Reconstruction of Preclinical PET Images via Chebyshev Polynomial Approximation of the Sinogram
Abstract
1. Introduction
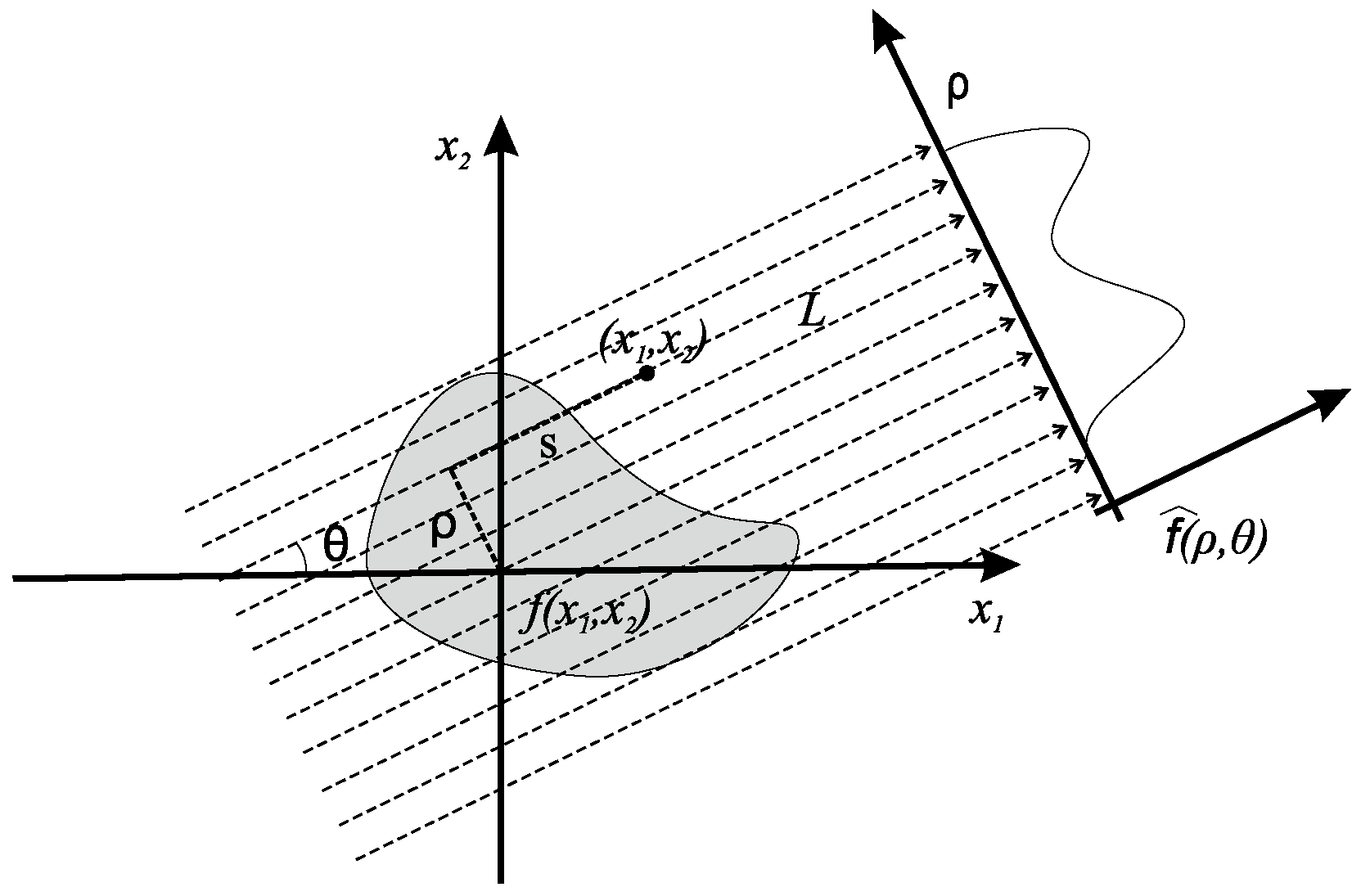
2. Mathematical Formulation
2.1. Inversion of the Radon Transform via Chebyshev Polynomials
2.2. Numerical Implementation of the Inversion of the Radon Transform via Chebyshev Polynomials
| Algorithm 1: Computational steps of the proposed Chebyshev-based reconstruction algorithm |
| Reconstruction algorithm: Chebyshev approximation of the sinogram |
| Input: given at and |
| Compute: for all , and , via Equation (2), for all , and , via Equation (2), for all n, via Equation (7), for all n, via Equation (12), for all n, via Equation (6) as in [28], for all and , for all and , for all and , for all , and , , , via numerical integration |
| Output: |
3. Materials and Methods
3.1. Phantom Study: Simulated Micro-PET IQ Phantom via the NEMA NU 4-2008 Protocol
3.2. Sinograms
3.3. Reconstructions
3.4. Image Metrics
3.4.1. Percentage Standard Deviation
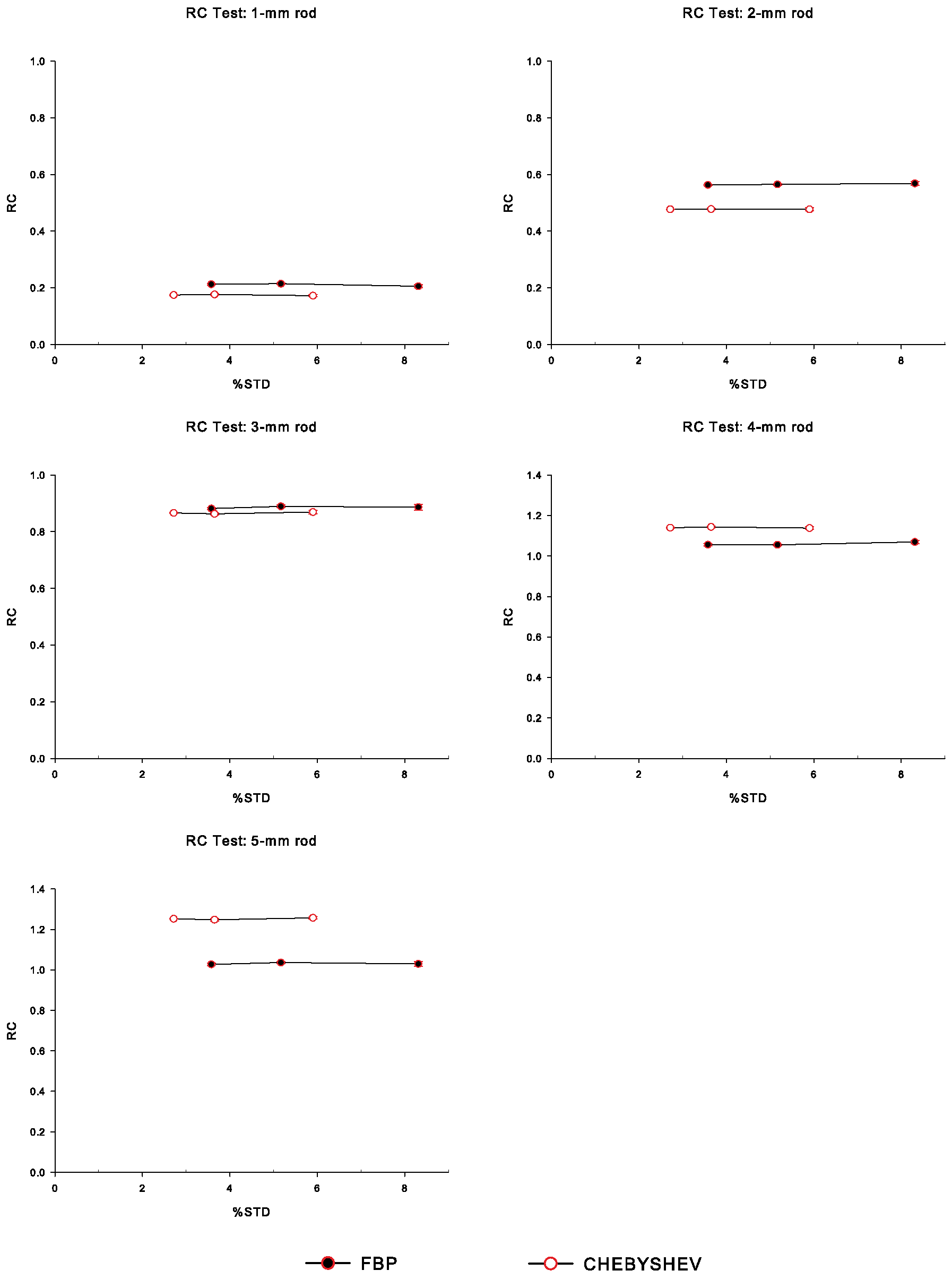
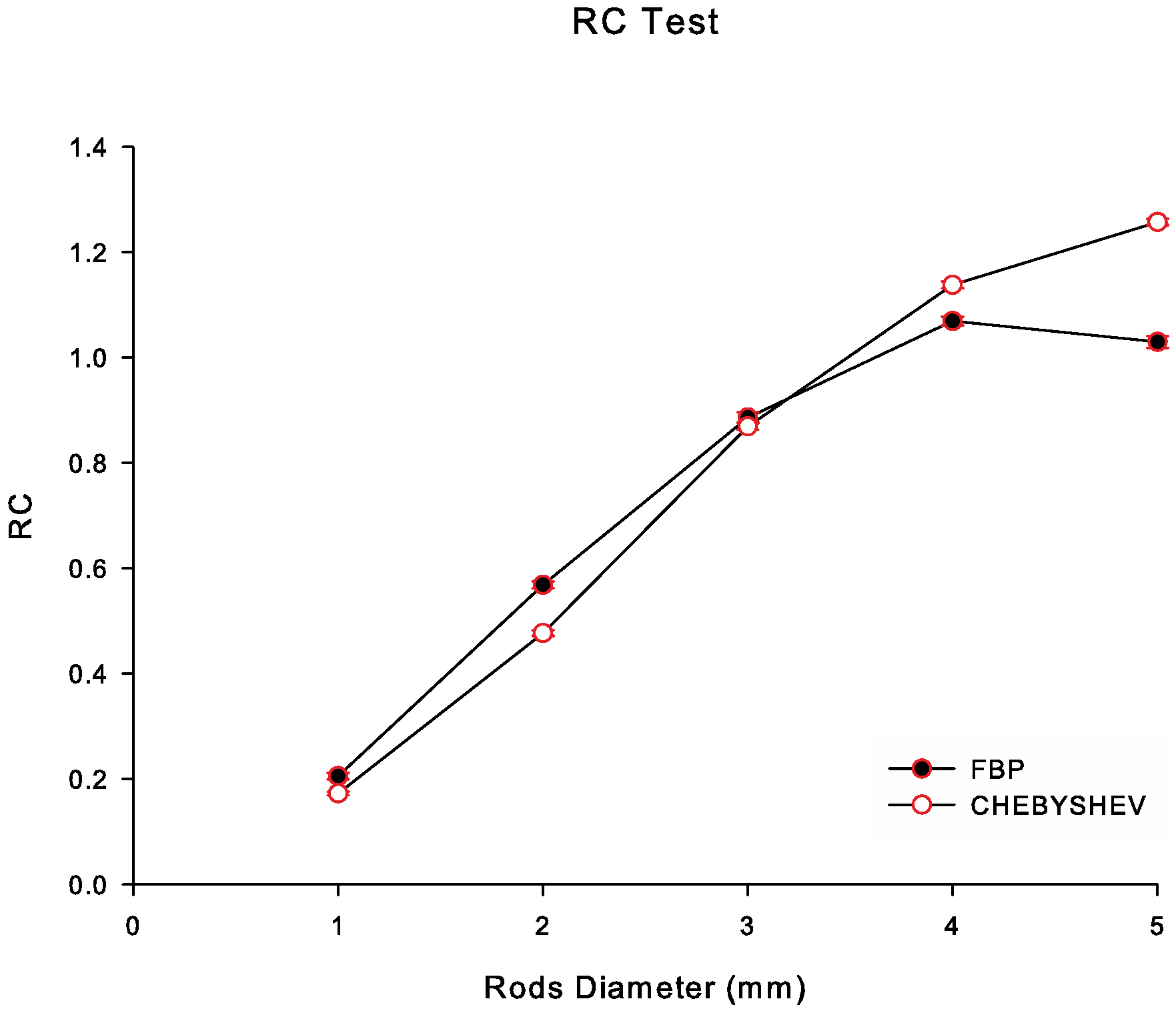
3.4.2. Recovery Coefficient
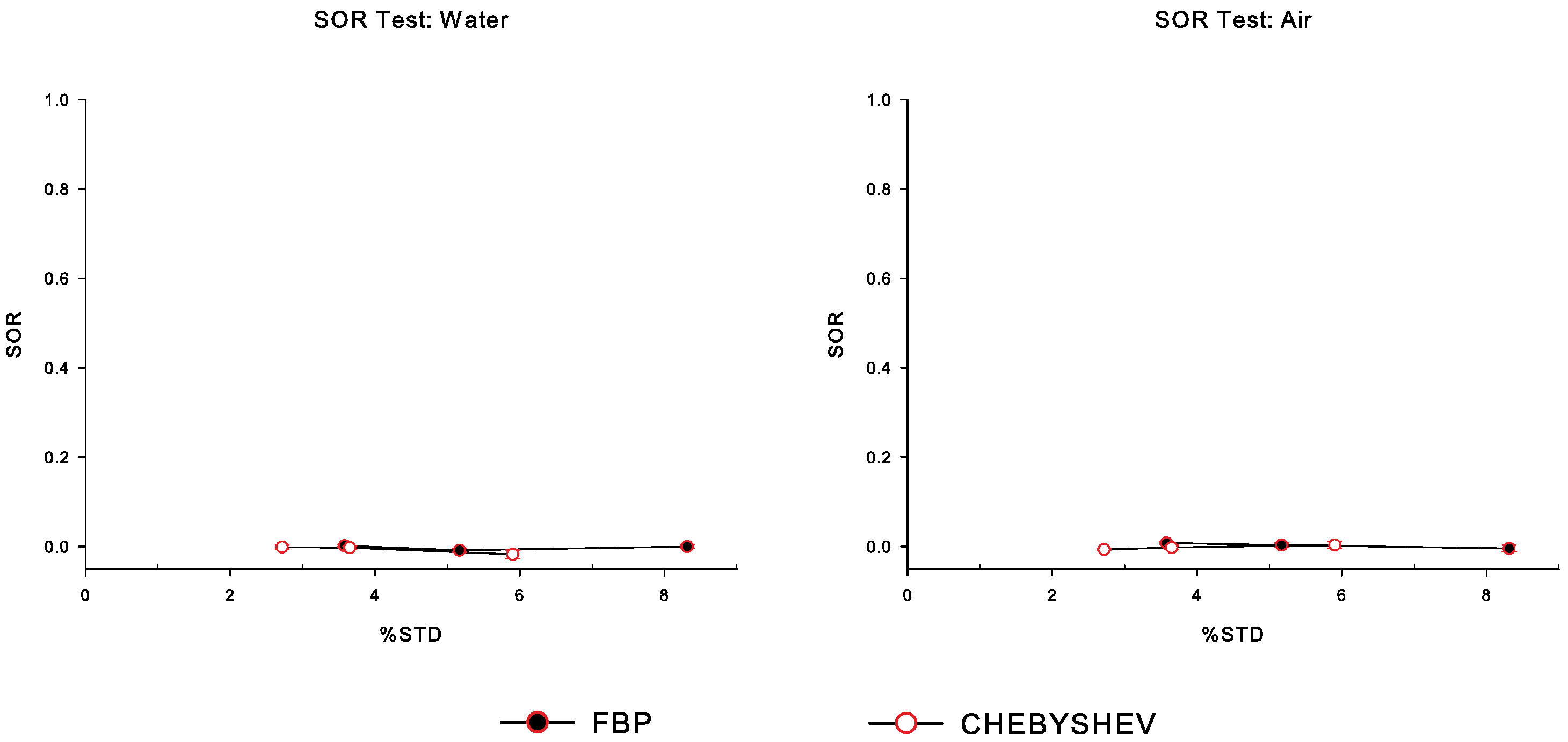
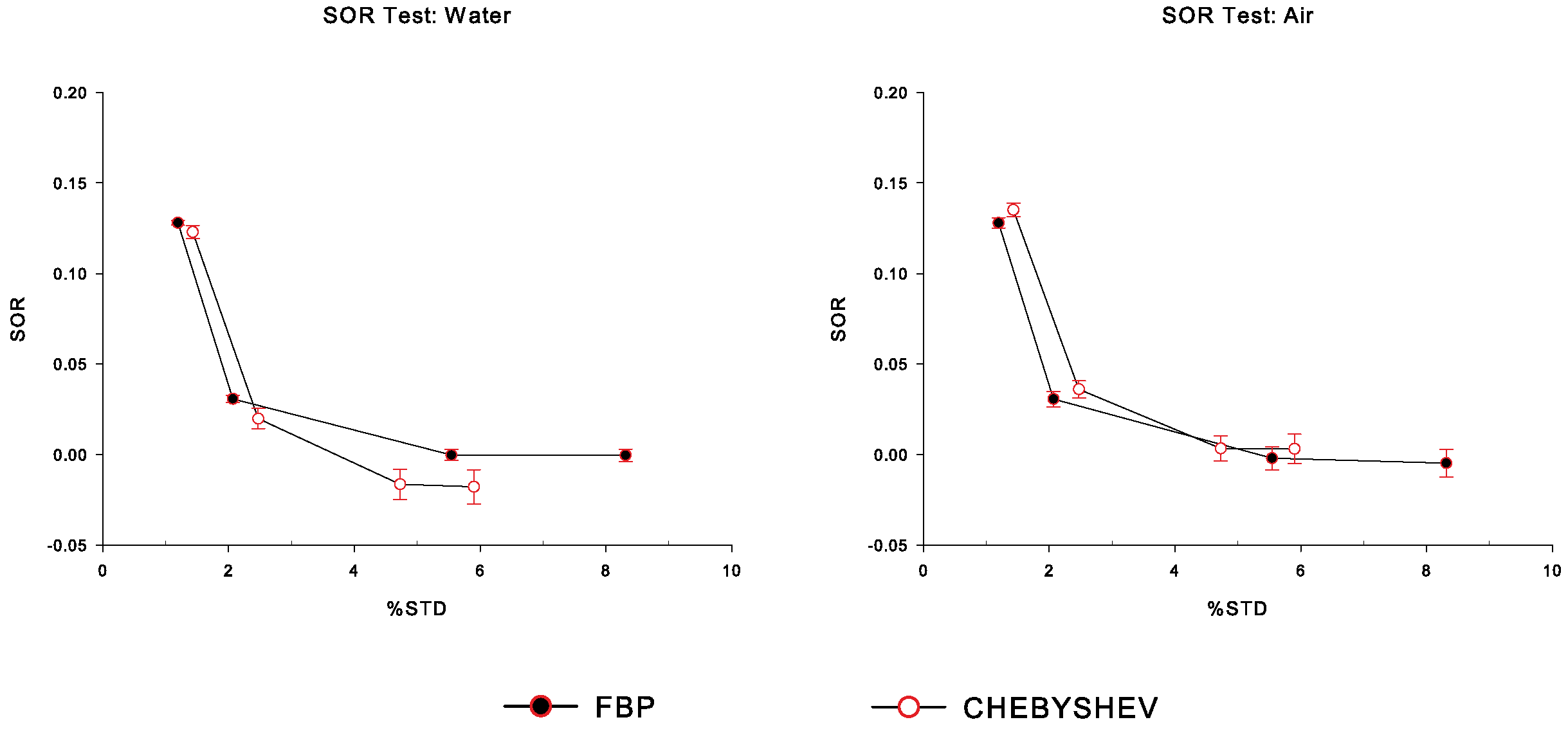
3.4.3. Spill-Over Ratio
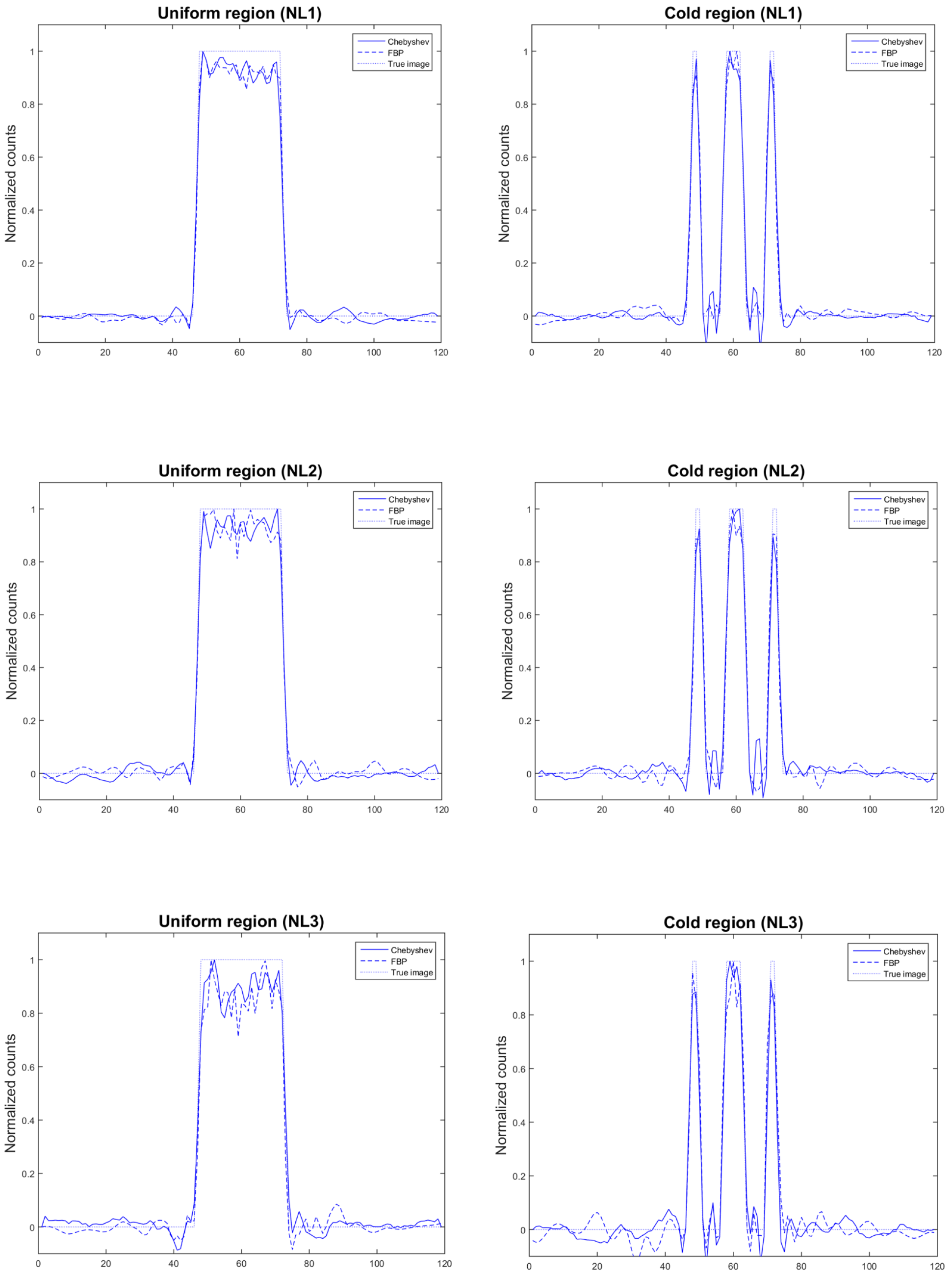
3.4.4. Image Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aulakh, G.K.; Kaur, M.; Brown, V.; Ekanayake, S.; Khan, B.; Fonge, H. Quantification of regional murine ozone-induced lung inflammation using [18F]F-FDG microPET/CT imaging. Sci. Rep. 2020, 10, 15699. [Google Scholar] [CrossRef] [PubMed]
- Kollenda, S.A.; Klose, J.; Knuschke, T.; Sokolova, V.; Schmitz, J.; Staniszewska, M.; Costa, P.F.; Herrmann, K.; Westendorf, A.M.; Fendler, W.P.; et al. In vivo biodistribution of calcium phosphate nanoparticles after intravascular, intramuscular, intratumoral, and soft tissue administration in mice investigated by small animal PET/CT. Acta Biomater. 2020, 109, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Iancu, S.D.; Albu, C.; Chiriac, L.; Moldovan, R.; Stefancu, A.; Moisoiu, V.; Coman, V.; Szabo, L.; Leopold, N.; Bálint, Z. Assessment of gold-coated iron oxide nanoparticles as negative T2 contrast agent in small animal MRI studies. Int. J. Nanomed. 2020, 15, 4811. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, K.; Periyasamy, V.; Pramanik, M. Non-invasive sentinel lymph node mapping and needle guidance using clinical handheld photoacoustic imaging system in small animal. J. Biophotonics 2018, 11, e201700061. [Google Scholar] [CrossRef]
- Johnson, L.C.; Guerraty, M.A.; Moore, S.C.; Metzler, S.D. Quantification of myocardial uptake rate constants in dynamic small-animal SPECT using a cardiac phantom. Phys. Med. Biol. 2019, 64, 065018. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Yang, K.; Wu, Y.; Boone, J.M.; Cherry, S.R. A microPET/CT system for in vivo small animal imaging. Phys. Med. Biol. 2007, 52, 3881. [Google Scholar] [CrossRef]
- Liu, Q.; Li, C.; Liu, J.; Krish, K.; Fu, X.; Zhao, J.; Chen, J.C. Performance evaluation of a small-animal PET/CT system based on NEMA NU 4–2008 standards. Med. Phys. 2021, 48, 5272–5282. [Google Scholar] [CrossRef]
- Yao, R.; Lecomte, R.; Crawford, E.S. Small-animal PET: What is it, and why do we need it? J. Nucl. Med. Technol. 2012, 40, 157–165. [Google Scholar] [CrossRef]
- Cherry, S.; Shao, Y.; Silverman, R.; Meadors, K.; Siegel, S.; Chatziioannou, A.; Young, J.; Jones, W.; Moyers, J.; Newport, D.; et al. MicroPET: A high resolution PET scanner for imaging small animals. IEEE Trans. Nucl. Sci. 1997, 44, 1161–1166. [Google Scholar] [CrossRef]
- Green, M.V.; Seidel, J.; Vaquero, J.J.; Jagoda, E.; Lee, I.; Eckelman, W. High resolution PET, SPECT and projection imaging in small animals. Comput. Med. Imaging Graph. 2001, 25, 79–86. [Google Scholar] [CrossRef]
- Kang, K.J.; Oh, S.J.; Nam, K.R.; Ahn, H.; Park, J.A.; Lee, K.C.; Choi, J.Y. Validation of Image Qualities of a Novel Four-Mice Bed PET System as an Oncological and Neurological Analysis Tool. J. Imaging 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Nose, N.; Nogami, S.; Koshino, K.; Chen, X.; Werner, R.A.; Kashima, S.; Rowe, S.P.; Lapa, C.; Fukuchi, K.; Higuchi, T. [18F] FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci. Rep. 2021, 11, 10896. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Alessio, A.M.; Kinahan, P.E. Image reconstruction for PET/CT scanners: Past achievements and future challenges. Imaging Med. 2010, 2, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Kim, K.; Cui, J.; Wu, D.; Li, Q. The evolution of image reconstruction in PET: From filtered back-projection to artificial intelligence. PET Clin. 2021, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Fokas, A.; Iserles, A.; Marinakis, V. Reconstruction algorithm for single photon emission computed tomography and its numerical implementation. J. R. Soc. Interface 2006, 3, 45–54. [Google Scholar] [CrossRef]
- Kastis, G.A.; Kyriakopoulou, D.; Gaitanis, A.; Fernández, Y.; Hutton, B.F.; Fokas, A.S. Evaluation of the spline reconstruction technique for PET. Med. Phys. 2014, 41, 042501. [Google Scholar] [CrossRef]
- Kastis, G.A.; Gaitanis, A.; Samartzis, A.P.; Fokas, A.S. The SRT reconstruction algorithm for semiquantification in PET imaging. Med. Phys. 2015, 42, 5970–5982. [Google Scholar] [CrossRef]
- Vrachliotis, A.; Kastis, G.A.; Protonotarios, N.E.; Fokas, A.; Nekolla, S.G.; Anagnostopoulos, C.D.; Costaridou, L.; Gaitanis, A. Evaluation of the spline reconstruction technique for preclinical PET imaging. Comput. Methods Programs Biomed. 2022, 217, 106668. [Google Scholar] [CrossRef]
- Protonotarios, N.E.; Fokas, A.S.; Kostarelos, K.; Kastis, G.A. The attenuated spline reconstruction technique for single photon emission computed tomography. J. R. Soc. Interface 2018, 15, 20180509. [Google Scholar] [CrossRef]
- Bortfeld, T.; Oelfke, U. Fast and exact 2D image reconstruction by means of Chebyshev decomposition and backprojection. Phys. Med. Biol. 1999, 44, 1105. [Google Scholar] [CrossRef]
- Fokas, A.S.; Marinakis, V. Reconstruction algorithm for the brain imaging techniques of PET and SPECT. HERMIS 2003, 4, 45–61. [Google Scholar]
- Bahri, M.A.; Plenevaux, A.; Warnock, G.; Luxen, A.; Seret, A. NEMA NU4-2008 image quality performance report for the microPET Focus 120 and for various transmission and reconstruction methods. J. Nucl. Med. 2009, 50, 1730–1738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NEMA. NEMA Standards Publication NU 4-2008: Performance Measurements of Small Animal Positron Emission Tomographs; Technical Report; National Electrical Manufacturers Association: Rosslyn, VA, USA, 2001. [Google Scholar]
- Barrett, H.H.; Myers, K.J. Foundations of Image Science; Wiley Series in Pure and Applied Optics; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Fokas, A.S.; Kastis, G.A. Mathematical methods in PET and SPECT imaging. In Handbook of Mathematical Methods in Imaging; Scherzer, O., Ed.; Springer: New York, NY, USA, 2015; pp. 903–936. [Google Scholar] [CrossRef]
- Gil, A.; Segura, J.; Temme, N.M. Numerical Methods for Special Functions; SIAM: Philadelphia, PA, USA, 2007. [Google Scholar]
- Notaris, S. Integral formulas for Chebyshev polynomials and the error term of interpolatory quadrature formulae for analytic functions. Math. Comput. 2006, 75, 1217–1231. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes 3rd Edition: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Thielemans, K.; Tsoumpas, C.; Mustafovic, S.; Beisel, T.; Aguiar, P.; Dikaios, N.; Jacobson, M.W. STIR: Software for Tomographic Image Reconstruction release 2. Phys. Med. Biol. 2012, 57, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Lajtos, I.; Czernin, J.; Dahlbom, M.; Daver, F.; Emri, M.; Farshchi-Heydari, S.; Forgacs, A.; Hoh, C.K.; Joszai, I.; Krizsan, A.K.; et al. Cold wall effect eliminating method to determine the contrast recovery coefficient for small animal PET scanners using the NEMA NU-4 image quality phantom. Phys. Med. Biol. 2014, 59, 2727. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Huang, S.C.; Phelps, M.E. Quantitation in positron emission computed tomography: 1. Effect of object size. J. Comput. Assist. Tomogr. 1979, 3, 299–308. [Google Scholar] [CrossRef]
- Kessler, R.M.; Ellis, J.R., Jr.; Eden, M. Analysis of emission tomographic scan data: Limitations imposed by resolution and background. J. Comput. Assist. Tomogr. 1984, 8, 514–522. [Google Scholar] [CrossRef]
- Zhu, Y.; Geng, C.; Huang, J.; Liu, J.; Wu, N.; Xin, J.; Xu, H.; Yu, L.; Geng, J. Measurement and evaluation of quantitative performance of PET/CT images before a multicenter clinical trial. Sci. Rep. 2018, 8, 9035. [Google Scholar] [CrossRef]
- Srinivas, S.M.; Dhurairaj, T.; Basu, S.; Bural, G.; Surti, S.; Alavi, A. A recovery coefficient method for partial volume correction of PET images. Ann. Nucl. Med. 2009, 23, 341–348. [Google Scholar] [CrossRef]
- Bettinardi, V.; Danna, M.; Savi, A.; Lecchi, M.; Castiglioni, I.; Gilardi, M.C.; Bammer, H.; Lucignani, G.; Fazio, F. Performance evaluation of the new whole-body PET/CT scanner: Discovery ST. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 867–881. [Google Scholar] [CrossRef]
- Tong, S.; Alessio, A.M.; Kinahan, P.E. Noise and signal properties in PSF-based fully 3D PET image reconstruction: An experimental evaluation. Phys. Med. Biol. 2010, 55, 1453–1473. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, A.; Kastis, G.A.; Vlastou, E.; Bouziotis, P.; Verginis, P.; Anagnostopoulos, C.D. Investigation of image reconstruction parameters of the Mediso nanoScan PC small-animal PET/CT scanner for two different positron emitters under NEMA NU 4-2008 standards. Mol. Imaging Biol. 2017, 19, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.P.; Disselhorst, J.A.; van Lier, M.G.; Laverman, P.; de Jong, G.M.; Oyen, W.J.; Boerman, O.C. Characterization and optimization of image quality as a function of reconstruction algorithms and parameter settings in a Siemens Inveon small-animal PET scanner using the NEMA NU 4-2008 standards. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2011, 629, 357–367. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. BMJ 2005, 331, 903. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.M.; Gambhir, S.S. AMIDE: A free software tool for multimodality medical image analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]
- Goertzen, A.L.; Bao, Q.; Bergeron, M.; Blankemeyer, E.; Blinder, S.; Cañadas, M.; Chatziioannou, A.F.; Dinelle, K.; Elhami, E.; Jans, H.S.; et al. NEMA NU 4-2008 comparison of preclinical PET imaging systems. J. Nucl. Med. 2012, 53, 1300–1309. [Google Scholar] [CrossRef]
- Luo, W.; Anashkin, E.; Matthews, C.G. Performance evaluation of a PEM scanner using the NEMA NU 4—2008 small animal PET standards. IEEE Trans. Nucl. Sci. 2010, 57, 94–103. [Google Scholar] [CrossRef]
- O’Sullivan, F.; Pawitan, Y.; Haynor, D. Reducing negativity artifacts in emission tomography: Post-processing filtered backprojection solutions. IEEE Trans. Med. Imaging 1993, 12, 653–663. [Google Scholar] [CrossRef]

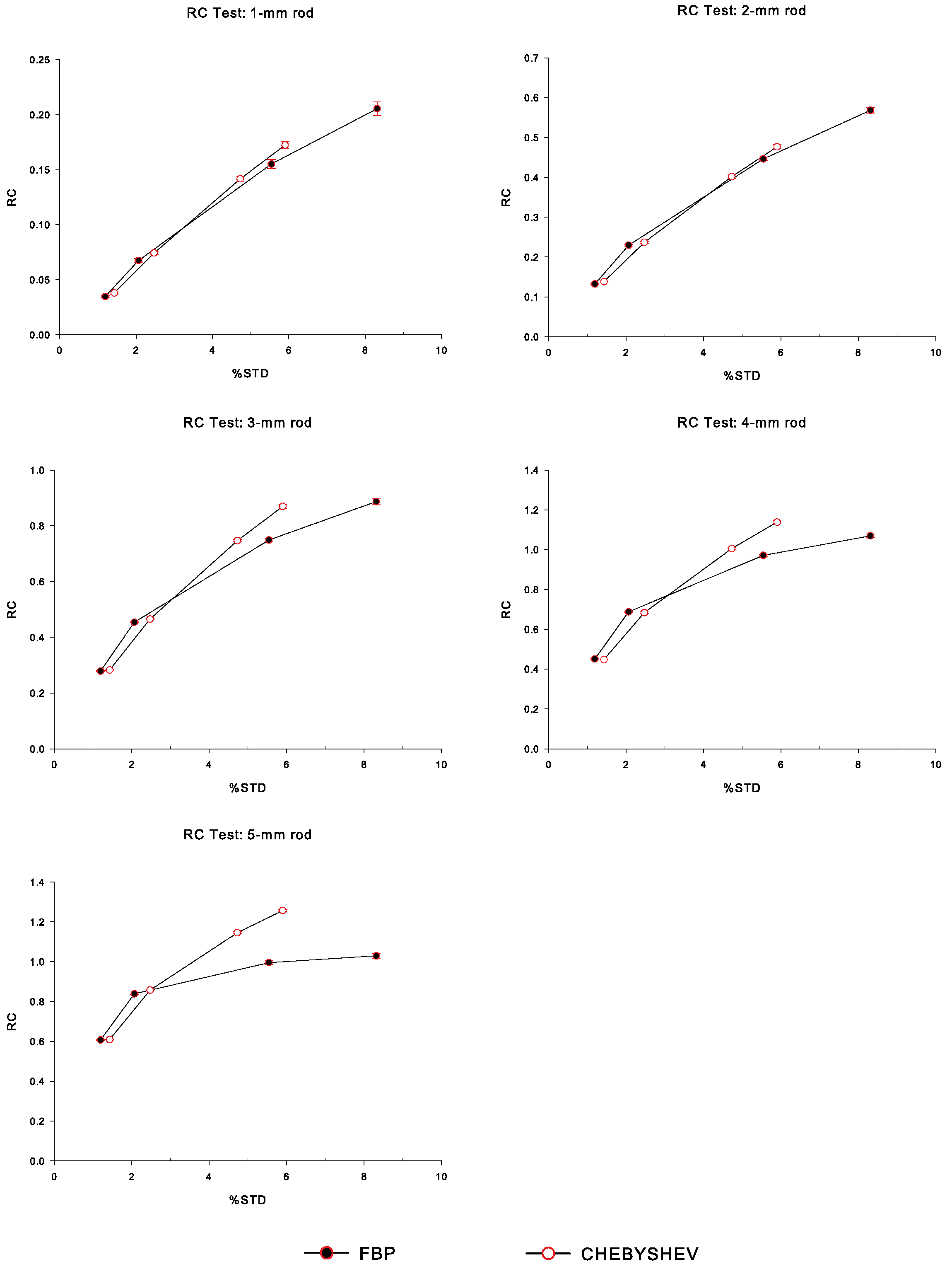
| NL1 | NL2 | NL3 | |
|---|---|---|---|
| Chebyshev | 2.72 | 3.65 | 5.90 |
| FBP | 3.58 | 5.17 | 8.31 |
| Rod | Noise Level | Chebyshev | FBP |
|---|---|---|---|
| 1 mm | NL1 | 6.62 | 5.94 |
| NL2 | 4.68 | 4.15 | |
| NL3 | 3.00 | 2.47 | |
| 2 mm | NL1 | 17.90 | 15.74 |
| NL2 | 12.91 | 10.93 | |
| NL3 | 8.03 | 6.84 | |
| 3 mm | NL1 | 32.42 | 24.65 |
| NL2 | 23.34 | 17.21 | |
| NL3 | 14.41 | 10.66 | |
| 4 mm | NL1 | 42.75 | 29.52 |
| NL2 | 30.62 | 20.42 | |
| NL3 | 18.85 | 12.86 | |
| 5 mm | NL1 | 46.84 | 28.73 |
| NL2 | 33.76 | 20.06 | |
| NL3 | 20.84 | 12.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protonotarios, N.E.; Fokas, A.S.; Vrachliotis, A.; Marinakis, V.; Dikaios, N.; Kastis, G.A. Reconstruction of Preclinical PET Images via Chebyshev Polynomial Approximation of the Sinogram. Appl. Sci. 2022, 12, 3335. https://doi.org/10.3390/app12073335
Protonotarios NE, Fokas AS, Vrachliotis A, Marinakis V, Dikaios N, Kastis GA. Reconstruction of Preclinical PET Images via Chebyshev Polynomial Approximation of the Sinogram. Applied Sciences. 2022; 12(7):3335. https://doi.org/10.3390/app12073335
Chicago/Turabian StyleProtonotarios, Nicholas E., Athanassios S. Fokas, Alexandros Vrachliotis, Vangelis Marinakis, Nikolaos Dikaios, and George A. Kastis. 2022. "Reconstruction of Preclinical PET Images via Chebyshev Polynomial Approximation of the Sinogram" Applied Sciences 12, no. 7: 3335. https://doi.org/10.3390/app12073335
APA StyleProtonotarios, N. E., Fokas, A. S., Vrachliotis, A., Marinakis, V., Dikaios, N., & Kastis, G. A. (2022). Reconstruction of Preclinical PET Images via Chebyshev Polynomial Approximation of the Sinogram. Applied Sciences, 12(7), 3335. https://doi.org/10.3390/app12073335









