Pregnancy Achievement by Medical Assisted Reproduction Is Correlated to the G Protein-Coupled Receptor 30 mRNA Abundance in Human Spermatozoa
Abstract
:1. Introduction
2. Materials and Methods
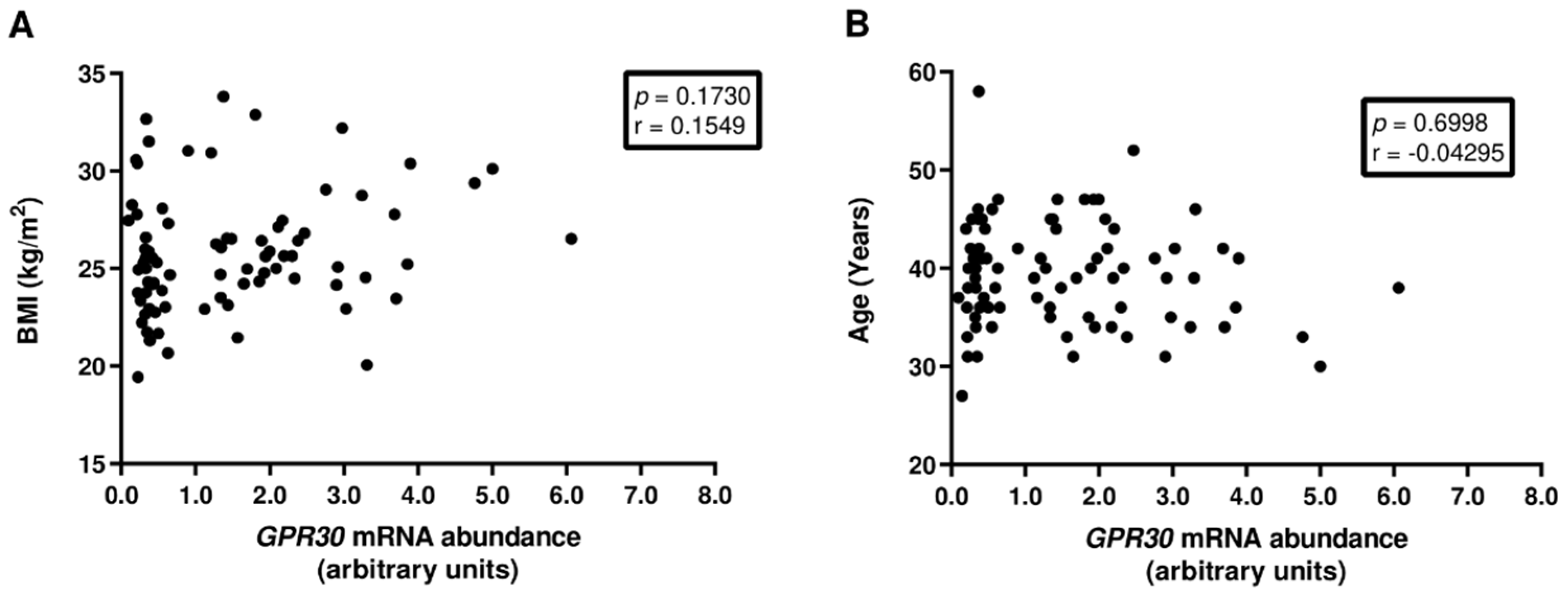
2.1. Patients’ Characterization
2.2. Sample Preparation for ART
2.3. ART Data Analysis
2.4. Reverse Transcriptase-Polymerase Chain Reaction and Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
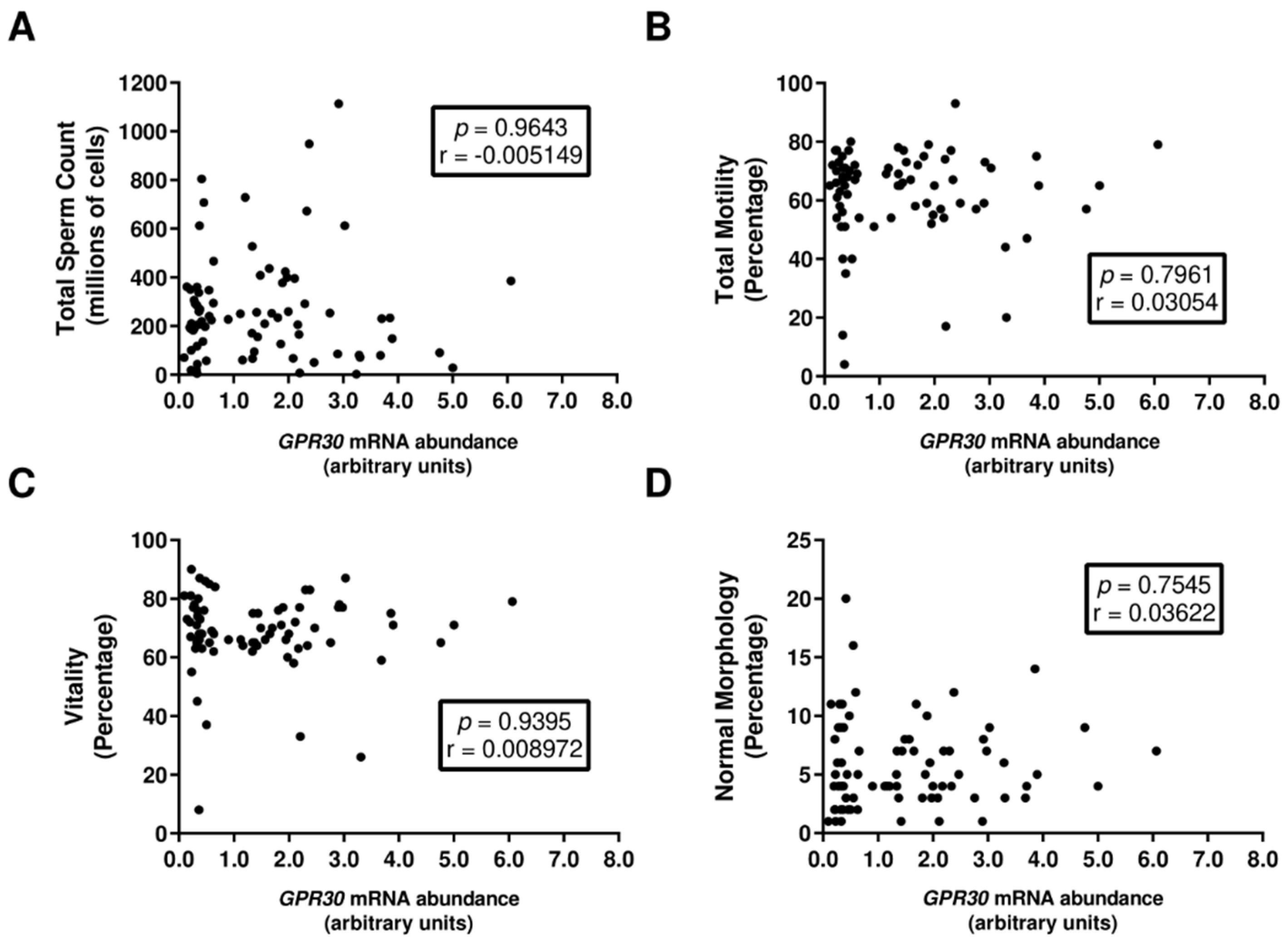
3.1. GPR30 mRNA Abundance in Human Spermatozoa Is Not Associated with Decreased Sperm Quality
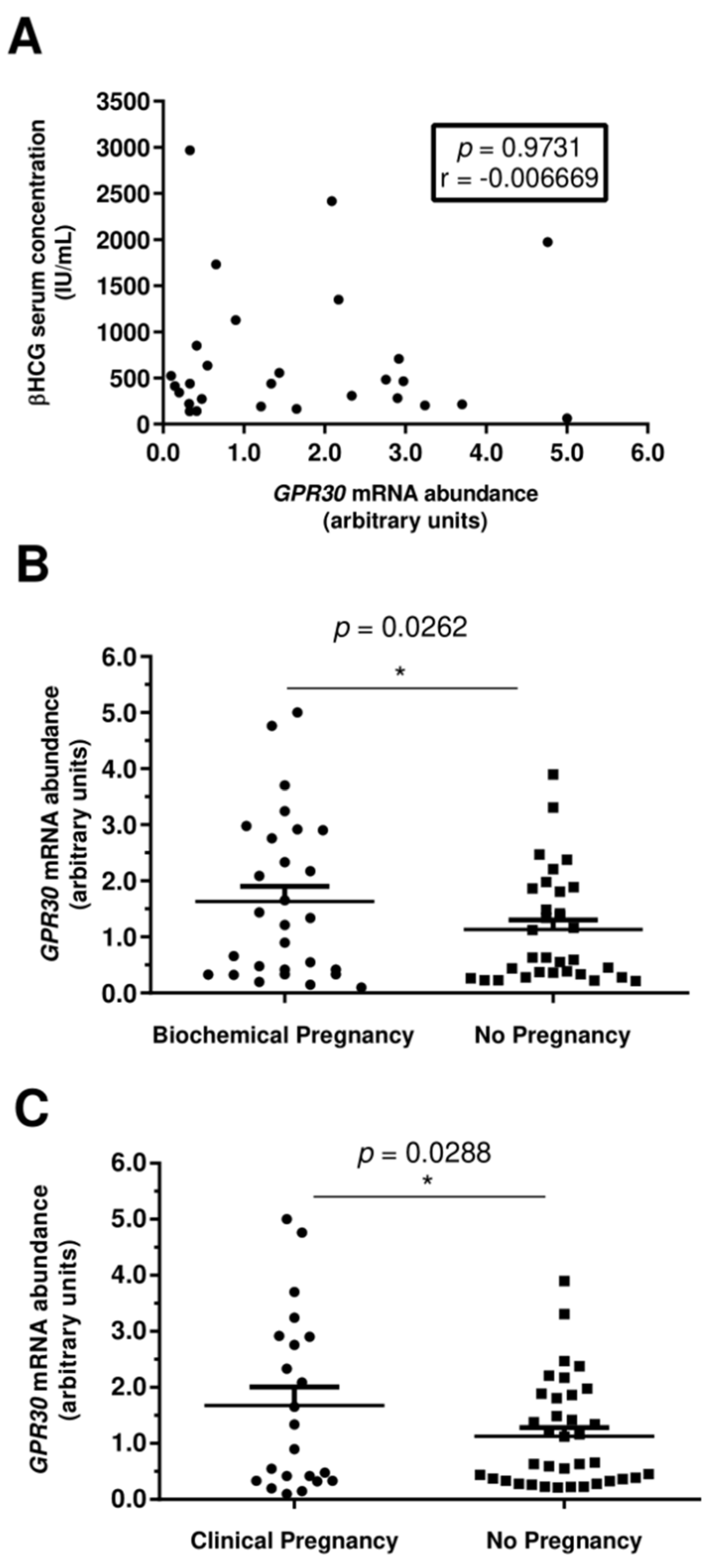
3.2. Increased GPR30 Abundance Levels in Human Spermatozoa Are Associated with Clinical Pregnancies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bernardino, R.L.; Carrageta, D.F.; Silva, A.M.; Calamita, G.; Alves, M.G.; Soveral, G.; Oliveira, P.F. Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9. Cells 2018, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Robertson, K.M.; Jones, M.E.; Simpson, E.R. Estrogen and Spermatogenesis. Endocr. Rev. 2001, 22, 289–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaco, J.E.B.; Laurentino, S.S.; Barros, A.; Sousa, M.; Socorro, S. Estrogen Receptors α and β in Human Testis: Both Isoforms are Expressed. Syst. Biol. Reprod. Med. 2009, 55, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G.; Martins, A.D.; Correia, S.; Bernardino, R.L.; Silva, J.; Barros, A.; Sousa, M.; Cavaco, J.E.; Socorro, S. Expression pattern of G protein-coupled receptor 30 in human seminiferous tubular cells. Gen. Comp. Endocrinol. 2014, 201, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Romeo, F.; Giordano, F.; Maggiolini, M.; Carpino, A. Identification of the estrogen receptor GPER in neoplastic and non-neoplastic human testes. Reprod. Biol. Endocrinol. 2011, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Katib, A. Mechanisms linking obesity to male infertility. Cent. Eur. J. Urol. 2015, 68, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Kramer, J.A.; Krawetz, S.A. RNA in spermatozoa: Implications for the alternative haploid genome. Mol. Hum. Reprod. 1997, 3, 473–478. [Google Scholar] [CrossRef]
- Abraham, K.A.; Bhargava, P.M. Nucleic acid metabolism of mammalian spermatozoa. Biochem. J. 1963, 86, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Fullston, T.; Teague, E.M.C.O.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef]
- Crisóstomo, L.; Jarak, I.; Rato, L.P.; Raposo, J.F.; Batterham, R.L.; Oliveira, P.F.; Alves, M.G. Inheritable testicular metabolic memory of high-fat diet causes transgenerational sperm defects in mice. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Crisóstomo, L.; Rato, L.; Jarak, I.; Silva, B.M.; Raposo, J.F.; Batterham, R.L.; Oliveira, P.F.; Alves, M.G. A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction 2019, 158, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo, L.; Videira, R.A.; Jarak, I.; Starčević, K.; Mašek, T.; Rato, L.; Raposo, J.F.; Batterham, R.L.; Oliveira, P.F.; Alves, M.G. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E1061–E1073. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-h.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.; Sousa, M.; Silva, B.; Monteiro, M.; Alves, M. Obesity, energy balance and spermatogenesis. Reproduction 2017, 153, R173–R185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, S.; Rato, L.; Sousa, M.; Alves, M.; Oliveira, P. Fertility and Sperm Quality in the Aging Male. Curr. Pharm. Des. 2017, 23, 4429–4437. [Google Scholar] [CrossRef]
- Bakos, H.W.; Henshaw, R.C.; Mitchell, M.; Lane, M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil. Steril. 2011, 95, 1700–1704. [Google Scholar] [CrossRef]
- Colaci, D.S.; Afeiche, M.; Gaskins, A.J.; Wright, D.L.; Toth, T.L.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil. Steril. 2012, 98, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Anifandis, G.; Dafopoulos, K.; Messini, C.I.; Polyzos, N.; Messinis, I.E. The BMI of men and not sperm parameters impact on embryo quality and the IVF outcome. Andrology 2013, 1, 85–89. [Google Scholar] [CrossRef]
- Frattarelli, J.; Miller, K.; Miller, B.; Hirsch, K.; Scott, R. Male age negatively impacts embryo development and reproductive outcome in donor oocyte art cycles. Fertil. Steril. 2007, 88, 97–103. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.S.; Natarajan, L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am. J. Obstet. Gynecol. 2004, 191, 507–514. [Google Scholar] [CrossRef]
- Pereira, S.C.; Martins, A.D.; Monteiro, M.P.; Pinto, S.; Barros, A.; Oliveira, P.F.; Alves, M.G. Expression of obesity-related genes in human spermatozoa affects the outcomes of reproductive treatments. F&S Sci. 2021, 2, 164–175. [Google Scholar]
- WHO Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Jameel, T. Sperm swim-up: A simple and effective technique of semen processing for intrauterine insemination. J. Pak. Med. Assoc. 2008, 58, 71. [Google Scholar]
- Annan, J.J.K.; Gudi, A.; Bhide, P.; Shah, A.; Hombrug, R. Biochemical pregnancy during assisted conception: A little bit pregnant. J. Clin. Med. Res. 2013, 5, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.; Martins, A.; Vaz, C.; Correia, S.; Moreira, P.; Oliveira, P.; Socorro, S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014, 171, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Giordano, F.; Brunelli, E.; Zito, D.; Aquila, S.; Carpino, A. Identification of G protein-coupled estrogen receptor in human and pig spermatozoa. J. Anat. 2014, 224, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Pasqualotto, F.F.; Sobreiro, B.P.; Hallak, J.; Pasqualotto, E.B.; Lucon, A.M. Sperm concentration and normal sperm morphology decrease and follicle-stimulating hormone level increases with age. BJU Int. 2005, 96, 1087–1091. [Google Scholar] [CrossRef]
- Verón, G.L.; Tissera, A.D.; Bello, R.; Beltramone, F.; Estofan, G.; Molina, R.I.; Vazquez-Levin, M.H. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil. Steril. 2018, 110, 68–75.e64. [Google Scholar] [CrossRef]
- Chimento, A.; Sirianni, R.; Delalande, C.; Silandre, D.; Bois, C.; Andò, S.; Maggiolini, M.; Carreau, S.; Pezzi, V. 17β-Estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ERα. Mol. Cell Endocrinol. 2010, 320, 136–144. [Google Scholar] [CrossRef]
- Vaucher, L.; Funaro, M.G.; Mehta, A.; Mielnik, A.; Bolyakov, A.; Prossnitz, E.R.; Schlegel, P.N.; Paduch, D.A. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS ONE 2014, 9, e92425. [Google Scholar] [CrossRef]
- Bernardino, R.L.; Alves, M.; Silva, J.; Barros, A.; Ferraz, L.; Sousa, M.; Sá, R.; Oliveira, P. Expression of estrogen receptors alpha (ER-α), beta (ER-β), and G protein-coupled receptor 30 (GPR30) in testicular tissue of men with Klinefelter syndrome. Horm. Metab. Res. 2016, 48, 413–415. [Google Scholar] [CrossRef]
- Cao, X.; Huang, J.; Zhang, G.; Zuo, W.; Lan, C.; Sun, Q.; Yang, D.; Gao, D.; Cheng, C.H.; Zhou, W.L. Functional expression of G protein-coupled receptor 30 in immature rat epididymal epithelium. Cell Biol. Int. 2017, 41, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Jarak, I.; Almeida, S.; Carvalho, R.A.; Sousa, M.; Barros, A.; Alves, M.G.; Oliveira, P.F. Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochim. Biophys. Acta 2018, 1864, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qu, T.; Zhang, S.; Yuan, D.; Xu, Q.; Zhang, J.; He, Y.; Yue, L. GPR30 Mediates the Fast Effect of Estrogen on Mouse Blastocyst and its Role in Implantation. Reprod. Sci. 2015, 22, 1312–1320. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Zhou, X.; Shi, S.; Qi, H.; Baker, P.; Zhang, H. Imbalance between proliferation and apoptosis−related impaired GPR30 expression is involved in preeclampsia. Cell Tissue Res. 2016, 366, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, P.; Siddiqui, M.F.; Lala, P.K. Restraint of Trophoblast Invasion of the Uterus by Decorin: Role in Pre-eclampsia. Am. J. Reprod. Immunol. 2016, 75, 351–360. [Google Scholar] [CrossRef]
- Boerke, A.; Dieleman, S.; Gadella, B. A possible role for sperm RNA in early embryo development. Theriogenology 2007, 68, S147–S155. [Google Scholar] [CrossRef]
- DiLuigi, A.; Weitzman, V.N.; Pace, M.C.; Siano, L.J.; Maier, D.; Mehlmann, L.M. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol. Reprod. 2008, 78, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Xin, Q.; Wang, X.; Wang, S.; Wang, H.; Zhang, W.; Yang, Y.; Zhang, Y.; Zhang, Z.; Wang, C.; et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 2017, 8, e2662. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes. J. Steroid Biochem. Mol. Biol. 2017, 167, 153–161. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.C.; Esperança, I.F.; Pinto, S.; Barros, A.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Pregnancy Achievement by Medical Assisted Reproduction Is Correlated to the G Protein-Coupled Receptor 30 mRNA Abundance in Human Spermatozoa. Appl. Sci. 2022, 12, 3240. https://doi.org/10.3390/app12073240
Pereira SC, Esperança IF, Pinto S, Barros A, Sousa M, Alves MG, Oliveira PF. Pregnancy Achievement by Medical Assisted Reproduction Is Correlated to the G Protein-Coupled Receptor 30 mRNA Abundance in Human Spermatozoa. Applied Sciences. 2022; 12(7):3240. https://doi.org/10.3390/app12073240
Chicago/Turabian StylePereira, Sara C., Inês F. Esperança, Soraia Pinto, Alberto Barros, Mário Sousa, Marco G. Alves, and Pedro F. Oliveira. 2022. "Pregnancy Achievement by Medical Assisted Reproduction Is Correlated to the G Protein-Coupled Receptor 30 mRNA Abundance in Human Spermatozoa" Applied Sciences 12, no. 7: 3240. https://doi.org/10.3390/app12073240
APA StylePereira, S. C., Esperança, I. F., Pinto, S., Barros, A., Sousa, M., Alves, M. G., & Oliveira, P. F. (2022). Pregnancy Achievement by Medical Assisted Reproduction Is Correlated to the G Protein-Coupled Receptor 30 mRNA Abundance in Human Spermatozoa. Applied Sciences, 12(7), 3240. https://doi.org/10.3390/app12073240







