance measurements of clamped battery cell connectors for cylindrical 18650 battery cells. J. Energy Storage 2017, 12, 29–36. [Google Scholar] [CrossRef]
- Ning, G.; Haran, B.; Popov, B.N. Capacity fade study of lithium-ion batteries cycled at high discharge rates. J. Power Sources 2003, 117, 160–169. [Google Scholar] [CrossRef]
- Andrea, D. Battery Management Systems for Large Lithium-Ion Battery Packs; Artech House: Norwood, MA, USA, 2010; p. 02062. ISBN 9781608071050. [Google Scholar]
- Baumann, M.; Wildfeuer, L.; Rohr, S.; Lienkamp, M. Parameter variations within Li-Ion battery packs–Theoretical investigations and experimental quantification. J. Energy Storage 2018, 18, 295–307. [Google Scholar] [CrossRef]
- Marcis, V.A.; Praneeth, A.V.J.S.; Patnaik, L.; Williamson, S.S. Analysis of CT-CV Charging Technique for Lithium-ion and NCM 18650 Cells Over Temperature Range. In Proceedings of the IEEE International Conference on Industrial Technology (ICIT), Buenos Aires, Argentina, 26–28 February 2020; pp. 947–952. [Google Scholar] [CrossRef]
- Tang, W.; Tam, W.C.; Yuan, L.; Dubaniewicz, T.; Thomas, R.; Soles, J. Estimation of the critical external heat leading to the failure of lithium-ion batteries. Appl. Therm. Eng. 2020, 179, 115665. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, B.H. Screening process-based modeling of the multi-cell battery string in series and parallel connections for high accuracy state-of-charge estimation. Energy 2013, 57, 581–599. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.O.; Chong, W.T.; Kuan, S.H.; Yew, M.C. Computational fluid dynamic and thermal analysis of Lithium-ion battery pack with air cooling. Appl. Energy 2016, 177, 783–792. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, F.; Fang, X.; Gao, X.; Zhang, Z. A hybrid thermal management system for lithium-ion batteries combining phase change materials with forced-air cooling. Appl. Energy 2015, 148, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.O.; Lee, P.Y.; Jang, M.; Yoo, K.; Kim, J. Comparison of internal parameters varied by environmental tests between high-power series/parallel battery packs with different shapes. J. Ind. Eng. Chem. 2019, 71, 260–269. [Google Scholar] [CrossRef]
- Yang, X.; Hu, X.; Chen, Z.; Chen, Y. Effect of ambient dissipation condition on thermal behavior of a lithium-ion battery using a 3D multi-partition model. Appl. Therm. Eng. 2020, 178, 115634. [Google Scholar] [CrossRef]
- Buidin, T.I.C.; Mariasiu, F. Modeling Approach of an Air-Based Battery Thermal Management System for an Electric Vehicle. Appl. Sci. 2021, 11, 7089. [Google Scholar] [CrossRef]


















| Camera | Flir E60bx | Flir E6 |
|---|---|---|
| IR resolution | 320 × 240 pixels | 160 × 120 pixels |
| Thermal sensitivity/NETD | <0.045 °C @ + 30 °C/45 mK | <0.06 °C/<60 mK |
| Field of view (FOV) | 25° × 19° | 45° × 34° |
| Spatial resolution (IFOV) | 1.36 mrad | 5.2 mrad |
| Spectral range | 7.5–13 μm | 7.5–13 μm |
| Object temperature range | −20 °C to +120 °C | −20 °C to +250 °C |
| Accuracy | ±2 °C or ±2% of reading, for ambient temperature 10 °C to 35 °C | ±2 °C or ±2% of reading, for ambient temperature 10 °C to 35 °C |
| Cell No. | Resistance R (mΩ) | Capacity C (mAh) | Group Capacity (mAh) | Remaining Capacity (mAh) | Cell Voltage U (V) | Discharge Time (min) |
|---|---|---|---|---|---|---|
| 1 | 114 | 351 | 1067 | 15.60 | 3.480 | 13 |
| 2 | 101 | 119 | 5.29 | 3.960 | 5 | |
| 3 | 114 | 350 | 15.56 | 3.640 | 15 | |
| 4 | 101 | 247 | 10.98 | 3.820 | 11 | |
| 5 | 141 | 268 | 1105 | 11.91 | 3.600 | 11 |
| 6 | 101 | 278 | 12.36 | 3.670 | 12 | |
| 7 | 103 | 259 | 11.51 | 3.740 | 11 | |
| 8 | 105 | 300 | 13.33 | 3.720 | 12 | |
| 9 | 101 | 277 | 934 | 12.31 | 3.640 | 12 |
| 10 | 98 | 285 | 12.67 | 3.710 | 12 | |
| 11 | 122 | 132 | 5.87 | 3.900 | 6 | |
| 12 | 106 | 240 | 10.67 | 3.810 | 10 | |
| 13 | 103 | 185 | 1411 | 8.22 | 3.880 | 8 |
| 14 | 90 | 895 | 39.78 | 3.550 | 29 | |
| 15 | 103 | 185 | 8.22 | 3.910 | 8 | |
| 16 | 122 | 146 | 6.49 | 3.990 | 6 | |
| 17 | 103 | 243 | 1193 | 10.80 | 3.754 | 10 |
| 18 | 106 | 248 | 11.02 | 3.685 | 10 | |
| 19 | 103 | 229 | 10.18 | 3.743 | 10 | |
| 20 | 101 | 473 | 21.02 | 3.556 | 17 | |
| 21 | 90 | 1101 | 1995 | 48.93 | 3.398 | 34 |
| 22 | 103 | 286 | 12.71 | 3.710 | 12 | |
| 23 | 99 | 292 | 12.98 | 3.789 | 12 | |
| 24 | 109 | 316 | 14.04 | 3.689 | 13 | |
| 25 | 95 | 353 | 1502 | 15.69 | 3.783 | 14 |
| 26 | 97 | 315 | 14.00 | 3.578 | 19 | |
| 27 | 95 | 529 | 23.51 | 3.607 | 13 | |
| 28 | 106 | 305 | 13.56 | 3.724 | 12 | |
| 29 | 111 | 229 | 1175 | 10.18 | 3.841 | 9 |
| 30 | 118 | 136 | 6.04 | 3.929 | 6 | |
| 31 | 91 | 258 | 11.47 | 3.634 | 11 | |
| 32 | 99 | 552 | 24.53 | 3.688 | 20 | |
| 33 | 101 | 267 | 762 | 11.87 | 3.601 | 11 |
| 34 | 112 | 128 | 5.69 | 3.776 | 6 | |
| 35 | 112 | 120 | 5.33 | 3.840 | 5 | |
| 36 | 99 | 247 | 10.98 | 3.769 | 10 | |
| 37 | 104 | 249 | 1051 | 11.07 | 3.860 | 11 |
| 38 | 97 | 260 | 11.56 | 3.830 | 11 | |
| 39 | 99 | 319 | 14.18 | 3.794 | 13 | |
| 40 | 102 | 223 | 9.91 | 3.778 | 9 |
| Cell No. | Resistance R (mΩ) | Capacity C (mAh) | Group Capacity (mAh) | Remaining Capacity (mAh) | Cell Voltage U (V) | Charge Time (min) |
|---|---|---|---|---|---|---|
| 1 | 113 | 115 | 937 | 5.11 | 4.140 | 16 |
| 2 | 101 | 340 | 15.11 | 4.120 | 34 | |
| 3 | 109 | 277 | 12.31 | 4.120 | 26 | |
| 4 | 101 | 205 | 9.11 | 4.100 | 23 | |
| 5 | 101 | 174 | 737 | 7.73 | 4.090 | 20 |
| 6 | 101 | 189 | 8.40 | 4.120 | 23 | |
| 7 | 103 | 156 | 6.93 | 4.100 | 18 | |
| 8 | 106 | 218 | 9.69 | 4.130 | 27 | |
| 9 | 101 | 207 | 668 | 9.20 | 4.190 | 23 |
| 10 | 98 | 226 | 10.04 | 4.140 | 25 | |
| 11 | 117 | 68 | 3.02 | 4.140 | 9 | |
| 12 | 111 | 167 | 7.42 | 4.120 | 20 | |
| 13 | 103 | 121 | 1131 | 5.38 | 4.130 | 16 |
| 14 | 95 | 831 | 36.93 | 4.110 | 61 | |
| 15 | 103 | 105 | 4.67 | 4.130 | 12 | |
| 16 | 122 | 74 | 3.29 | 4.130 | 11 | |
| 17 | 105 | 162 | 899 | 7.20 | 4.120 | 19 |
| 18 | 105 | 163 | 7.24 | 4.110 | 20 | |
| 19 | 109 | 160 | 7.11 | 4.110 | 21 | |
| 20 | 105 | 414 | 18.40 | 4.130 | 37 | |
| 21 | 89 | 1049 | 1693 | 46.62 | 4.140 | 14 |
| 22 | 103 | 203 | 9.02 | 4.150 | 23 | |
| 23 | 93 | 211 | 9.38 | 4.130 | 23 | |
| 24 | 103 | 230 | 10.22 | 4.130 | 25 | |
| 25 | 90 | 310 | 1297 | 13.78 | 4.130 | 32 |
| 26 | 106 | 246 | 10.93 | 4.130 | 25 | |
| 27 | 92 | 520 | 23.11 | 4.140 | 46 | |
| 28 | 106 | 221 | 9.82 | 4.110 | 25 | |
| 29 | 117 | 141 | 993 | 6.27 | 4.110 | 17 |
| 30 | 118 | 94 | 4.18 | 4.130 | 14 | |
| 31 | 101 | 193 | 8.58 | 4.120 | 21 | |
| 32 | 100 | 565 | 25.11 | 4.140 | 48 | |
| 33 | 103 | 207 | 654 | 9.20 | 4.110 | 24 |
| 34 | 109 | 84 | 3.73 | 4.110 | 11 | |
| 35 | 109 | 92 | 4.09 | 4.140 | 14 | |
| 36 | 103 | 271 | 12.04 | 4.110 | 30 | |
| 37 | 109 | 146 | 760 | 6.49 | 4.130 | 17 |
| 38 | 103 | 187 | 8.31 | 4.110 | 21 | |
| 39 | 103 | 262 | 11.64 | 4.130 | 29 | |
| 40 | 103 | 165 | 7.33 | 4.140 | 19 |
| 10s | ||||||||||
| 4p | 1049 | 831 | 520 | 262 | 565 | 271 | 414 | 277 | 310 | 340 |
| 68 | 92 | 146 | 226 | 115 | 211 | 162 | 205 | 189 | 167 | |
| 74 | 94 | 156 | 230 | 121 | 218 | 163 | 207 | 193 | 174 | |
| 84 | 105 | 160 | 246 | 141 | 221 | 165 | 207 | 203 | 187 | |
| sum (mAh) | 1275 | 1122 | 982 | 964 | 942 | 921 | 904 | 896 | 895 | 868 |
| Δ to avg.(mAh) | 298 | 145 | 5 | −13 | −35 | −56 | −73 | −81 | −82 | −109 |
| % | 30.51 | 14.85 | 0.52 | −1.32 | −3.57 | −5.72 | −7.46 | −8.28 | −8.38 | −11.15 |
| Cell No. | Start Voltage Us (V) | Cell Resistance R (mΩ) | Cell Capacity (mAh) | Discharge Time td | End Voltage Ue (V) | Charge Time tc (min) |
|---|---|---|---|---|---|---|
| 41 | 4.22 | 189 | 2283 | 1 h 15 m | 3.60 | 16 |
| 42 | 4.28 | 175 | 2412 | 1 h 20 m | 3.61 | 34 |
| 43 | 4.23 | 165 | 2326 | 1 h 17 m | 3.60 | 26 |
| 44 | 4.28 | 158 | 2412 | 1 h 20 m | 3.45 | 23 |
| 45 | 4.25 | 172 | 2381 | 1 h 19 m | 3.57 | 20 |
| 46 | 4.22 | 162 | 2255 | 1 h 14 m | 3.53 | 23 |
| 47 | 4.21 | 239 | 820 | 27 m 28 s | 3.78 | 18 |
| 48 | 4.24 | 202 | 840 | 27 m 59 s | 3.71 | 27 |
| 49 | 4.23 | 226 | 1094 | 36 m 25 s | 3.78 | 23 |
| 50 | 4.25 | 206 | 951 | 31 m 39 s | 3.81 | 25 |
| 51 | 4.17 | 215 | 387 | 12 m 57 s | 3.77 | 9 |
| 52 | 4.21 | 206 | 566 | 18 m 53 s | 3.60 | 20 |
| 53 | 4.24 | 135 | 2696 | 1 h 29 m | 3.34 | 16 |
| 54 | 4.21 | 185 | 1328 | 44 m 09 s | 3.70 | 61 |
| 55 | 4.23 | 187 | 953 | 31 m 43 s | 3.45 | 12 |
| 56 | 4.23 | 168 | 2117 | 1 h 10 m | 3.40 | 11 |
| 57 | 4.22 | 186 | 2128 | 1 h 10 m | 3.44 | 19 |
| 58 | 4.24 | 141 | 1009 | 33 m 43 s | 3.60 | 20 |
| 59 (14) | 4.24 | 137 | 1318 | 43 m 05 s | 3.54 | 21 |
| 60 (21) | 4.25 | 160 | 1455 | 48 m 33 s | 3.50 | 37 |
| 10s | ||||||||||
| 2p | 2117 | 2326 | 2255 | 2283 | 2412 | 2128 | 2381 | 2696 | 2412 | 1455 |
| 1318 | 951 | 1009 | 953 | 820 | 1094 | 840 | 387 | 566 | 1328 | |
| sum (mAh) | 3435 | 3277 | 3264 | 3236 | 3232 | 3222 | 3221 | 3083 | 2978 | 2783 |
| Δ to avg.(mAh) | 262 | 104 | 91 | 63 | 59 | 49 | 48 | −90 | −195 | −390 |
| % | 8.25 | 3.27 | 2.86 | 1.98 | 1.86 | 1.54 | 1.51 | −2.84 | −6.15 | −12.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavaš, H.; Barić, T.; Karakašić, M.; Keser, T. Application of Infrared Thermography in E-Bike Battery Pack Detail Analysis—Case Study. Appl. Sci. 2022, 12, 3444. https://doi.org/10.3390/app12073444
Glavaš H, Barić T, Karakašić M, Keser T. Application of Infrared Thermography in E-Bike Battery Pack Detail Analysis—Case Study. Applied Sciences. 2022; 12(7):3444. https://doi.org/10.3390/app12073444
Chicago/Turabian StyleGlavaš, Hrvoje, Tomislav Barić, Mirko Karakašić, and Tomislav Keser. 2022. "Application of Infrared Thermography in E-Bike Battery Pack Detail Analysis—Case Study" Applied Sciences 12, no. 7: 3444. https://doi.org/10.3390/app12073444
APA StyleGlavaš, H., Barić, T., Karakašić, M., & Keser, T. (2022). Application of Infrared Thermography in E-Bike Battery Pack Detail Analysis—Case Study. Applied Sciences, 12(7), 3444. https://doi.org/10.3390/app12073444










