Partial Replacement of Petroleum Coke with Modified Biocoke during Production of Anodes Used in the Aluminum Industry: Effect of Additive Type
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. XPS
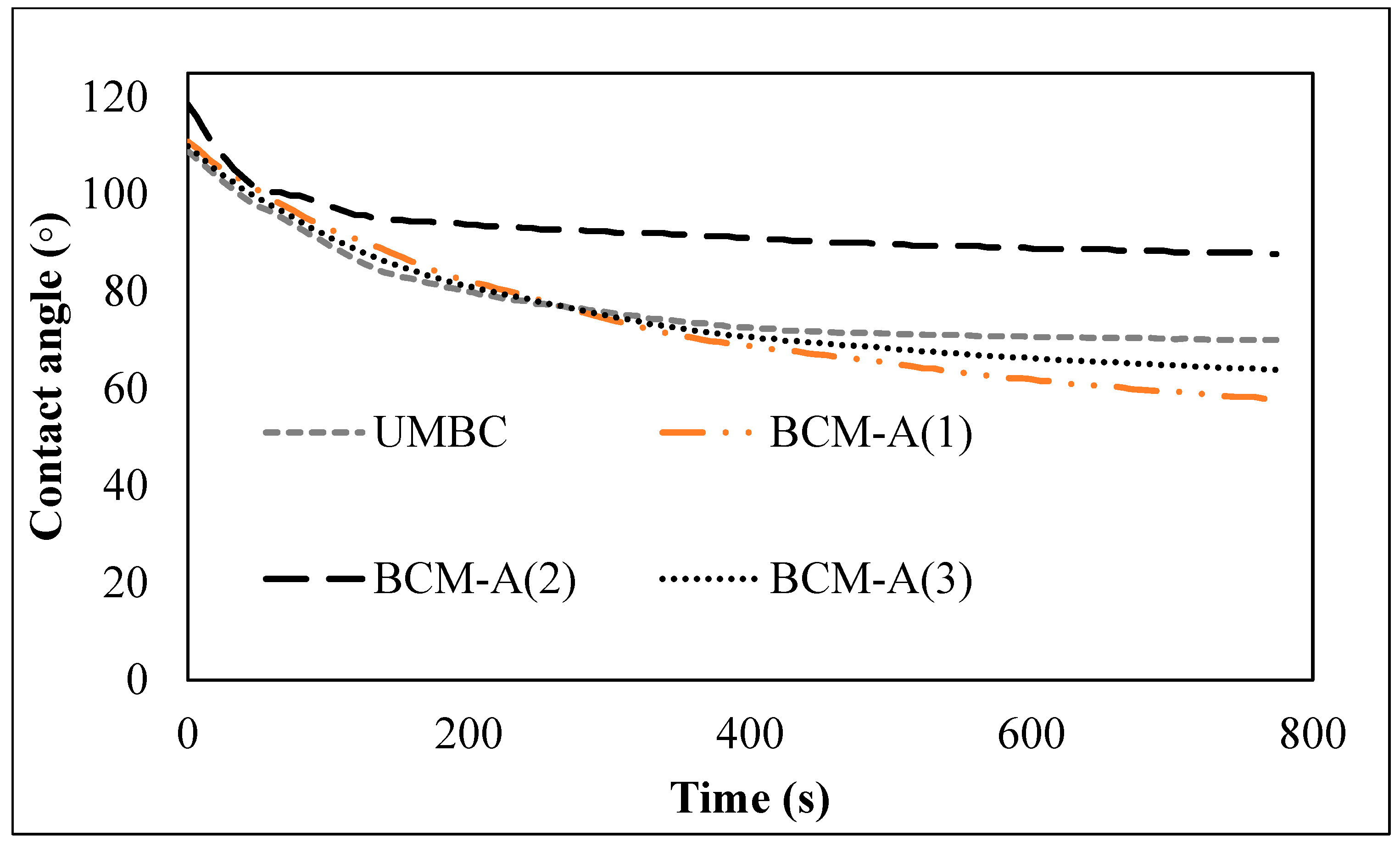
2.3. Wettability
2.4. Laboratory Anode Production
2.5. Measurement of Anode Properties
3. Results and Discussion
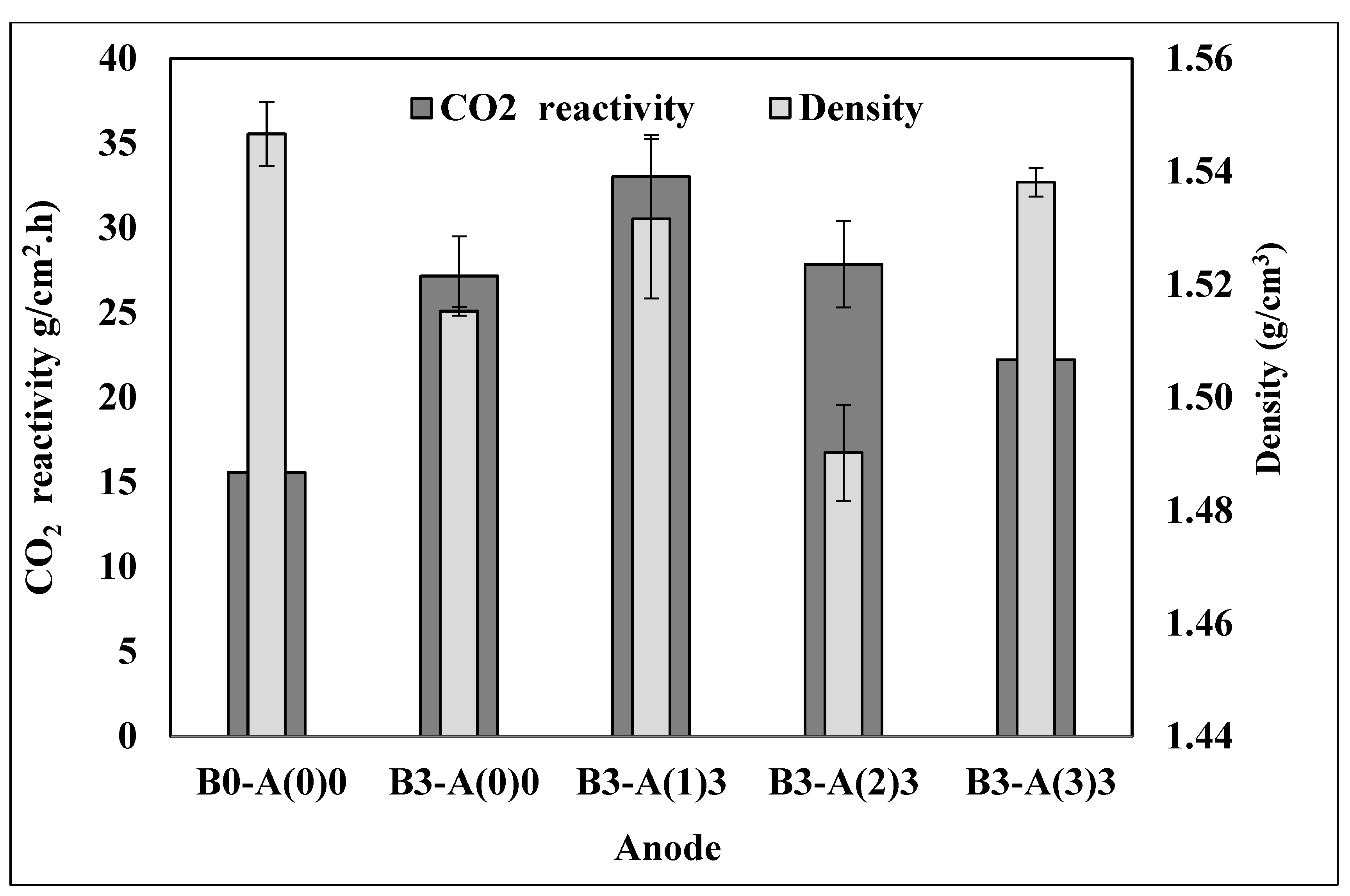
3.1. Density
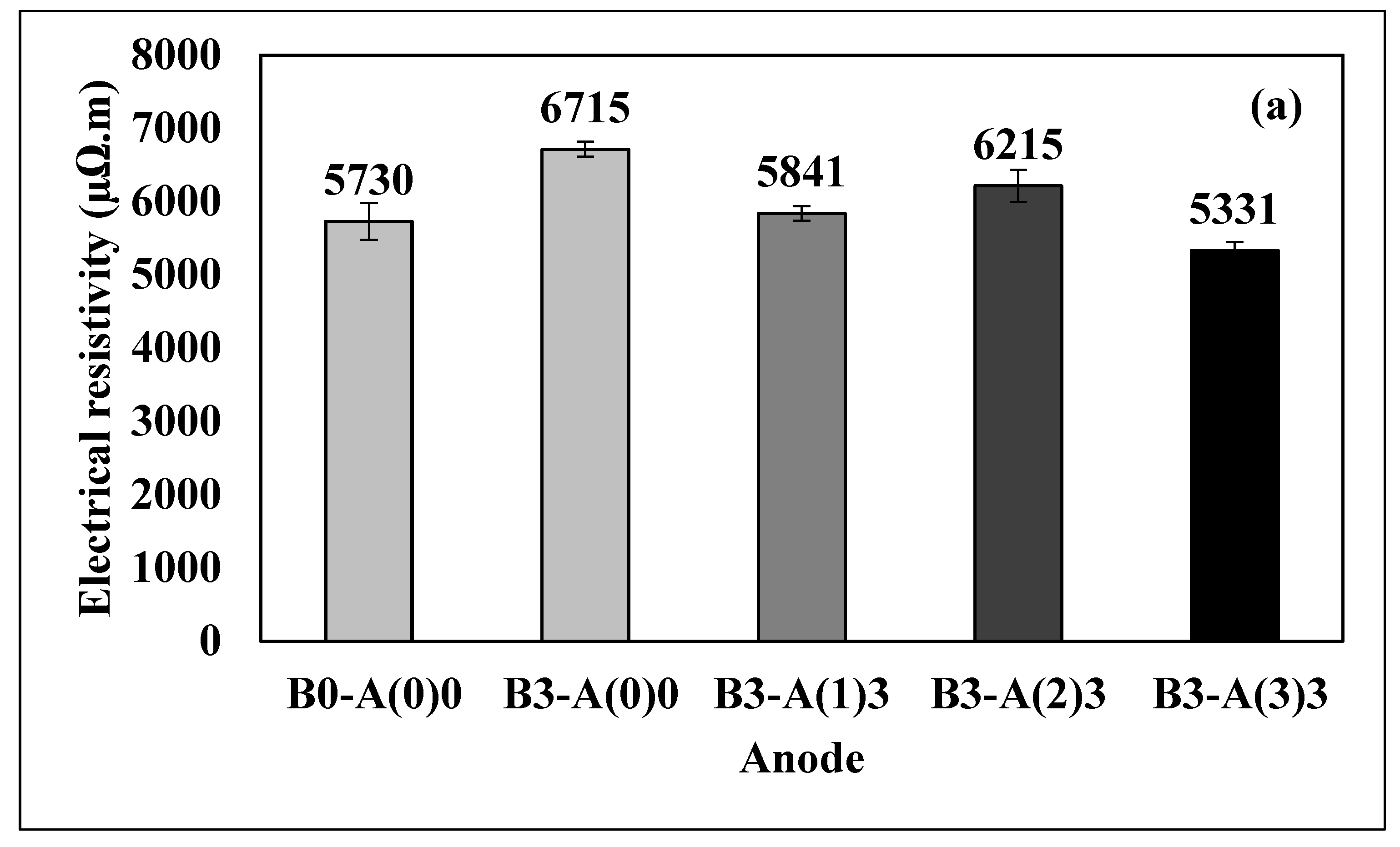
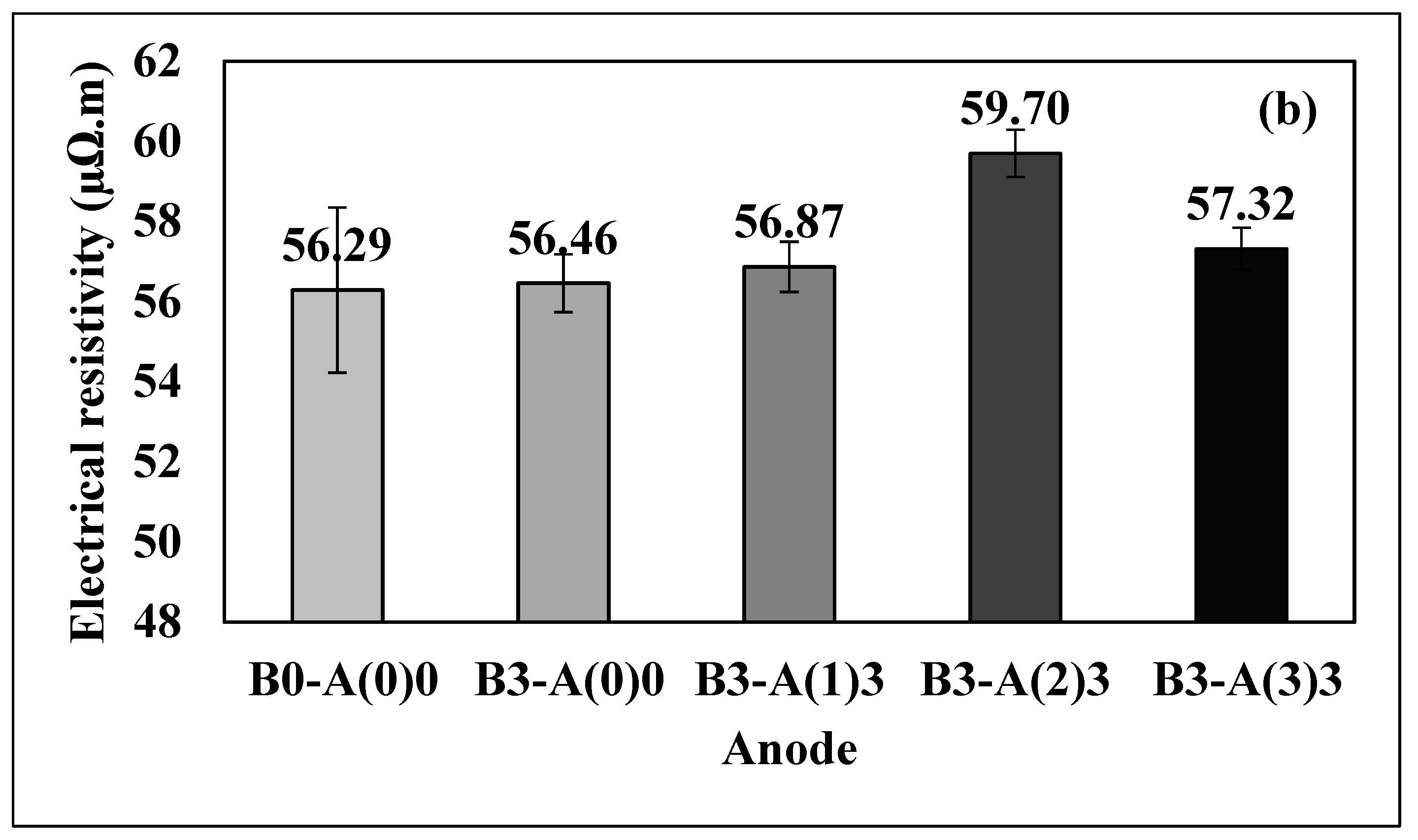
3.2. Electrical Resistivity
3.3. Air and CO2 Reactivities
3.3.1. Air Reactivity
3.3.2. Carbon Dioxide (CO2) Reactivity
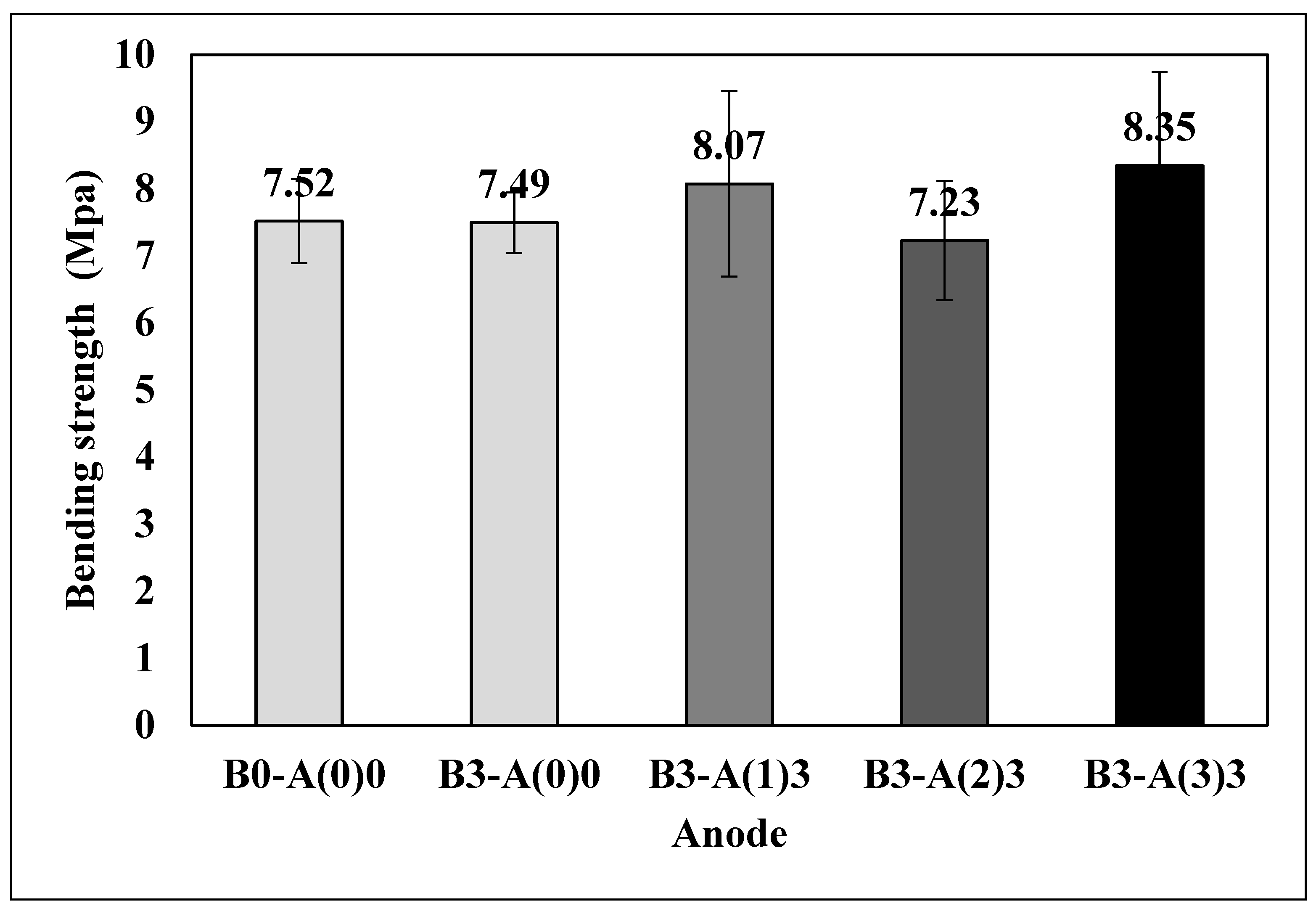
3.4. Bending Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saevarsdottir, G.; Halvor, K.; Barry, J.W. Aluminum production in the times of climate change: The global challenge to reduce the carbon footprint and prevent carbon leakage. JOM 2020, 72, 296–308. [Google Scholar] [CrossRef]
- Aluminium Association of Canada. Une Intensité de GES Figurant par les plus Basses au Monde. 2018. Available online: https://aac.metrio.net/indicators/environnement/emissions/emissions_ges (accessed on 10 December 2021).
- Charette, A.; Kocaefe, Y.S.; Kocaefe, D. Le Carbone dans l’Industrie de l’Aluminium; Les Presses de l’Aluminium: Chicoutimi, QC, Canada, 2012. [Google Scholar]
- Amara, B. Effet du Soufre sur la Réactivité des Anodes en Carbone. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2017. [Google Scholar]
- Hulse, K.L. Anode Manufacture: Raw Materials, Formulation and Processing Parameters; R & D Carbon Ltd.: Sierre, Switzerland, 2000. [Google Scholar]
- Edwards, L.; Backhouse, N.; Darmstadt, H.; Dion, M.J. Evolution of Anode Grade Coke Quality. In Light Metals; Springer: Cham, Switzerland, 2012; pp. 1207–1212. [Google Scholar]
- The 2020 State of Canada’s Forests Annual Report: An Overview 2020. Available online: https://www.nrcan.gc.ca/2020-state-canadas-forests-annual-report-overview/22925 (accessed on 2 February 2022).
- Overview of Canada’s Forest Industry. Available online: https://www.nrcan.gc.ca/our-natural-resources/forests-forestry/forest-industry-trade/overview-canadas-forest-industry/13311 (accessed on 2 February 2022).
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D. Wettability of bio-coke by coal tar pitch for its use in carbon anodes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 133–144. [Google Scholar] [CrossRef]
- Burgess, J.M. Biomass and renewables as alternative energy sources andreductants in the minerals industry. Fremantle 2004, 2004, 9–13. [Google Scholar]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D. Interaction of bio-coke with different coal tar pitches. Fuel 2016, 179, 179–192. [Google Scholar] [CrossRef]
- Huang, X.; Kocaefe, D.; Bhattacharyay, D.; Kocaefe, Y.; Morais, B. Characterization of carbon anode materials by image analysis. In Light Metals; Springer: Cham, Switzerland, 2016; pp. 859–864. [Google Scholar]
- Huang, X.; Kocaefe, D.; Kocaefe, Y. Utilization of Biocoke as a Raw Material for Carbon Anode Production. Energy Fuels 2018, 32, 8537–8544. [Google Scholar] [CrossRef]
- Hussein, A. A Bio-Coke for Anode Production and the Manufacturing Method Thereof. Master’s Thesis, Université Laval, Québec City, QC, Canada, 2014. [Google Scholar]
- Hussein, A.; Fafard, M.; Ziegler, D.; Alamdari, H. Effects of Charcoal Addition on the Properties of Carbon Anodes. Metals 2017, 7, 98. [Google Scholar] [CrossRef]
- Monsen, B.E.; Ratvik, A.P.; Lossius, L.P. Charcoal in anodes for aluminium production. In Light Metals; Springer: Cham, Switzerland, 2010; pp. 929–934. [Google Scholar]
- Ozturk, S.; Kocaefe, D.; Bhattacharyay, D.; Kocaefe, Y.; Morais, B. Modification of coke by different additives to improve anode properties. Fuel 2018, 211, 102–109. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Yetunde Omolayo, Y.; Spatari, S. Continuous calcination of biocoke/petcoke blends in a rotary tube furnace. ACS Sustain. Chem. Eng. 2021, 9, 695–703. [Google Scholar] [CrossRef]
- Amara, B.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D.; Côté, J.; Gilbert, A. Effect of Coke Type on the Partial Replacement of Coke with Modified Biocoke in Anodes Used in Primary Aluminum Production. In Light Metals; Springer: Cham, Switzerland, 2022; pp. 818–825. [Google Scholar]
- An American National Standard, ASTM D5502-00 (2005); Standard Test Methodes for Apparent Density by Physical Measurements of Manufactured Anode and Cathode Carbon Used by the Aluminium Industry. American National Standards Institute: New York, NY, USA, 2010.
- An American National Standard, ASTM D6120-97 (2007); Standard Test Method for Electrical Resistivity of Anode and Cathode Carbon Material at Room Temperature. American National Standards Institute: New York, NY, USA, 2010.
- An American National Standard, ASTM D6559-00 (Reapproved 2005); Standard Test Method for Determination of TGA Air Reactivity of Baked Carbon Anodes and Cathode Blocks. American National Standards Institute: New York, NY, USA, 2010.
- An American National Standard, ASTM D6558-00 (Reapproved 2005); Standard Test Method for Determination of TGA CO2 Reactivity of Baked Carbon Anodes and Cathode Blocks. American National Standards Institute: New York, NY, USA, 2010.
- ISO 12986-1: 2014; Carbonaceous Materials Used in the Production of Aluminium—Prebaked Anodes and Cathode Blocks—Part 1: Determination of Bending/Shear Strength by the Three-Point Method. International Organization for Standardization: Geneva, Switzerland, 2014.
- Suriyapraphadilok, U. Characterization of Coal- and Petroleum-Derived Binder Pitches and the Interaction of Pitch/Coke Mixtures in Pre-baked Carbon Anodes. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2008; 354p. [Google Scholar]
- Kocaefe, D.; Sarkar, A.; Das, S.; Amrani, S.; Bhattacharyay, D.; Sarkar, D.; Kocaefe, Y.S.; Morais, B.; Gagnon, M. Review of different techniques to study the interactions between coke and pitch in anode manufacturing. In TMS Light Metals; Springer: Cham, Switzerland, 2013; pp. 1045–1050. [Google Scholar]
- Hume, S.M. Anode Reactivity: Influence of Raw Material Properties, 2nd ed.; R & D Carbon Ltd.: Sierre, Switzerland, 1999. [Google Scholar]
- Kuang, Z.; Thonstad, J.; Sørlie, M. Effects of additives on the electrolytic consumption of carbon anodes in aluminium electrolysis. Carbon 1995, 33, 1479–1484. [Google Scholar] [CrossRef]
- Sørlie, M. Effect of sulphur on anode reactivity and electrolytic consumption. In Light Metals; Springer: Cham, Switzerland, 1994; pp. 659–665. [Google Scholar]
- Amara, B.; Faouzi, F.E.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D.; Côté, J.; Gilbert, A. Modification of biocoke destined for the fabrication of anodes used in primary aluminum production. Fuel 2021, 304, 121352. [Google Scholar] [CrossRef]


| Abbreviation | |
|---|---|
| UMBC | Unmodified biocoke |
| BCM-A(1) | Biocoke modified with 3% A(1) |
| BCM-A(2) | Biocoke modified with 3% A(2) |
| BCM-A(3) | Biocoke modified with 3% A(3) |
| Anode | Percent Biocoke (%) | Additive | Percent Additive (%) |
|---|---|---|---|
| B0-A(0)0 * | 0 | 0 | 0 |
| B3-A(0)0 | 3 | 0 | 0 |
| B3-A(1)3 | 3 | 1 | 3 |
| B3-A(2)3 | 3 | 2 | 3 |
| B3-A(3)3 | 3 | 3 | 3 |
| Sample | Carbon C1s Components (%) | ||||||
|---|---|---|---|---|---|---|---|
| Aromatic | Aliphatic | Ether Alcohol | Carbonyl | Carboxyl, Imide, Ester | Amine, Epoxy | ||
| C=C | C–C | C–O | C=O | COO/COON | CN | ||
| UMBC | 89.8 | 0 | 1.5 | 1.2 | 1.2 | - | |
| BCM-A(1) | 88.3 | 0 | 1.7 | 2.2 | 2.2 | - | |
| BCM-A(2) | 56.24 | 25.43 | 5.41 | 1.7 | 2.07 | - | |
| BCM-A(3) | 73.06 | 9.82 | 4.82 | 2.09 | 1.34 | ||
| Pitch | 66.1 | 25.8 | 0.9 | 0.4 | 0.4 | 0.5 | |
| Coke | 89.4 | 4.9 | 1.1 | 1 | 0.9 | - | |
| Simple | Atomic Percentage | ||||||
| Heteroatoms | |||||||
| C(%) | O(%) | S(%) | N(%) | K(%) | |||
| UMBC | 93.8 | 4.9 | 0.1 | - | 1.1 | ||
| BCM-A(1) | 91.9 | 6.3 | - | - | 1.2 | ||
| BCM-A(2) | 92.41 | 7.59 | - | - | - | ||
| BCM-A(3) | 92.18 | 7.82 | - | - | - | ||
| Pitch | 94.8 | 3.6 | 0.1 | 2.2 | - | ||
| Coke | 97.3 | 2.5 | 0.2 | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amara, B.; Kocaefe, D.; Kocaefe, Y.; Bhattacharyay, D.; Côté, J.; Gilbert, A. Partial Replacement of Petroleum Coke with Modified Biocoke during Production of Anodes Used in the Aluminum Industry: Effect of Additive Type. Appl. Sci. 2022, 12, 3426. https://doi.org/10.3390/app12073426
Amara B, Kocaefe D, Kocaefe Y, Bhattacharyay D, Côté J, Gilbert A. Partial Replacement of Petroleum Coke with Modified Biocoke during Production of Anodes Used in the Aluminum Industry: Effect of Additive Type. Applied Sciences. 2022; 12(7):3426. https://doi.org/10.3390/app12073426
Chicago/Turabian StyleAmara, Belkacem, Duygu Kocaefe, Yasar Kocaefe, Dipankar Bhattacharyay, Jules Côté, and André Gilbert. 2022. "Partial Replacement of Petroleum Coke with Modified Biocoke during Production of Anodes Used in the Aluminum Industry: Effect of Additive Type" Applied Sciences 12, no. 7: 3426. https://doi.org/10.3390/app12073426
APA StyleAmara, B., Kocaefe, D., Kocaefe, Y., Bhattacharyay, D., Côté, J., & Gilbert, A. (2022). Partial Replacement of Petroleum Coke with Modified Biocoke during Production of Anodes Used in the Aluminum Industry: Effect of Additive Type. Applied Sciences, 12(7), 3426. https://doi.org/10.3390/app12073426








