Relationship between Subjective and Biological Responses to Comfortable and Uncomfortable Sounds
Abstract
:1. Introduction
2. Methods
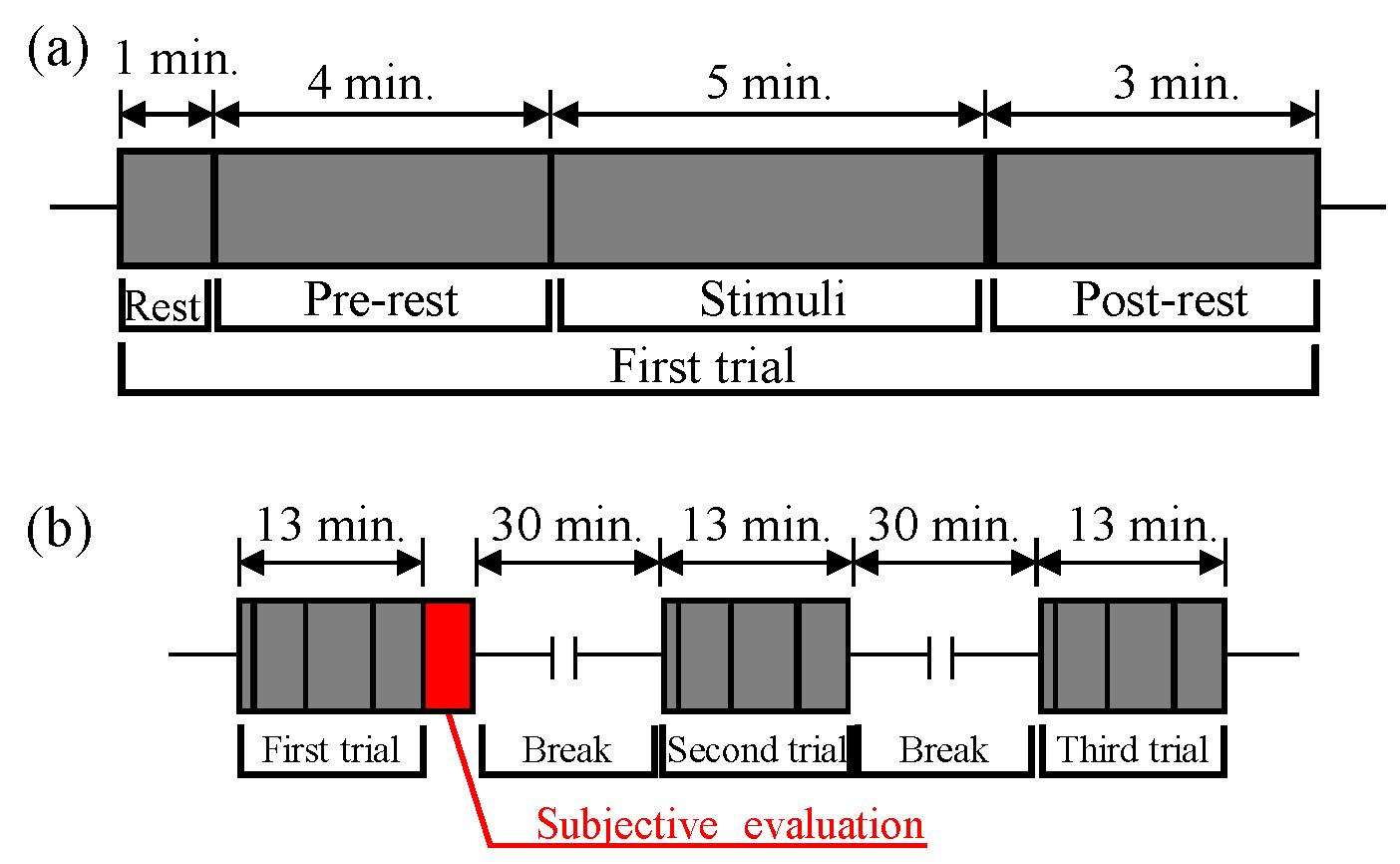
2.1. Outline of the Experiment
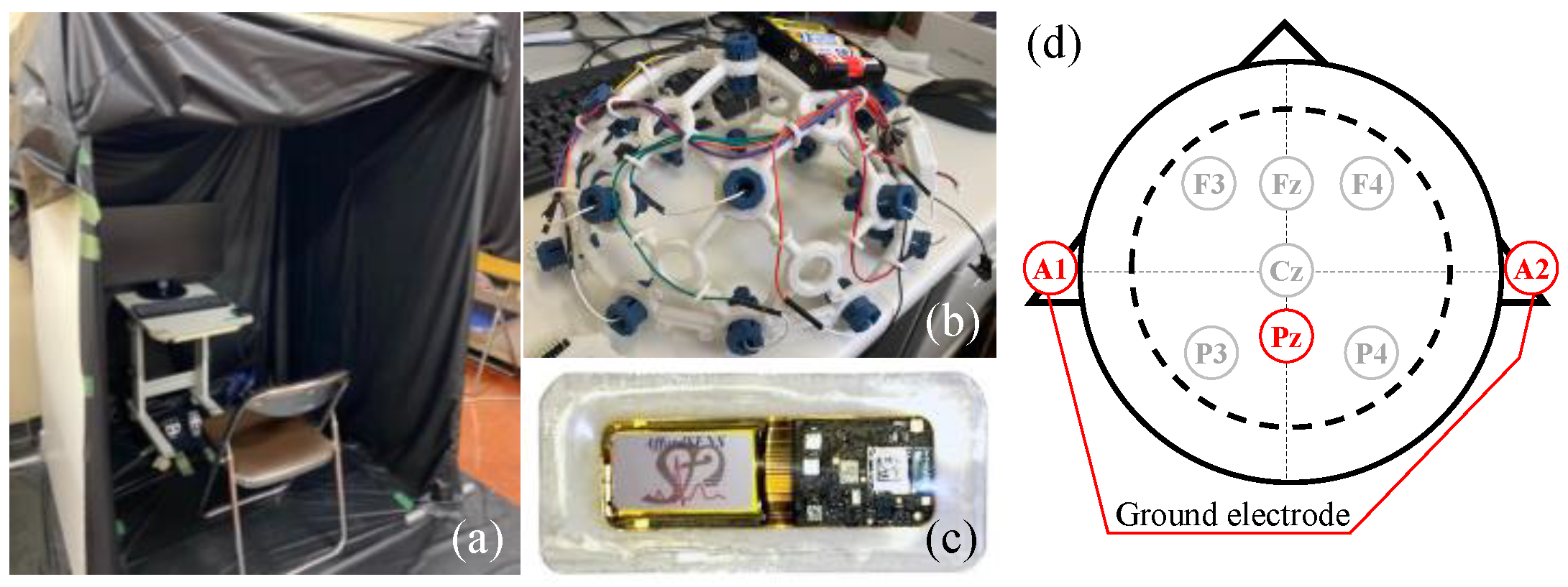
2.2. Physiological Measurement
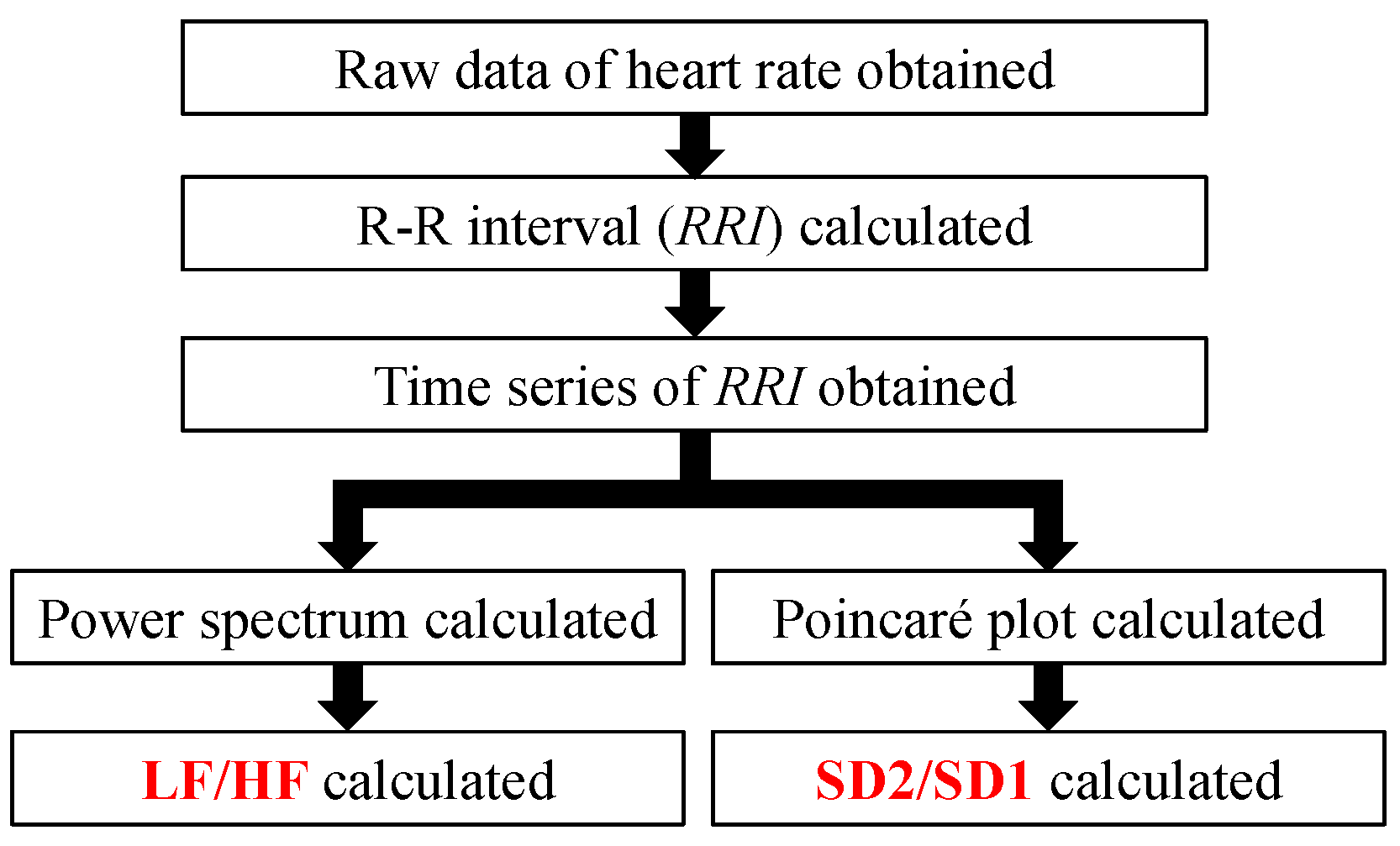
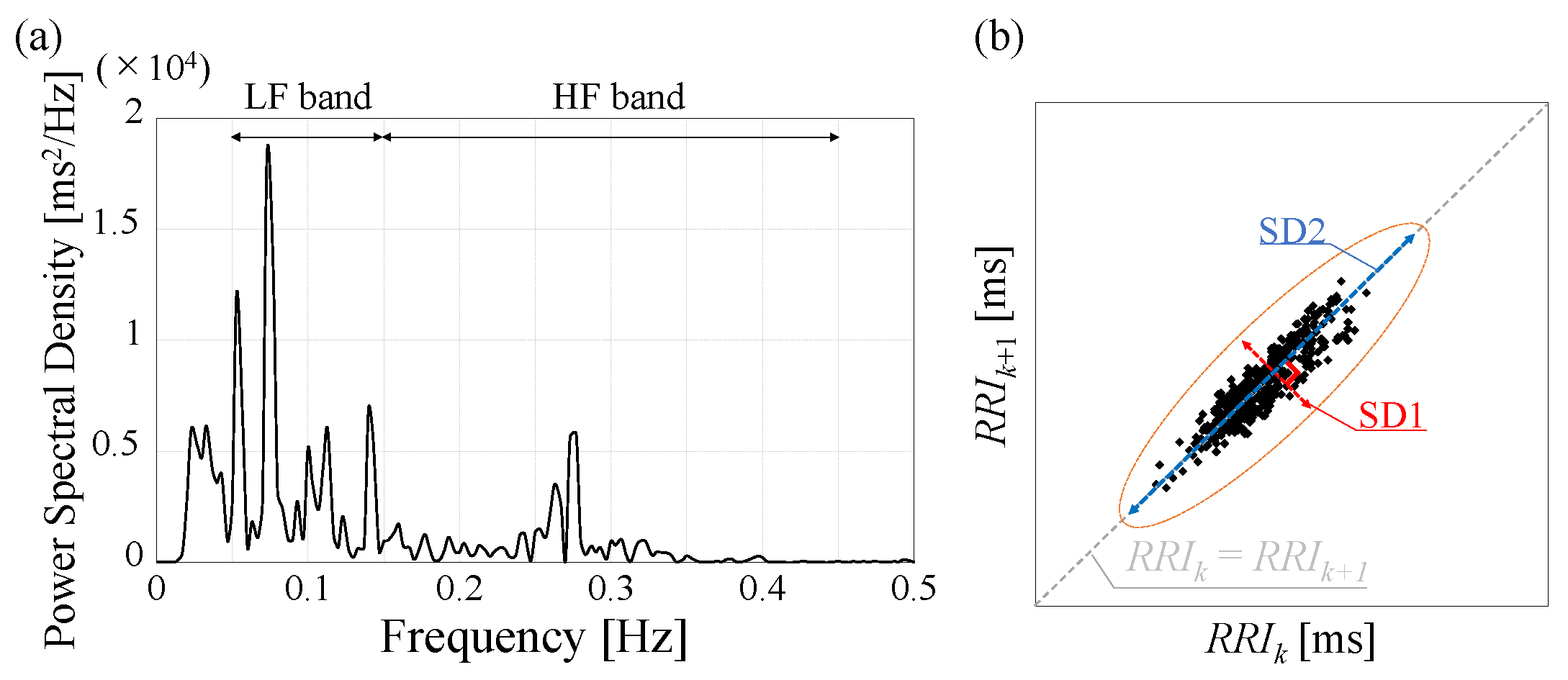
2.3. Analysis
2.4. Subjective Evaluation Experiment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Profile Analysis
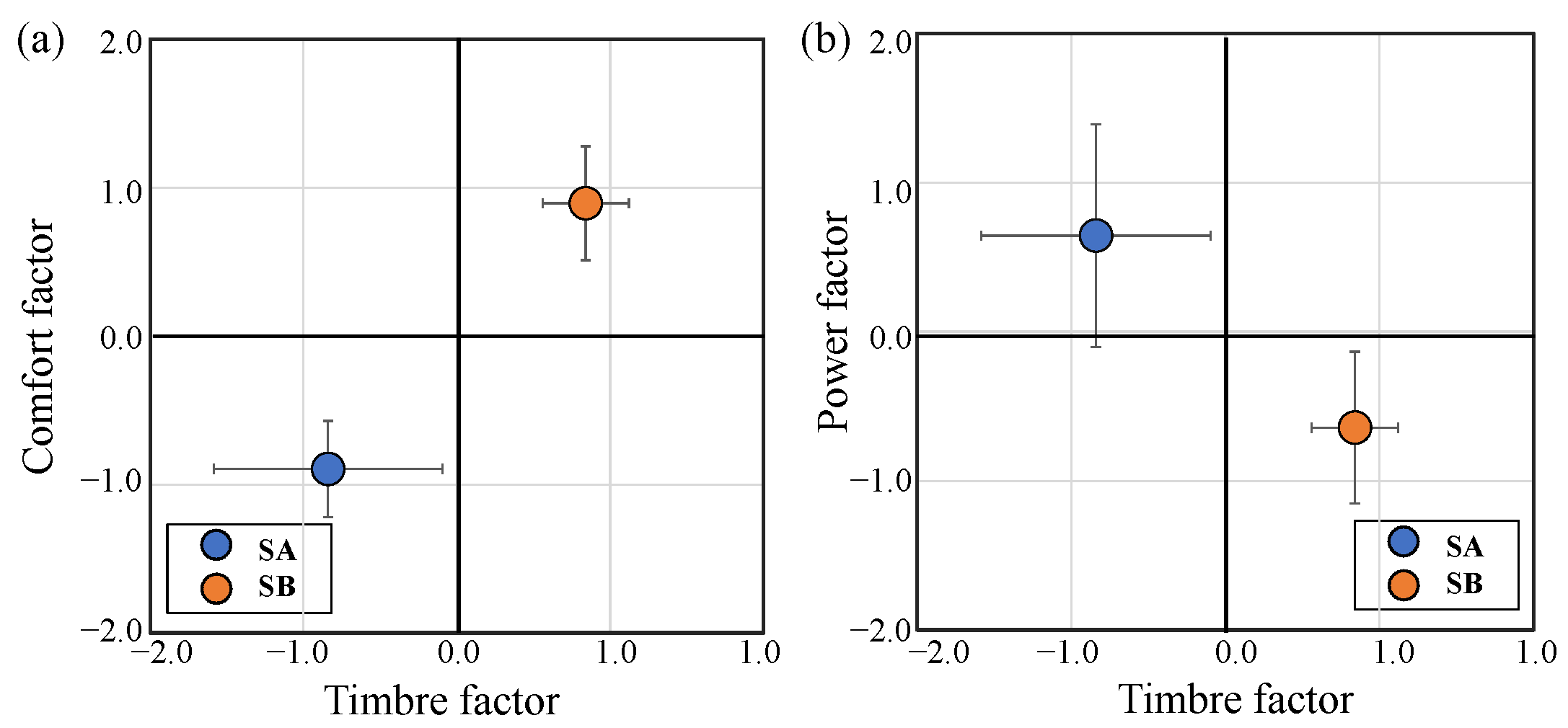
3.2. Factor Analysis
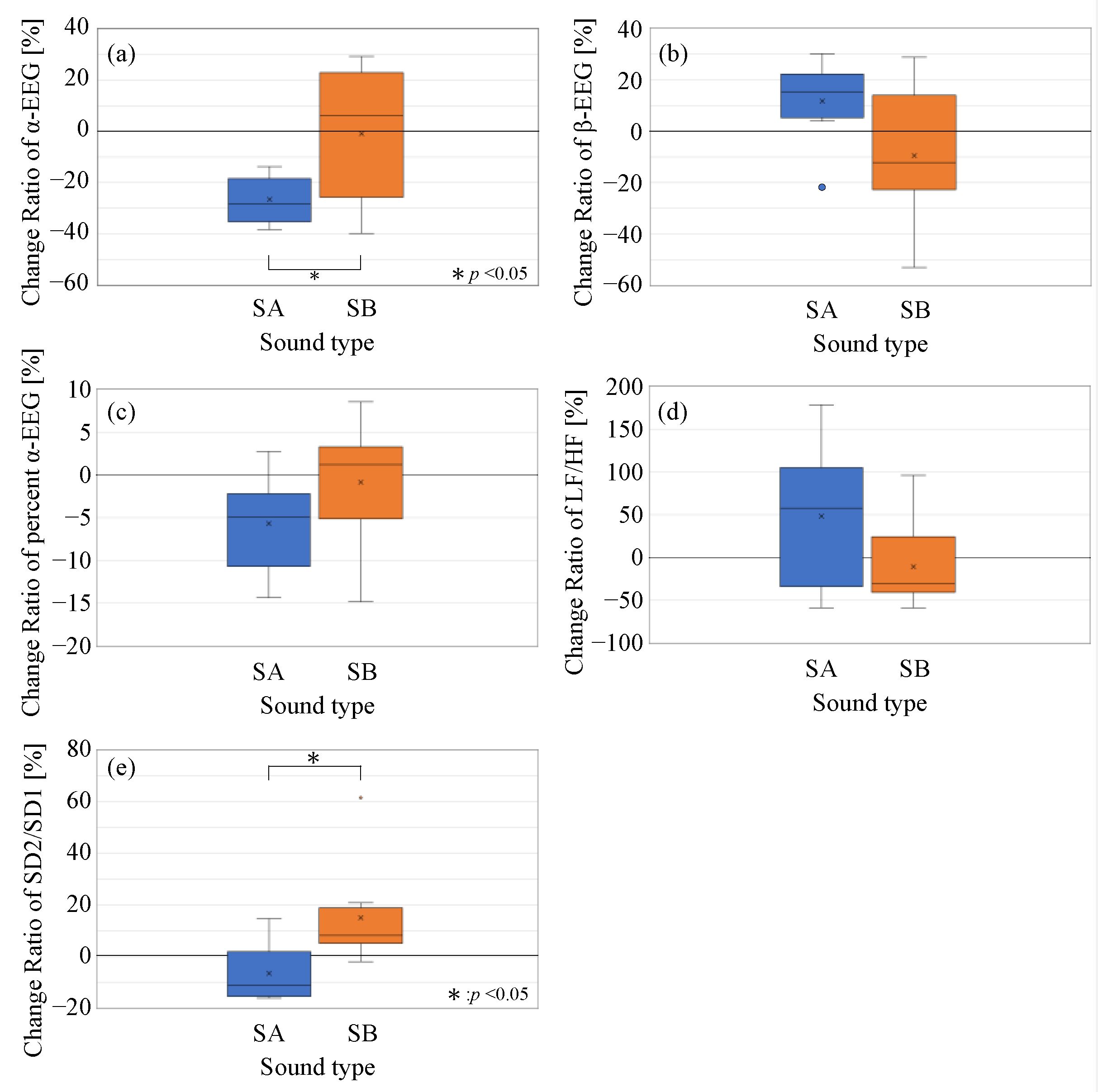
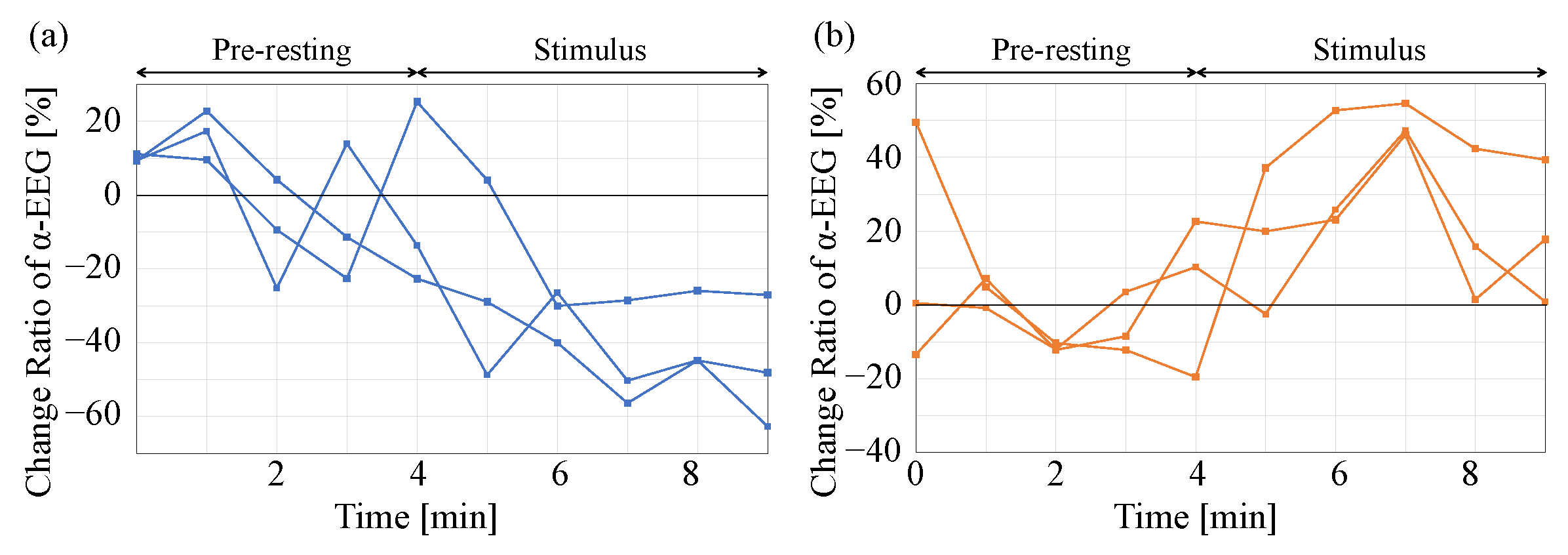
3.3. Physiological Response
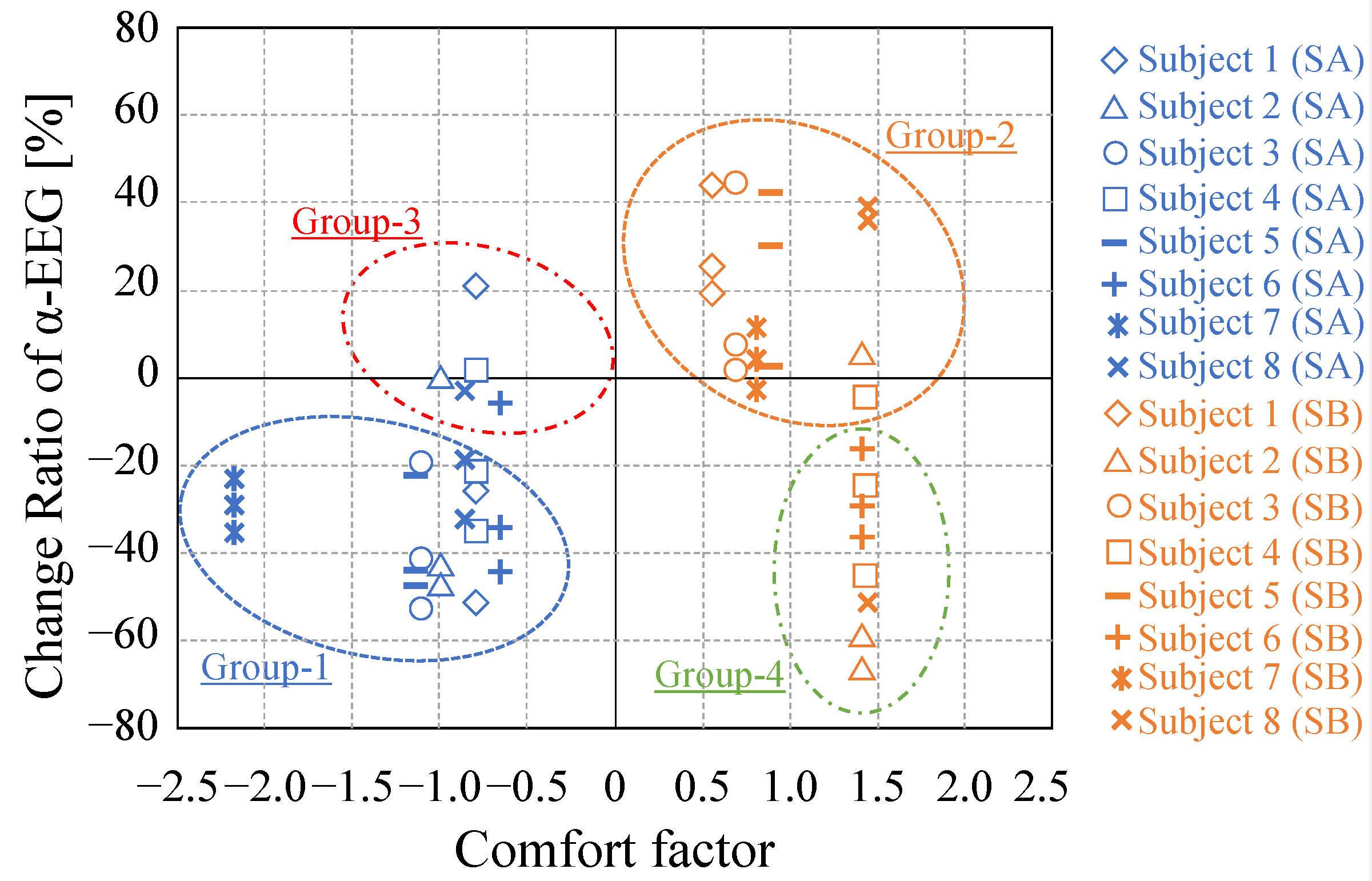
3.4. Relationship between Subjective and Biological Responses
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.; Kessler, R.C.; Gordon, L.U. Strategies for measuring stress in studies of psychiatric and physical disorders. In Measuring Stress: A Guide for Health and Social Scientists; Cohen, S., Kessler, R.C., Gordon, L.U., Eds.; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Lampert, R. ECG signatures of psychological stress. J. Electrocardiol. 2015, 48, 1000–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blons, E.; Arsac, L.M.; Grivel, E.; Lespinet-Najib, V.; Deschodt-Arsac, V. Physiological resonance in empathic stress: Insights from nonlinear dynamics of heart rate variability. Int. J. Environ. Res. Public Health. 2021, 18, 2081. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Mendes, W.B. A large-scale study of stress, emotions, and blood pressure in daily life using a digital platform. Proc. Natl. Acad. Sci. USA 2021, 118, e2105573118. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.D.; Lisete, C.M.; De-Pei, L.; Hui-Lin, P. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am. J. Physiol. Heart Circulat. Phys. 2018, 315, H1200–H1214. [Google Scholar]
- Suess, W.M.; Alexander, A.B.; Smith, D.D.; Sweeney, H.W.; Marion, R.J. The Effects of Psychological Stress on Respiration: A Preliminary Study of Anxiety and Hyperventilation. Psychophysiology 1980, 17, 535–540. [Google Scholar] [CrossRef]
- Baker, L.M.; Taylor, W.M. The relationship under stress between changes in skin temperature, electrical skin resistance, and pulse rate. J. Exp. Psychol. 1954, 48, 361–366. [Google Scholar] [CrossRef]
- Blore, D. Use of eye movement to reduce stress after trauma. Nurs. Times 1996, 92, 43–45. [Google Scholar]
- Li, T.M.; Chao, H.C.; Zhang, J. Emotion classification based on brain wave: A survey. Hum. Cent. Comput. Inf. Sci. 2019, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Gerrard, P.; Malcolm, R. Mechanisms of modafinil: A review of current research. Neuropsychiatr. Dis. Treat. 2007, 3, 349–364. [Google Scholar] [PubMed]
- Haas, L.F. Hans Berger (1873–1941), Richard Caton (1842–1926), and electroencephalography. J. Neurol. Neurosurg. Psychiatry. 2003, 74, 9. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.H.; Byun, S.; Park, M.S.; Park, J.H. Reliability of Physiological Responses Induced by Basic Emotions: A Pilot Study. J. Physiol. Anthropol. 2019, 38, 15. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.V.; Mewes, R.; Nater, U.M. Preliminary evidence: The stress-reducing effect of listening to water sounds depends on somatic complaints: A randomized trial. Medicine 2018, 97, e9851. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, S.; Walter, A.S. Towards a neural basis of music perception. Trends Cogn. Sci. 2005, 9, 578–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, H.; Humphries, E.; Peña, S.; Mekic, A.; Hurless, N.; Nichols, D.F. Music genre preference and tempo alter alpha and beta waves in human non-musicians. Impuls. Prem. Undergrad. Neurosci. J. 2013, 22, 1–11. [Google Scholar]
- Norbert, J.; Katarina, H. The influence of Mozart sonata K.448 on brain activity during the performance of spiritual rotation and numerical tasks. Brain Topogr. 2005, 17, 207–218. [Google Scholar]
- Caldwell, G.N.; Riby, L.M. The effects of music exposure and own genre preference on conscious and unconscious cognitive processes: A pilot ERP study. Conscious. Cogn. 2007, 16, 992–996. [Google Scholar] [CrossRef]
- Park, S.A.; Song, C.; Choi, J.Y.; Son, K.C.; Miyazaki, Y. Foliage Plants cause physiological and psychological relaxation as evidenced by measurements of prefrontal cortex activity and profile of mood states. HortScience 2016, 51, 1308–1312. [Google Scholar] [CrossRef]
- Acharya, R.; Joseph, P.; Kannathal, N.; Lim, C.M.; Suri, J. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.; Laguna, P.; Bartra, A.; Bailón, R. Drowsiness detection using heart rate variability. Med. Biol. Eng. Comput. 2016, 54, 927–937. [Google Scholar] [CrossRef]
- Spangler, D.; Alam, S.; Rahman, S.; Crone, J.; Robucci, R.; Banerjee, N.; Kerick, S.; Brooks, J. Multilevel longitudinal analysis of shooting performance as a function of stress and cardiovascular responses. IEEE Trans. Affect. Comput. 2020, 12, 648–665. [Google Scholar] [CrossRef]
- Fukuba, Y.; Sato, H.; Sakiyama, T.; Endo, Y.M.; Yamada, M.; Ueoka, H.; Miura, A.; Koga, S. Autonomic nervous activities assessed by heart rate variability in pre- and post-adolescent Japanese. J. Physiol. Anthropol. 2009, 28, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, A.; Jaime, V.; Beatriz, R.; Pedro, M.; Ángel, M.M. The relationship between heart rate variability and electroencephalography functional connectivity variability is associated with cognitive flexibility. Front. Hum. Neurosci. 2019, 13, 64. [Google Scholar]
- Piper, D.; Schiecke, K.; Leistritz, L.; Pester, B.; Benninger, F.; Feucht, M.; Ungureanu, G.; Strungaru, R.; Witte, H. Synchronization analysis between heart rate variability and EEG activity before, during, and after epileptic seizure. Biomed. Eng. 2014, 59, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Frey, J. Comparison of an open-hardware electroencephalography amplifier with medical grade device in brain-computer interface applications. arXiv 2016, arXiv:1606.02438. [Google Scholar]
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Guzik, P.; Piskorski, J.; Krauze, T.; Schneider, R.; Wesseling, K.H.; Wykretowicz, A.; Wysocki, H. Correlations between the Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 2007, 57, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Behbahani, S.; Dabanloo, N.J.; Nasrabadi, A.M. Ictal heart rate variability assessment with focus on secondary generalized and complex partial epileptic seizures. Adv. Biores. 2012, 4, 50–58. [Google Scholar]
- Brigo, F.; Ausserer, H.; Nardone, R.; Tezzon, F.; Manganotti, P.; Bongiovann, L.G. Subclinical rhythmic electroencephalogram discharge of adults occurring during sleep: A diagnostic challenge. Clin. EEG Neurosci. 2013, 44, 227–231. [Google Scholar] [CrossRef]
- Sorel, L.; Ranwez, R. Basis and use of a true monopolar derivation: The neo-electroencephalography (N.E.E.G.). Clin. Electroencephalogr. 1984, 15, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.W. Naming of the waves in the ECG, with a brief account of their genesis. Circulation 1998, 98, 1937–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltola, M.A. Role of editing of R-R intervals in the analysis of heart rate variability. Front. Physiol. 2012, 3, 148. [Google Scholar] [CrossRef] [Green Version]
- Camm, J. Task force of the European Society of Cardiology the North American Society of Pacing Electrophysiology; Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Wheeler, T.; Watkins, P.J. Cardiac denervation in diabetes. BMJ 1973, 4, 584–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, A. Acoustic quality of theatres: Correlations between experimental measures and subjective evaluations. Appl. Acoust. 2001, 62, 889–916. [Google Scholar] [CrossRef]
- Romero, V.P.; Maffei, L.; Brambilla, G.; Ciaburro, G. Modelling the soundscape quality of urban waterfronts by artificial neural networks. Appl. Acoust. 2016, 111, 121–128. [Google Scholar] [CrossRef]
- Puyana Romero, V.; Maffei, L.; Brambilla, G.; Ciaburro, G. Acoustic, visual and spatial indicators for the description of the soundscape of waterfront areas with and without road traffic flow. Int. J. Environ. Res. Public Health 2016, 13, 934. [Google Scholar] [CrossRef]
- Parizet, E.; Bolmont, A.; Fingerhuth, S. Subjective evaluation of tonalness and relation between tonalness and unpleasantness. In INTER-NOISE and NOISE-CON Congress and Conference Proceedings; Institute of Noise Control Engineering: Washington, DC, USA, 2009; Volume 2009, pp. 934–941. [Google Scholar]
- Osgood, C.E.; Tzeng, O. Language, Meaning, and Culture: The Selected Papers of C.E. Osgood; Praeger Publishers: Westport, CT, USA, 1990. [Google Scholar]
- Yamada, T.; Kuwano, S.; Ebisu, S.; Hayashi, M. Statistical analysis for subjective and objective evaluations of dental drill sounds. PLoS ONE 2016, 11, e0159926. [Google Scholar] [CrossRef]
- ISO 10551; Ergonomics of the Thermal Environment—Assessment of the Influence of the Thermal Environment Using Subjective Judgement Scales. ISO: Geneva, Switzerland, 1995.
- ISO/TS 15666; Acoustics—Assessment of Noise Annoyance by Means of Social and Socio-Acoustic Surveys. ISO: Geneva, Switzerland, 2003.
- Yang, W.; Moon, H.J.; Jeon, J.Y. Comparison of Response Scales as Measures of Indoor Environmental Perception in Combined Thermal and Acoustic Conditions. Sustainability 2019, 11, 3975. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.Y.; Jeon, J.Y. Designing sound and visual components for enhancement of urban soundscapes. J. Acoust. Soc. Am. 2013, 134, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Guttman, L. A new approach to factor analysis: The Radex. In Mathematical Thinking in the Social Sciences; Lazarsfeld, P.F., Ed.; Free Press: New York, NY, USA, 1954; pp. 258–348. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Saito, K. Effect of noise on higher nervous activity. J. Sound Vib. 1988, 127, 419–424. [Google Scholar] [CrossRef]
- Matsui, K.; Kawai, J.; Sawamura, K.; Kohara, Y.; Matsumoto, K. A psychophysiological study on biological reaction using music stimuli. J. Clin. Educ. Psychol. 2003, 29, 43–57. [Google Scholar]
- Hamid, N.H.A.; Sulaiman, N.; Murat, Z.H.; Taib, M.N. Brainwaves stress pattern based on perceived stress scale test. In Proceedings of the IEEE 6th Control and System Graduate Research Colloquium, Shah Alam, Malaysia, 10–11 August 2015; pp. 135–140. [Google Scholar]
- Kropotov, J.D. Quantitative EEG, Event-Related Potentials and Neurotherapy, 1st ed.; Academic Press: Moscow, Russia, 2010. [Google Scholar]
- Nishifuji, S.; Sato, M.; Maino, D.; Tanaka, S. Effect of acoustic stimuli and mental task on alpha, beta and gamma rhythms in brain wave. In Proceedings of the SICE Annual Conference 2010, Taipei, Taiwan, 18–21 August 2010; pp. 1548–1554. [Google Scholar]
- Roque, A.; Valenti, V.; Guida, H.; Campos, M.; Knap, A.; Vanderlei, L.; Ferreira, L.; Ferreira, C.; Abreu, L. The effects of auditory stimulation with music on heart rate variability in healthy women. Clinics 2013, 68, 960–967. [Google Scholar] [CrossRef]
- Toichi, M.; Sugiura, T.; Murai, T.; Sengoku, A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R–R interval. J. Auton. Nerv. Syst. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Mäkikallio, T.H.; Takala, T.E.; Seppänen, T.; Huikuri, H.V. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am. J. Physiol. 1996, 271, 244–252. [Google Scholar] [CrossRef]
- Lee, G.S.; Chen, M.L.; Wang, G.Y. Evoked response of heart rate variability using short-duration white noise. Auton. Neurosci. 2010, 155, 94–97. [Google Scholar] [CrossRef]




| (a) | |
| Channels | 8 |
| Quantization bit rate | 24-bit |
| Possible gain | 1, 2, 4, 6, 8, 12, 24 |
| Operation voltage | 3.3 V |
| Amplifier | Texas Instruments ADS1299 ADC |
| Microcontroller | PIC32MX250F128B |
| (b) | |
| Sampling frequency | 128, 256, 512, 1024 Hz |
| Quantization bit rate | 10-bit |
| Radio communication | Bluetooth 4.0 wireless technology |
| Power supply | Rechargeable Li-ion battery |
| Terminal OS | iOS 8.0 and above |
| Factor 1 | Factor 2 | Factor 3 | |||
|---|---|---|---|---|---|
| Beautiful | - | Dirty | 0.78 | 0.18 | −0.13 |
| Clear | - | Dull | 0.73 | 0.16 | −0.19 |
| Comfortable | - | Uncomfortable | 0.55 | 0.32 | −0.24 |
| Smooth | - | Rough | 1.12 | −0.35 | −0.14 |
| Unsentimental | - | Sentimental | −0.43 | −0.24 | −0.37 |
| Loud | - | Calm | −0.74 | −0.21 | 0.05 |
| Bright | - | Dark | 0.43 | 0.38 | −0.18 |
| Low-pitched | - | High-pitched | 0.47 | 0.32 | 0.38 |
| Moist | - | Dry | 0.45 | 0.48 | −0.17 |
| Poor | - | Rich | 0.01 | −0.70 | −0.07 |
| Large | - | Small | 0.10 | 0.81 | −0.04 |
| Soft | - | Hard | 0.21 | 0.67 | −0.07 |
| Metallic | - | Not metallic | −0.23 | −0.70 | −0.49 |
| Powerless | - | Powerful | −0.12 | 0.13 | −0.87 |
| Quiet | - | Noisy | 0.12 | −0.42 | −0.69 |
| Contribution ratio | 0.34 | 0.28 | 0.18 | ||
| Cumulative contribution ratio | 0.34 | 0.62 | 0.80 | ||
| Comfort Factor | Timbre Factor | Power Factor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corr. Coeff. | p Value | Corr. Coeff. | p Value | Corr. Coeff. | p Value | ||||
| a-EEG | 0.48 | 0.05 | * | −0.56 | 0.02 | * | 0.52 | 0.04 | * |
| b-EEG | 0.40 | 0.12 | −0.54 | 0.03 | * | −0.37 | 0.15 | ||
| Percent a-EEG | 0.24 | 0.36 | −0.21 | 0.43 | −0.43 | 0.03 | * | ||
| LF/HF | −0.44 | 0.09 | ** | 0.37 | 0.16 | 0.40 | 0.13 | ||
| SD2/SD1 | 0.44 | 0.09 | ** | −0.58 | 0.02 | * | −0.33 | 0.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asakura, T. Relationship between Subjective and Biological Responses to Comfortable and Uncomfortable Sounds. Appl. Sci. 2022, 12, 3417. https://doi.org/10.3390/app12073417
Asakura T. Relationship between Subjective and Biological Responses to Comfortable and Uncomfortable Sounds. Applied Sciences. 2022; 12(7):3417. https://doi.org/10.3390/app12073417
Chicago/Turabian StyleAsakura, Takumi. 2022. "Relationship between Subjective and Biological Responses to Comfortable and Uncomfortable Sounds" Applied Sciences 12, no. 7: 3417. https://doi.org/10.3390/app12073417
APA StyleAsakura, T. (2022). Relationship between Subjective and Biological Responses to Comfortable and Uncomfortable Sounds. Applied Sciences, 12(7), 3417. https://doi.org/10.3390/app12073417






