Erythropoietin Nanobots: Their Feasibility for the Controlled Release of Erythropoietin and Their Neuroprotective Bioequivalence in Central Nervous System Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Nanoparticles Using the Chemical Coprecipitation Method
2.2. Fabrication of Erythropoietin-Nanobots Using a Nanospray Dryer
2.3. Release of Erythropoietin from the Erythropoietin Nanobots Using Preconditioning Sonication
2.4. Injured Neuronal Cell Model
2.5. Neuronal Cell Viability
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Quick Release of Erythropoietin from Erythropoietin Nanobots
3.2. Neuronal Cell Viability after Erythropoietin Nanobot Treatment and Erythropoietin Treatment
3.3. Erythropoietin Receptor Signaling Pathway Activation
3.4. Proapoptotic Signaling Pathway Deactivation
3.5. Apoptotic Signaling Pathway Deactivation
4. Discussion
Limitations and Future Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buemi, M.; Caccamo, C.; Nostro, L.; Cavallaro, E.; Floccari, F.; Grasso, G. Brain and cancer: The protective role of erythropoietin. Med. Res. Rev. 2005, 25, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Gokmen, N.; Erbayraktar, S.; Akhisaroglu, M.; Konakc, S.; Ulukus, C.; Genc, S.; Genc, K.; Sagiroglu, E.; Cerami, A.; et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc. Natl. Acad. Sci. USA 2002, 99, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Juul, S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr. Suppl. 2002, 91, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.W. Erythropoietin: Physiology and pharmacology update. Exp. Biol. Med. 2003, 228, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.N.; Shim, J.H.; Won, Y.J.; Yoo, J.Y.; Hwang, C.H. Therapeutic time window for the effects of erythropoietin on astrogliosis and neurite outgrowth in an in vitro model of spinal cord injury. Medicine 2018, 97, e9913. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Okon, E.B.; Plunet, W.; Baptiste, D.; Fouad, K.; Hillyer, J.; Weaver, L.C.; Fehlings, M.G.; Tetzlaff, W. A systematic review of directly applied biologic therapies for acute spinal cord injury. J. Neurotrauma 2011, 28, 1589–1610. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Yang, M.T.; Kang, K.H.; Liou, H.C.; Lu, D.H.; Fu, W.M.; Lin, W.L. Targeted delivery of erythropoietin by transcranial focused ultrasound for neuroprotection against ischemia/reperfusion-induced neuronal injury: A long-term and short-term study. PLoS ONE 2014, 9, e90107. [Google Scholar] [CrossRef]
- Sirén, A.L.; Fratelli, M.; Brines, M.; Goemans, C.; Casagrande, S.; Lewczuk, P.; Keenan, S.; Gleiter, C.; Pasquali, C.; Capobianco, A.; et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA 2001, 98, 4044–4049. [Google Scholar] [CrossRef]
- Ehrenreich, H.; Hasselblatt, M.; Dembowski, C.; Cepek, L.; Lewczuk, P.; Stiefel, M.; Rustenbeck, H.H.; Breiter, N.; Jacob, S.; Knerlich, F.; et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002, 8, 495–505. [Google Scholar] [CrossRef]
- Sakanaka, M.; Wen, T.C.; Matsuda, S.; Masuda, S.; Morishita, E.; Nagao, M.; Sasaki, R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 1998, 95, 4635–4640. [Google Scholar] [CrossRef]
- Lippi, G.; Franchini, M.; Favaloro, E.J. Thrombotic complications of erythropoiesis-stimulating agents. Semin. Thromb. Hemost. 2010, 36, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Bischoff, P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin. Ther. Pat. 2010, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooke, M.J.; Morshead, C.M.; Shoichet, M.S. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012, 33, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Patel, G.M.; Patel, G.C.; Patel, R.B.; Patel, J.K.; Patel, M. Nanorobot: A versatile tool in nanomedicine. J. Drug Target. 2006, 14, 63–67. [Google Scholar] [CrossRef]
- Lammers, T. Improving the efficacy of combined modality anticancer therapy using HPMA copolymer-based nanomedicine formulations. Adv. Drug Deliv. Rev. 2010, 62, 203–230. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, C.R.; Le, T.H.; Koo, K.I.; Hwang, C.H. Magnetically guided targeted delivery of erythropoietin using magnetic nanoparticles: Proof of concept. Medicine 2020, 99, e19972. [Google Scholar] [CrossRef]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef]
- Kwok, C.S.; Mourad, P.D.; Crum, L.A.; Ratner, B.D. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J. Biomed. Mater. Res. 2001, 57, 151–164. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Harsha, S.N.; Aldhubiab, B.E.; Nair, A.B.; Alhaider, I.A.; Attimarad, M.; Venugopala, K.N.; Srinivasan, S.; Gangadhar, N.; Asif, A.H. Nanoparticle formulation by Buchi B-90 Nano Spray Dryer for oral mucoadhesion. Drug Des. Devel. Ther. 2015, 9, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, F.; Moztarzadeh, F. Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloids Surf. B Biointerfaces 2017, 153, 132–140. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Yoshino, H.; Kumai, Y.; Kashiwakura, I. Effects of endoplasmic reticulum stress on apoptosis induction in radioresistant macrophages. Mol. Med. Rep. 2017, 15, 2867–2872. [Google Scholar] [CrossRef]
- Yoshida, I.; Monji, A.; Tashiro, K.; Nakamura, K.; Inoue, R.; Kanba, S. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochem. Int. 2006, 48, 696–702. [Google Scholar] [CrossRef]
- Pham, T.D.; Ma, W.; Miller, D.; Kazakova, L.; Benchimol, S. Erythropoietin inhibits chemotherapy-induced cell death and promotes a senescence-like state in leukemia cells. Cell Death Dis. 2019, 10, 22. [Google Scholar] [CrossRef]
- Li, G.; Ma, R.; Huang, C.; Tang, Q.; Fu, Q.; Liu, H.; Hu, B.; Xiang, J. Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci. Lett. 2008, 442, 143–147. [Google Scholar] [CrossRef]
- Sun, Z.-K.; Yang, H.-Q.; Wang, Z.-Q.; Pan, J.; Hong, Z.; Chen, S.-D. Erythropoietin prevents PC12 cells from beta-amyloid-induced apoptosis via PI3KAkt pathway. Transl. Neurodegener. 2012, 1, 7. [Google Scholar]
- Ma, R.; Xiong, N.; Huang, C.; Tang, Q.; Hu, B.; Xiang, J.; Li, G. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology 2009, 56, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Pitt, W.G.; Sun, H.; Nelson, J.L. Drug delivery in polymeric micelles: From in vitro to in vivo. J. Control. Release Off. J. Control. Release Soc. 2003, 91, 85–95. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, G.; Gao, Y.; Zhou, Y.; Liu, J.; Zhang, L.; Long, A.; Zhang, L.; Tang, P. A sequential delivery system employing the synergism of EPO and NGF promotes sciatic nerve repair. Colloids Surf. B Biointerfaces 2017, 159, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, Y.; Zhou, Y.; Liu, J.; Zhang, L.; Long, A.; Zhang, L.; Tang, P. Localized and sustained delivery of erythropoietin from PLGA microspheres promotes functional recovery and nerve regeneration in peripheral nerve injury. BioMed Res. Int. 2015, 2015, 478103. [Google Scholar] [PubMed]
- Hwang, C.H. Targeted Delivery of Erythropoietin Hybridized with Magnetic Nanocarriers for the Treatment of Central Nervous System Injury: A Literature Review. Int. J. Nanomed. 2020, 15, 9683–9701. [Google Scholar] [CrossRef]
- Matis, G.K.; Birbilis, T.A. Erythropoietin in spinal cord injury. Eur. Spine J. 2009, 18, 314–323. [Google Scholar] [CrossRef]
- Vitellaro-Zuccarello, L.; Mazzetti, S.; Madaschi, L.; Bosisio, P.; Gorio, A.; De Biasi, S. Erythropoietin-mediated preservation of the white matter in rat spinal cord injury. Neuroscience 2007, 144, 865–877. [Google Scholar] [CrossRef]
- Morishita, E.; Masuda, S.; Nagao, M.; Yasuda, Y.; Sasaki, R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1996, 76, 105–116. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kim, G.; Park, S.; Sim, J.H.; Won, Y.J.; Hwang, C.H.; Yoo, J.Y.; Hong, H.N. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem. Biophys. Res. Commun. 2012, 417, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Rhee, V.; Ho, O.L. Kainic acid: A powerful neurotoxic analogue of glutamate. Brain Res. 1974, 77, 507–512. [Google Scholar] [CrossRef]
- Matute, C.; Domercq, M.; Sánchez-Gómez, M.V. Glutamate-mediated glial injury: Mechanisms and clinical importance. Glia 2006, 53, 212–224. [Google Scholar] [CrossRef] [PubMed]
- David, J.C.; Yamada, K.A.; Bagwe, M.R.; Goldberg, M.P. AMPA receptor activation is rapidly toxic to cortical astrocytes when desensitization is blocked. J. Neurosci. 1996, 16, 200–209. [Google Scholar] [CrossRef]
- Nagańska, E.; Taraszewska, A.; Matyja, E.; Grieb, P.; Rafałowska, J. Neuroprotective effect of erythropoietin in amyotrophic lateral sclerosis (ALS) model in vitro. Ultrastructural study. Folia Neuropathol. 2010, 48, 35–44. [Google Scholar]
- Miller, J.L.; Rai, M.; Frigon, N.L.; Pandolfo, M.; Punnonen, J.; Spencer, J.R. Erythropoietin and small molecule agonists of the tissue-protective erythropoietin receptor increase FXN expression in neuronal cells in vitro and in Fxn-deficient KIKO mice in vivo. Neuropharmacology 2017, 123, 34–45. [Google Scholar] [CrossRef]
- Watowich, S.S. The erythropoietin receptor: Molecular structure and hematopoietic signaling pathways. J. Investig. Med. 2011, 59, 1067–1072. [Google Scholar] [CrossRef]
- Murua, A.; Orive, G.; Hernandez, R.M.; Pedraz, J.L. Emerging technologies in the delivery of erythropoietin for therapeutics. Med. Res. Rev. 2011, 31, 284–309. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Hwang, C.H.; Hong, H.N. A Model of Glial Scarring Analogous to the Environment of a Traumatically Injured Spinal Cord Using Kainate. Ann. Rehabil. Med. 2016, 40, 757–768. [Google Scholar] [CrossRef]
- Rasheva, V.I.; Domingos, P.M. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 2009, 14, 996–1007. [Google Scholar] [CrossRef]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, A.; Fernández, L.; Rico-Bautista, E.; Umana, A.; Negrín, C.; Zhang, J.-G.; Norstedt, G. Endoplasmic Reticulum Stress Prolongs GH-Induced Janus Kinase (JAK2)/Signal Transducer and Activator of Transcription (STAT5) Signaling Pathway. Mol. Endocrinol. 2001, 15, 1471–1483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [PubMed]
- Lindner, P.; Christensen, S.B.; Nissen, P.; Møller, J.V.; Engedal, N. Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components. Cell Commun. Signal. 2020, 18, 12. [Google Scholar] [CrossRef]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 2009, 11, 2307–2316. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Shergalis, A.G.; Hu, S.; Bankhead, A., 3rd; Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef]
- Klymenko, O.; Huehn, M.; Wilhelm, J.; Wasnick, R.; Shalashova, I.; Ruppert, C.; Henneke, I.; Hezel, S.; Guenther, K.; Mahavadi, P.; et al. Regulation and role of the ER stress transcription factor CHOP in alveolar epithelial type-II cells. J. Mol. Med. 2019, 97, 973–990. [Google Scholar] [CrossRef]
- Chaudhari, S.; Li, W.; Wang, Y.; Jiang, H.; Ma, Y.; Davis, M.E.; Zuckerman, J.E.; Ma, R. Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells. Am. J. Physiol. Ren. Physiol. 2017, 313, F729–F739. [Google Scholar] [CrossRef]
- Ming, M.; Manzini, I.; Le, W.; Krieglstein, K.; Spittau, B. Thapsigargin-induced Ca2+ increase inhibits TGFβ1-mediated Smad2 transcriptional responses via Ca2+/calmodulin-dependent protein kinase II. J. Cell. Biochem. 2010, 111, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Tsavlis, D.; Anestakis, D.; Tzoumaka, A.; Symeonidou, C.; Kouzi-Koliakou, K.; Tektonidou, A.; Spandou, E. Erythropoietin (EPO) attenuates the immunohistochemical expression of tumor growth factor-ß (TGF-ß) in bleomycin (BLM)-induced pulmonary fibrosis (PF) in rats. Eur. Respir. J. 2018, 52, PA996. [Google Scholar]
- Okumura, N.; Hashimoto, K.; Kitahara, M.; Okuda, H.; Ueda, E.; Watanabe, K.; Nakahara, M.; Sato, T.; Kinoshita, S.; Tourtas, T.; et al. Activation of TGF-β signaling induces cell death via the unfolded protein response in Fuchs endothelial corneal dystrophy. Sci. Rep. 2017, 7, 6801. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Pinkernelle, J.; Calatayud, P.; Goya, G.F.; Fansa, H.; Keilhoff, G. Magnetic nanoparticles in primary neural cell cultures are mainly taken up by microglia. BMC Neurosci. 2012, 13, 32. [Google Scholar] [CrossRef]
- Yu, J.; Jin, D.; Chan, K.F.; Wang, Q.; Yuan, K.; Zhang, L. Active generation and magnetic actuation of microrobotic swarms in bio-fluids. Nat. Commun. 2019, 10, 5631. [Google Scholar] [CrossRef]
- Andhari, S.S.; Wavhale, R.D.; Dhobale, K.D.; Tawade, B.V.; Chate, G.P.; Patil, Y.N.; Khandare, J.J.; Banerjee, S.S. Self-Propelling Targeted Magneto-Nanobots for Deep Tumor Penetration and pH-Responsive Intracellular Drug Delivery. Sci. Rep. 2020, 10, 4703. [Google Scholar] [CrossRef]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef]
- Ma, S.W.; Fan, Y.J.; Li, H.Y.; Su, L.; Wang, Z.L.; Zhu, G. Flexible Porous Polydimethylsiloxane/Lead Zirconate Titanate-Based Nanogenerator Enabled by the Dual Effect of Ferroelectricity and Piezoelectricity. ACS Appl. Mater. Interfaces 2018, 10, 33105–33111. [Google Scholar] [CrossRef]
- Panja, S.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. A Smart Magnetically Active Nanovehicle for on-Demand Targeted Drug Delivery: Where van der Waals Force Balances the Magnetic Interaction. ACS Appl. Mater. Interfaces 2015, 7, 24229–24241. [Google Scholar] [CrossRef]

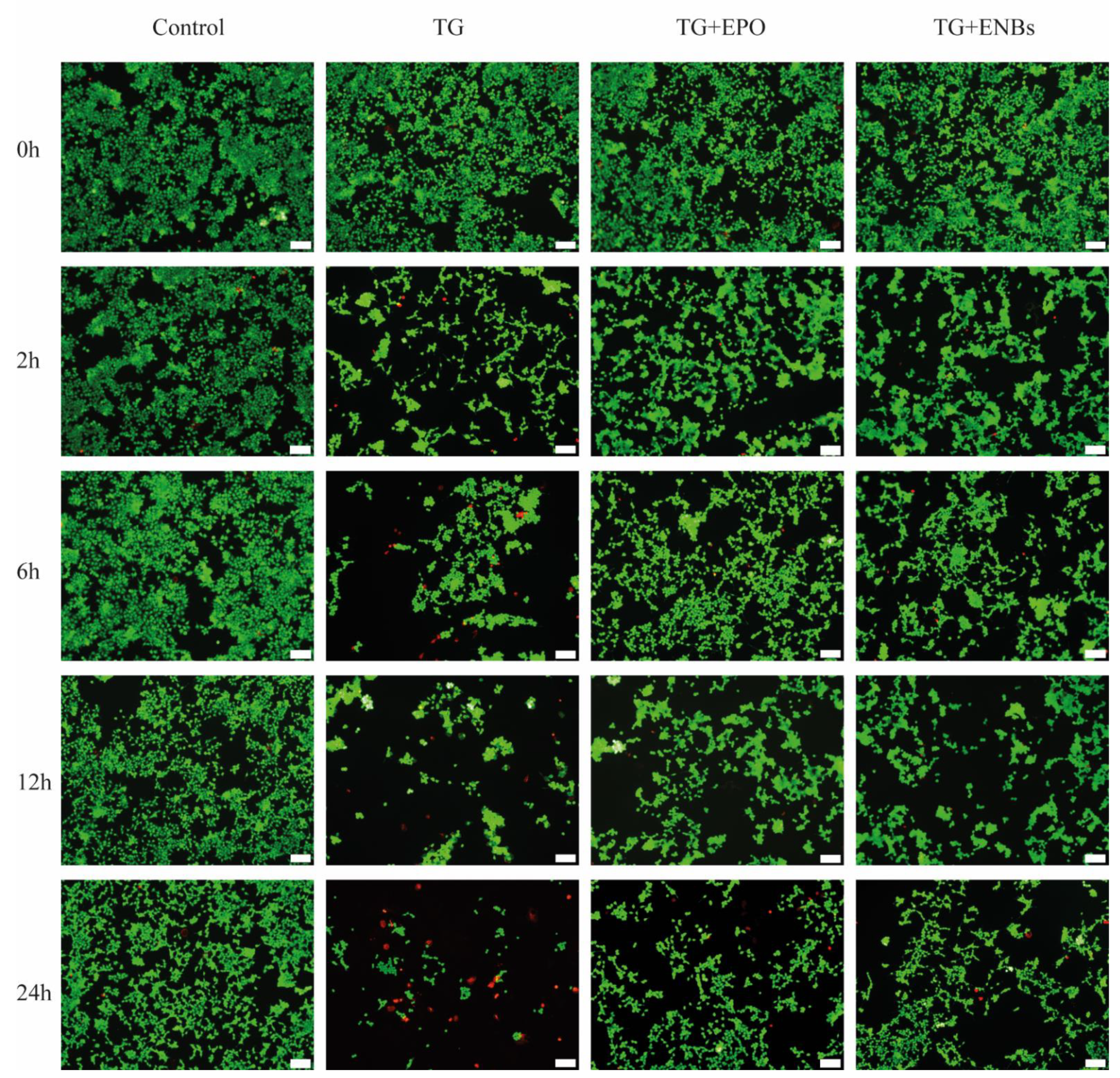



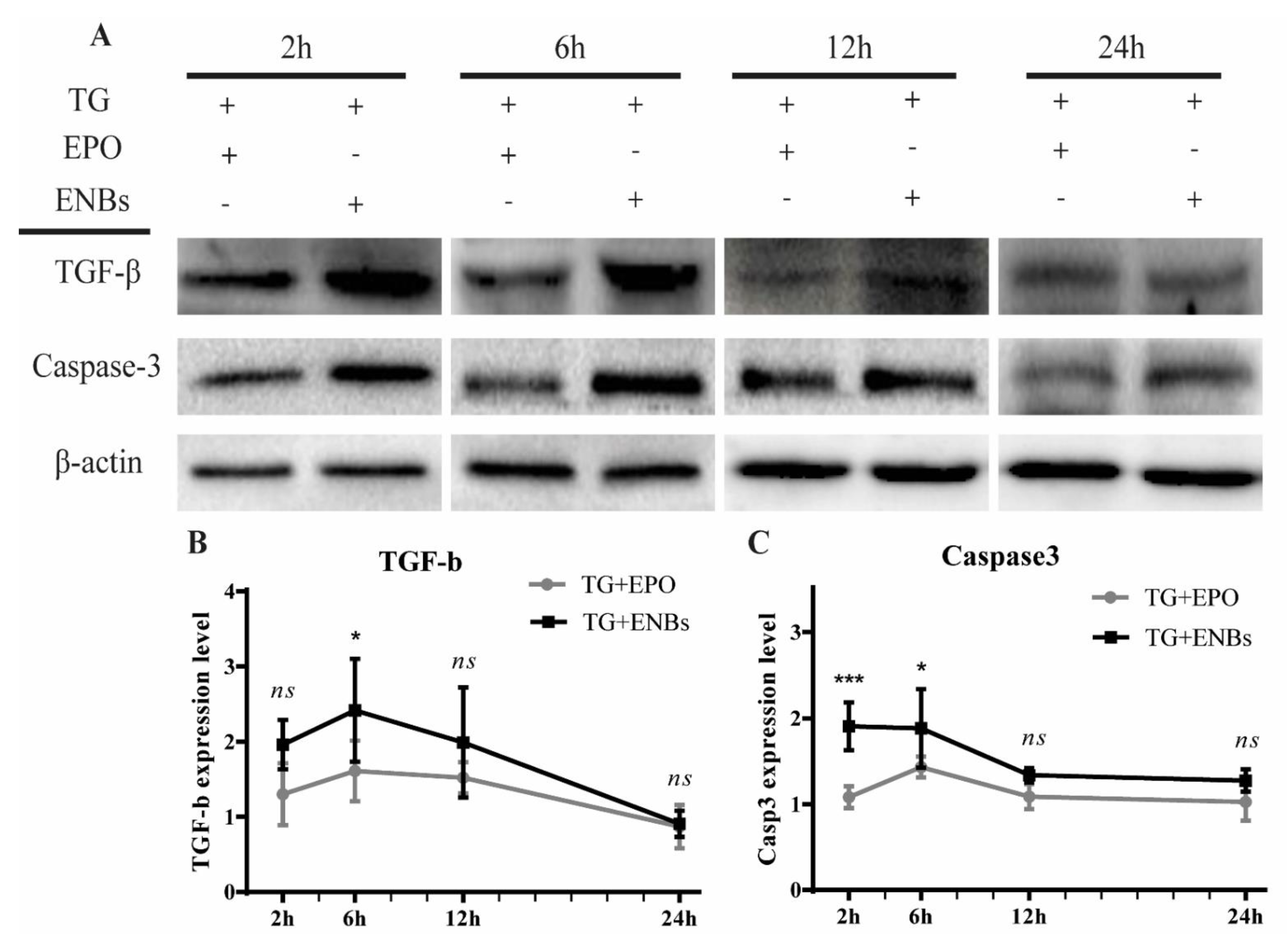
| Cell Only | TG | TG + EPO | TG + ENBs | |
|---|---|---|---|---|
| Culture media | 10 mL | 9.995 mL | 9.975 mL | 9.945 mL |
| 1 mM/mL TG | - | 5 μL | 5 μL | 5 μL |
| 250 IU/mL EPO | - | - | 20 μL | - |
| 100 IU EPO/mL-nanobots | - | - | - | 50 μL |
| Total volume | 10 mL | 10 mL | 10 mL | 10 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.H.; Nguyen, C.T.; Koo, K.-i.; Hwang, C.H. Erythropoietin Nanobots: Their Feasibility for the Controlled Release of Erythropoietin and Their Neuroprotective Bioequivalence in Central Nervous System Injury. Appl. Sci. 2022, 12, 3351. https://doi.org/10.3390/app12073351
Le TH, Nguyen CT, Koo K-i, Hwang CH. Erythropoietin Nanobots: Their Feasibility for the Controlled Release of Erythropoietin and Their Neuroprotective Bioequivalence in Central Nervous System Injury. Applied Sciences. 2022; 12(7):3351. https://doi.org/10.3390/app12073351
Chicago/Turabian StyleLe, Thi Huong, Chanh Trung Nguyen, Kyo-in Koo, and Chang Ho Hwang. 2022. "Erythropoietin Nanobots: Their Feasibility for the Controlled Release of Erythropoietin and Their Neuroprotective Bioequivalence in Central Nervous System Injury" Applied Sciences 12, no. 7: 3351. https://doi.org/10.3390/app12073351
APA StyleLe, T. H., Nguyen, C. T., Koo, K.-i., & Hwang, C. H. (2022). Erythropoietin Nanobots: Their Feasibility for the Controlled Release of Erythropoietin and Their Neuroprotective Bioequivalence in Central Nervous System Injury. Applied Sciences, 12(7), 3351. https://doi.org/10.3390/app12073351






