Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Biometric
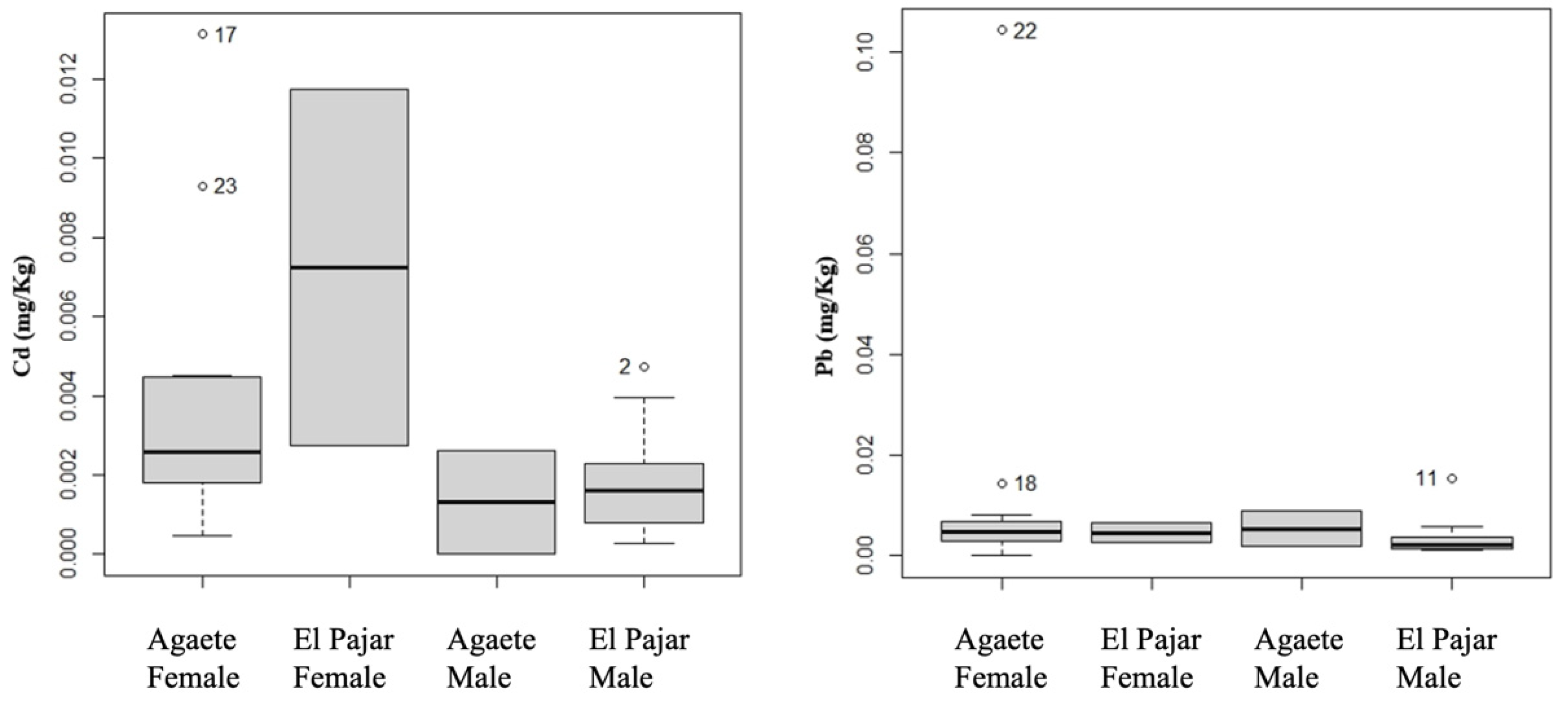
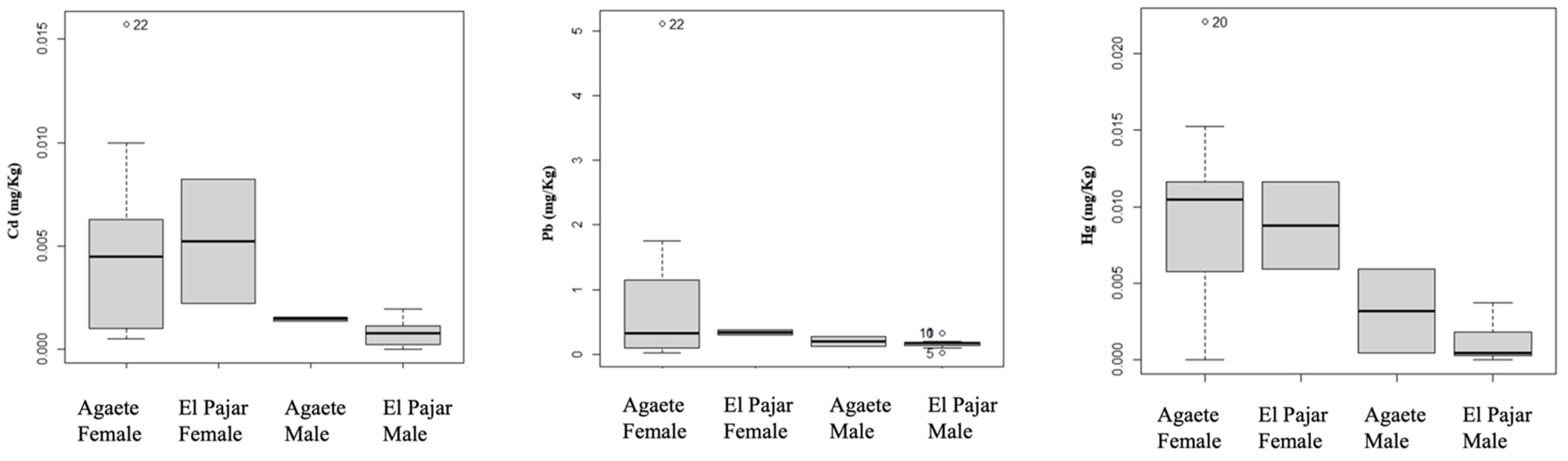
4.2. Metal Concentrations
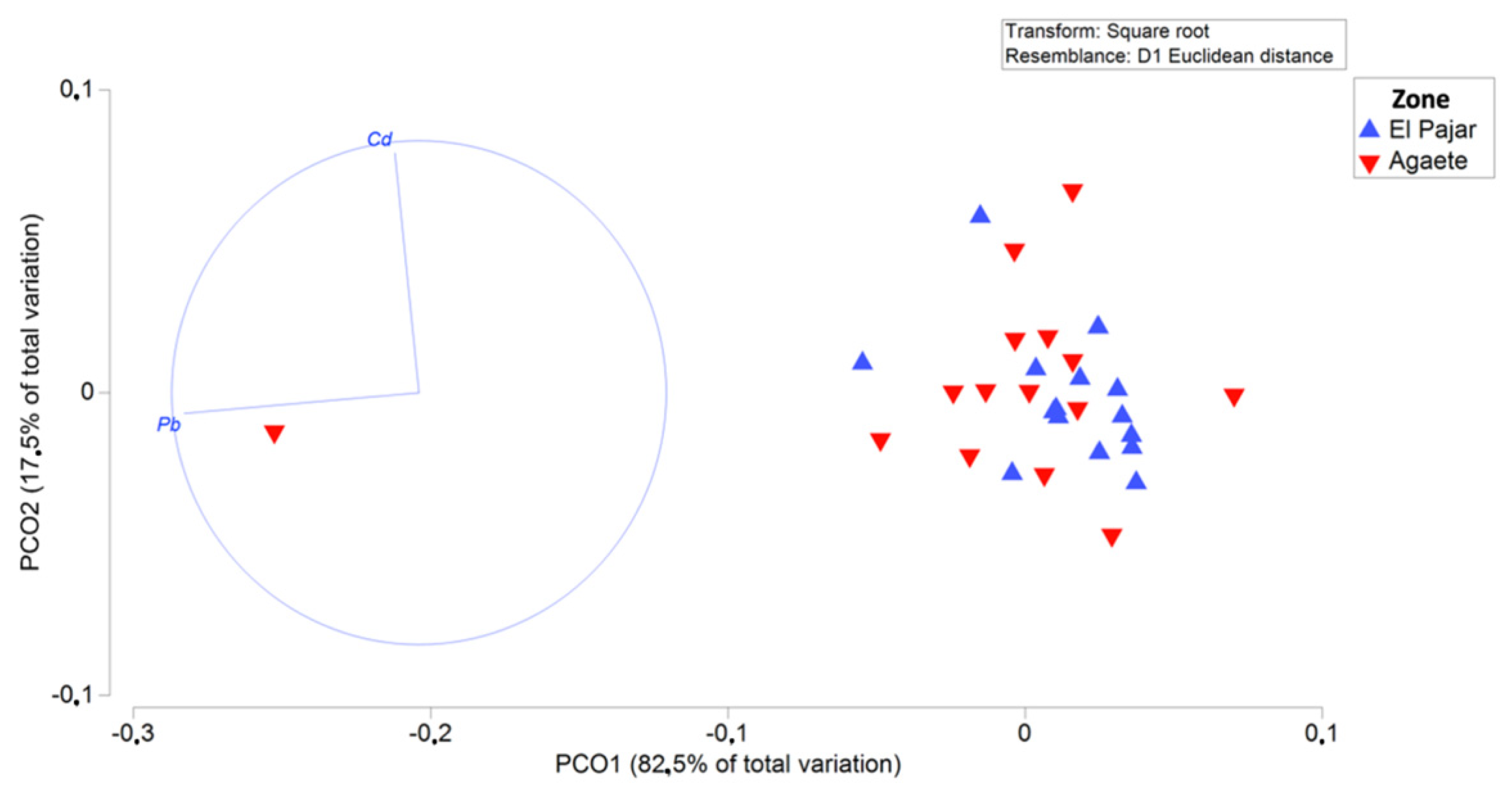
4.3. Statistical Analyses
5. Discussion
5.1. General Features
5.2. Comparison with Other Studies
5.3. Estimated Daily Intake
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozano-Bilbao, E.; Espinosa, J.M.; Jurado-Ruzafa, A.; Lozano, G.; Hardisson, A.; Rubio, C.; Weller, D.G.; Gutiérrez, Á.J.; González Weller, D.; Gutiérrez, Á.J. Inferring Class of organisms in the Central-East Atlantic from eco-toxicological characterization. Reg. Stud. Mar. Sci. 2020, 35, 101190. [Google Scholar] [CrossRef]
- Metian, M.; Bustamante, P.; Hédouin, L.; Warnau, M. Accumulation of nine metals and one metalloid in the tropical scallop Comptopallium radula from coral reefs in New Caledonia. Environ. Pollut. 2008, 152, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Dalman, Ö.; Demirak, A.; Balcı, A. Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern Aegean Sea (Turkey) by atomic absorption spectrometry. Food Chem. 2006, 95, 157–162. [Google Scholar] [CrossRef]
- Beiras, R.; Bellas, J.; Fernández, N.; Lorenzo, J.I.; Cobelo-García, A. Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussels (Mytilus galloprovincialis) versus embryo–larval bioassays using Paracentrotus lividus and Ciona intestinalis. Mar. Environ. Res. 2003, 56, 531–553. [Google Scholar] [CrossRef]
- Bonin-Font, F.; Campos, M.M.; Carrasco, P.N.; Codina, G.O.; Font, E.G.; Fidalgo, E.G. Towards a new methodology to evaluate the environmental impact of a marine outfall using a lightweight AUV. In Proceedings of the OCEANS 2017–Aberdeen, Aberdeen, UK, 19–22 June 2017; pp. 1–8. [Google Scholar]
- Ruilian, Y.; Xing, Y.; Zhao, Y.; Hu, G.; Tu, X. Heavy metal pollution in intertidal sediments from Quanzhou Bay, China. J. Environ. Sci. 2008, 20, 664–669. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nithila, P.; Reddy, S.J. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health Part A 1996, 31, 283–289. [Google Scholar]
- Verma, R.; Dwivedi, P. Heavy metal water pollution—A case study. Recent Res. Sci. Technol. 2013, 5, 98–99. [Google Scholar]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Hosono, T.; Su, C.C.; Delinom, R.; Umezawa, Y.; Toyota, T.; Kaneko, S.; Taniguchi, M. Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations. Estuar. Coast. Shelf Sci. 2011, 92, 297–306. [Google Scholar] [CrossRef]
- Topcuo, S. Heavy metal monitoring of marine algae from the Turkish Coast of the Black Sea, 1998–2000. Chemosphere 2003, 52, 1683–1688. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Kim, J. Metal-bearing molten sulfur collected from a submarine volcano: Implications for vapor transport of metals in seafl oor hydrothermal systems. Geology 2011, 39, 351–354. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Clemente, S.; Espinosa, J.M.; Jurado-Ruzafa, A.; Lozano, G.; Raimundo, J.; Hardisson, A.; Rubio, C.; González-Weller, D.; Jiménez, S.; et al. Inferring trophic groups of fish in the central-east Atlantic from eco-toxicological characterization. Chemosphere 2019, 229, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Barrento, S.; Marques, A.; Teixeira, B.; Anacleto, P.; Carvalho, M.L.; Vaz-Pires, P.; Nunes, M.L. Macro and trace elements in two populations of brown crab Cancer pagurus: Ecological and human health implications. J. Food Compos. Anal. 2009, 22, 65–71. [Google Scholar] [CrossRef]
- Semedo, M.; Reis-Henriques, M.A.; Rey-Salgueiro, L.; Oliveira, M.; Delerue-Matos, C.; Morais, S.; Ferreira, M. Metal accumulation and oxidative stress biomarkers in octopus (Octopus vulgaris) from Northwest Atlantic. Sci. Total Environ. 2012, 433, 230–237. [Google Scholar] [CrossRef]
- Kurun, A.; Balk, H.; Balk, N. Accumulations of total metal in dominant shrimp species (Palaemon adspersus, Palaemon serratus, Parapenaeus longirostris) and bottom surface sediments obtained from the Northern Inner Shelf of the Sea of Marmara. Environ. Monit. Assess. 2007, 135, 353–367. [Google Scholar] [CrossRef]
- Marcovecchio, J.E. Trace metal residues in tissues of two crustacean species from the Bahia Blanca estuary, Argentina. Environ. Monit. Assess. 1994, 29, 65–73. [Google Scholar] [CrossRef]
- Suciu, M.C.; Tavares, D.C.; Zalmon, I.R. Comparative evaluation of crustaceans as bioindicators of human impact on Brazilian sandy beaches. J. Crustac. Biol. 2018, 38, 420–428. [Google Scholar] [CrossRef]
- Tian, Y.; Boulangé-Lecomte, C.; Benamar, A.; Giusti-Petrucciani, N.; Duflot, A.; Olivier, S.; Frederick, C.; Forget-Leray, J.; Portet-Koltalo, F. Application of a crustacean bioassay to evaluate a multi-contaminated (metal, PAH, PCB) harbor sediment before and after electrokinetic remediation using eco-friendly enhancing agents. Sci. Total Environ. 2017, 607, 944–953. [Google Scholar] [CrossRef]
- White, S.L.; Rainbow, P.S. Heavy metal concentrations and size effects in the mesopelagic decapod crustacean Systellaspis debilis. Mar. Ecol. Prog. Ser. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; González-Delgado, S.; Alcázar-Treviño, J. Use of survival rates of the barnacle Chthamalus stellatus as a bioindicator of pollution. Environ. Sci. Pollut. Res. 2021, 28, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Caney, S. Climate change. In The Routledge Handbook of Global Ethics; Routledge: London, UK, 2015; pp. 384–398. [Google Scholar]
- Genthe, B.; Kapwata, T.; Le Roux, W.; Chamier, J.; Wright, C.Y. The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere 2018, 199, 1–9. [Google Scholar] [CrossRef]
- Anderson, L.G.; Rocliffe, S.; Haddaway, N.R.; Dunn, A.M. The role of tourism and recreation in the spread of non-native species: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0140833. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Hulme, P.E. Non-native species, ecosystem services, and human well-being. In Impact of Biological Invasions on Ecosystem Services; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–14. [Google Scholar]
- Chapman, D.S.; Makra, L.; Albertini, R.; Bonini, M.; Páldy, A.; Rodinkova, V.; Šikoparija, B.; Weryszko-Chmielewska, E.; Bullock, J.M. Modelling the introduction and spread of non-native species: International trade and climate change drive ragweed invasion. Glob. Chang. Biol. 2016, 22, 3067–3079. [Google Scholar] [CrossRef] [PubMed]
- Grosholz, E.D.; Ruiz, G.M. Biological invasions drive size increases in marine and estuarine invertebrates. Ecol. Lett. 2003, 6, 700–705. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Mačić, V.; Beqiraj, S.; Poursanidis, D.; Kashta, L. Invading the Adriatic: Spatial patterns of marine alien species across the Ionian Adriatic boundary. Aquat. Biol. 2011, 13, 107–118. [Google Scholar] [CrossRef][Green Version]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Ontogenic and seasonal variations of metal content in a small pelagic fish (Trachurus picturatus) in northwestern African waters. Mar. Pollut. Bull. 2020, 156, 111251. [Google Scholar] [CrossRef]
- Triay Portella, R.; Martín García, J.A.; González Pérez, J.A.; Lorenzo Nespereira, J.M. Conocer al invasor: Dispersión del Decápodo donativo Cronius ruber en los Ecosistemas Marinos de Gran Canaria (Islas Canarias). In Proceedings of the VI International Symposium on Marine Sciences (ISMS), Vigo, Spain, 20–22 June 2018; Volume 1, p. 102. [Google Scholar]
- Pajuelo, J.G.; González, J.A.; Triay-Portella, R.; Martín, J.A.; Ruiz-Díaz, R.; Lorenzo, J.M.; Luque, Á. Introduction of non-native marine fish species to the Canary Islands waters through oil platforms as vectors. J. Mar. Syst. 2016, 163, 23–30. [Google Scholar] [CrossRef]
- Verlaque, M.; Afonso-Carrillo, J.; Gil-Rodriguez, M.C.; Durand, C.; Boudouresque, C.F.; Le Parco, Y. Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (north-east Atlantic). Biol. Invasions 2004, 6, 269–281. [Google Scholar] [CrossRef]
- AESAN. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) Regarding Criteria for the Estimation of Concentrations for the Discussion Proposals for Migration Limits of Certain Heavy Metals and Other Elements from Cerami. 2012. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_cc_ingles/FOOD_CONTACT_MATERIALOS_.pdf (accessed on 11 January 2022).
- FESNAD. Ingestas Dietéticas de Referencia (IDR) para la Población Española. Act. Diet 2010, 14, 196–197. [Google Scholar]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.-G.; Paz, S.; Gutiérrez, Á.J. Seasonal and ontogenic variations of metal content in the European pilchard (Sardina pilchardus) in northwestern African waters. Environ. Pollut. 2020, 266, 115113. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Jurado-Ruzafa, A.; Lozano, G.; Jiménez, S.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Development stage and season influence in the metal content of small pelagic fish in the North-West Africa. Chemosphere 2020, 261, 127692. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Braak, C. Ter Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.R. The Resource for the Power Industry Professional. Proc. ASME Power 2004, 32. [Google Scholar]
- Lozano-Bilbao, E.; Herranz, I.; González-Lorenzo, G.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Paz, S.; Gutiérrez, Á.J. Limpets as bioindicators of element pollution in the coasts of Tenerife (Canary Islands). Environ. Sci. Pollut. Res. 2021, 28, 42999–43006. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Udy, J.; Longstaff, B.; Jones, A. Using nitrogen stable isotope ratios (??15N) of macroalgae to determine the effectiveness of sewage upgrades: Changes in the extent of sewage plumes over four years in Moreton Bay, Australia. Mar. Pollut. Bull. 2005, 51, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Daoji, L.; Daler, D. Ocean Pollution from Land-based Sources: East China Sea, China. AMBIO A J. Hum. Environ. 2004, 33, 107–113. [Google Scholar] [CrossRef]
- Karunasagar, D.; Krishna, M.V.B.; Anjaneyulu, Y.; Arunachalam, J. Studies of mercury pollution in a lake due to a thermometer factory situated in a tourist resort: Kodaikkanal, India. Environ. Pollut. 2006, 143, 153–158. [Google Scholar] [CrossRef]
- Cornish, A.S.; Ng, W.C.; Ho, V.C.M.; Wong, H.L.; Lam, J.C.W.; Lam, P.K.S.; Leung, K.M.Y. Trace metals and organochlorines in the bamboo shark Chiloscyllium plagiosum from the southern waters of Hong Kong, China. Sci. Total Environ. 2007, 376, 335–345. [Google Scholar] [CrossRef]
- Jakimska, A.; Konieczka, P.; Skóra, K.; Namieśnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part I: The Role and Impact of Heavy Metals on Organisms. Pol. J. Environ. Stud. 2011, 20, 1117–1125. [Google Scholar]
- Olgunoğlu, M.P.; Olgunoğlu, İ.A.; Bayhan, Y.K. Heavy Metal Concentrations (Cd, Pb, Cu, Zn, Fe) in Giant Red Shrimp (Aristaeomorpha foliacea Risso 1827) from the Mediterranean Sea. Polish J. Environ. Stud. 2015, 24, 631–635. [Google Scholar] [CrossRef]
- González, J.A.; Triay-Portella, R.; Escribano, A.; Cuesta, J.A. Northernmost record of the pantropical portunid crab Cronius ruber in the eastern Atlantic (Canary Islands): Natural range extension or human-mediated introduction? Sci. Mar. 2017, 81, 81–89. [Google Scholar] [CrossRef]
- Khan, A.T.; Forester, D.M.; Mielke, H.W. Heavy metal concentrations in two populations of crayfish. Vet. Hum. Toxicol. 1995, 37, 426–428. [Google Scholar] [PubMed]
- Khan, F.R.; Bury, N.R.; Hogstrand, C. Cadmium bound to metal rich granules and exoskeleton from Gammarus pulex causes increased gut lipid peroxidation in zebrafish following single dietary exposure. Aquat. Toxicol. 2010, 96, 124–129. [Google Scholar] [CrossRef]
- Bergey, L.L.; Weis, J.S. Molting as a mechanism of depuration of metals in the fiddler crab, Uca pugnax. Mar. Environ. Res. 2007, 64, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.A.; Pedersen, B. Trace metals in fish used for time trend analysis and as environmental indicators. Mar. Pollut. Bull. 1994, 28, 24–32. [Google Scholar] [CrossRef]
- Woo, P.T.K.; Sin, Y.M.; Wong, M.K. The effects of short-term acute cadmium exposure on blue tilapia, Oreochromis aureus. Environ. Biol. Fishes 1993, 37, 67–74. [Google Scholar] [CrossRef]
- Chan, H.M.; Rainbow, P.S. The accumulation of dissolved zinc by the shore crab Carcinus maenas (L.). Ophelia 1993, 38, 13–30. [Google Scholar] [CrossRef]
- Bjerregaard, P.; Depledge, M. Trace metal concentrations and contents in the tissues of the shore crab Carcinus maenas: Effects of size and tissue hydration. Mar. Biol. 2002, 141, 741–752. [Google Scholar]
- Butler, B.; Zou, E. Cadmium is deposited to the exoskeleton during post-ecdysial mineralization in the blue crab, Callinectes sapidus. Sci. Total Environ. 2021, 798, 149358. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Bastami, A.A.; Khoei, J.K.; Esmailian, M.; Songhori, E.J.; Najafzadeh, M. Concentrations of heavy metals in selected tissues of blue swimming crab, Portunus pelagicus (Linnaeus, 1758) and sediments from Persian Gulf. World Appl. Sci. J. 2012, 19, 1398–1405. [Google Scholar]
- Jerome, F.C.; Hassan, A.; Omoniyi-Esan, G.O.; Odujoko, O.O.; Chukwuka, A.V. Metal uptake, oxidative stress and histopathological alterations in gills and hepatopancreas of Callinectes amnicola exposed to industrial effluent. Ecotoxicol. Environ. Saf. 2017, 139, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Yasunaga, Y.; Iwata, H.; Ichihashi, H.; Tanabe, S.; Tatsukawa, R. Concentrations of heavy metals, organochlorines, and organotins in horseshoe crab, Tachypleus tridentatus, from Japanese coastal waters. Arch. Environ. Contam. Toxicol. 1995, 28, 40–47. [Google Scholar] [CrossRef]
- Zhu, Q.-H.; Zhou, Z.-K.; Tu, D.-D.; Zhou, Y.-L.; Wang, C.; Liu, Z.-P.; Gu, W.-B.; Chen, Y.-Y.; Shu, M.-A. Effect of cadmium exposure on hepatopancreas and gills of the estuary mud crab (Scylla paramamosain): Histopathological changes and expression characterization of stress response genes. Aquat. Toxicol. 2018, 195, 1–7. [Google Scholar] [CrossRef]
- Kumar, K.A.; Achyuthan, H. Heavy metal accumulation in certain marine animals along the East Coast of Chennai, Tamil Nadu, India. J. Environ. Biol. 2007, 28, 637–643. [Google Scholar]
- Botello, A.V. Golfo de México: Contaminación e Impacto Ambiental: Diagnóstico y Tendencias; Universidad Juárez Autónoma de Tabasco: Villahermosa, Mexico, 2005. [Google Scholar]
- Knowlton, M.F.; Boyle, T.P.; Jones, J.R. Uptake of lead from aquatic sediment by submersed macrophytes and crayfish. Arch. Environ. Contam. Toxicol. 1983, 12, 535–541. [Google Scholar] [CrossRef]
- Franke, C.; Studinger, G.; Berger, G.; Böhling, S.; Bruckmann, U.; Cohors-Fresenborg, D.; Jöhncke, U. The assessment of bioaccumulation. Chemosphere 1994, 29, 1501–1514. [Google Scholar] [CrossRef]
- Li, N.; Hou, Y.; Ma, D.; Jing, W.; Dahms, H.-U.; Wang, L. Lead accumulation, oxidative damage and histopathological alteration in testes and accessory glands of freshwater crab, Sinopotamon henanense, induced by acute lead exposure. Ecotoxicol. Environ. Saf. 2015, 117, 20–27. [Google Scholar] [CrossRef]
- Campos-Campos, N.H.; Dueñas-Ramírez, P.R.; Genes, N. Malformación en cangrejos de la superfamilia Xanthoidea (Crustacea: Brachyura) en la bahía de Cispatá (Córdoba, Colombia). Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2015, 39, 91–99. [Google Scholar] [CrossRef]
- Gutiérrez, A.J.; Lozano, G.; Rubio, C.; Martín, V.; Hardisson, A.; Revert, C. Heavy Metals in Black Crabs in the Atlantic Coast (Tenerife, Spain)—Human Risk Assessment. CLEAN-Soil Air Water 2017, 45. [Google Scholar] [CrossRef]
- Gil, M.N.; Torres, A.; Harvey, M.; Esteves, J.L. Metales pesados en organismos marinos de la zona costera de la Patagonia argentina continental Heavy metals in marine organisms from the coastal zone of Continental Argentine Patagonia. Rev. Biol. Mar. Oceanogr. 2006, 41, 167–176. [Google Scholar] [CrossRef]
- Younis, A.M.; El-Zokm, G.M.; Okbah, M.A. Spatial variation of acid-volatile sulfide and simultaneously extracted metals in Egyptian Mediterranean Sea lagoon sediments. Environ. Monit. Assess. 2014, 186, 3567–3579. [Google Scholar] [CrossRef]
- Kilgour, B.W. Cadmium uptake from cadmium-spiked sediments by four freshwater invertebrates. Bull. Environ. Contam. Toxicol. 1991, 47, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.M.; Amin, H.F.; Alkaladi, A.; Mosleh, Y.Y.I. Bioaccumulation of heavy metals in fish, squids and crustaceans from the Red Sea, Jeddah Coast, Saudi Arabia. Open J. Mar. Sci. 2015, 5, 369. [Google Scholar] [CrossRef]
- Mohapatra, A.; Rautray, T.R.; Patra, A.K.; Vijayan, V.; Mohanty, R.K. Trace element-based food value evaluation in soft and hard shelled mud crabs. Food Chem. Toxicol. 2009, 47, 2730–2734. [Google Scholar] [CrossRef]
- Falusi, B.A.; Olanipekun, E.O. Bioconcentration Factors of Heavy Metals in Tropical Crab (Carcinus sp) from River Aponwe, Ado-Ekiti, Nigeria. 2007. Available online: https://tspace.library.utoronto.ca/handle/1807/51574 (accessed on 11 January 2022).
- Turoczy, N.J.; Mitchell, B.D.; Levings, A.H.; Rajendram, V.S. Cadmium, copper, mercury, and zinc concentrations in tissues of the King Crab (Pseudocarcinus gigas) from southeast Australian waters. Environ. Int. 2001, 27, 327–334. [Google Scholar] [CrossRef]
- Gutiérrez-Peña, L.V.; Picón, D.; Gutiérrez, I.A.; Prada, M.; Carrero, P.E.; Delgado-Cayama, Y.J.; Gutiérrez, E.O.; Morón, M.; González, C.E.; Lara, N.D. Heavy metals in soft tissue of blue crab (Callinectes sapidus) of Puerto Concha, Colon Municipality, Zulia State. Av. Biomed. 2018, 7, 17–22. [Google Scholar]
- AECOSAN. Spanish Diet Model for the Determination of Consumer Exposure to Chemical Substances; Ministry of Health and Consumption: Madrid, Spain, 2006. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2010, 8, 1570. [Google Scholar]
- Reglamento (CE) No 1881/2006. Reglamento (CE) No 1881/2006 de la Comisión de 19 de Diciembre de 2006 por el Que Se Fija el Contenido Máximo de Determinados Contaminantes en los Productos Alimenticios. 2006. Available online: https://www.boe.es/doue/2006/364/L00005-00024.pdf (accessed on 11 January 2022).
- Reglamento (UE) 2015/1005. Reglamento (UE) 2015/1005 de la Comisión de 25 de Junio de 2015 Que Modifica el Reglamento (CE) no. 1881/2006 por lo Que Respecta al Contenido Máximo de Plomo en Determinados Productos Alimenticios. 2015. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:32015R1005 (accessed on 11 January 2022).
- Reglamento (CE) No 420/2011. Reglamento (CE) No 420/2011 de la Comisión de 29 de Abril de 2011 Que Modifica el Reglamento (CE) no 1881/2006, por el Que se Fija el Contenido Máximo de Determinados Contaminantes en los Productos Alimenticios. Diario Oficial de la Unión Europeas. 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:111:0003:0006:ES:PDF (accessed on 11 January 2022).
- Reglamento (UE) No 488/2014. Reglamento (UE) No 488/2014 de la Comisión de 12 de Mayo de 2014 Que Modifica el Reglamento (CE) no. 1881/2006 por lo Que Respecta al Contenido Máximo de Cadmio en los PRoductos Alimenticios. 2014. Available online: https://www.boe.es/doue/2014/138/L00075-00079.pdf (accessed on 11 January 2022).

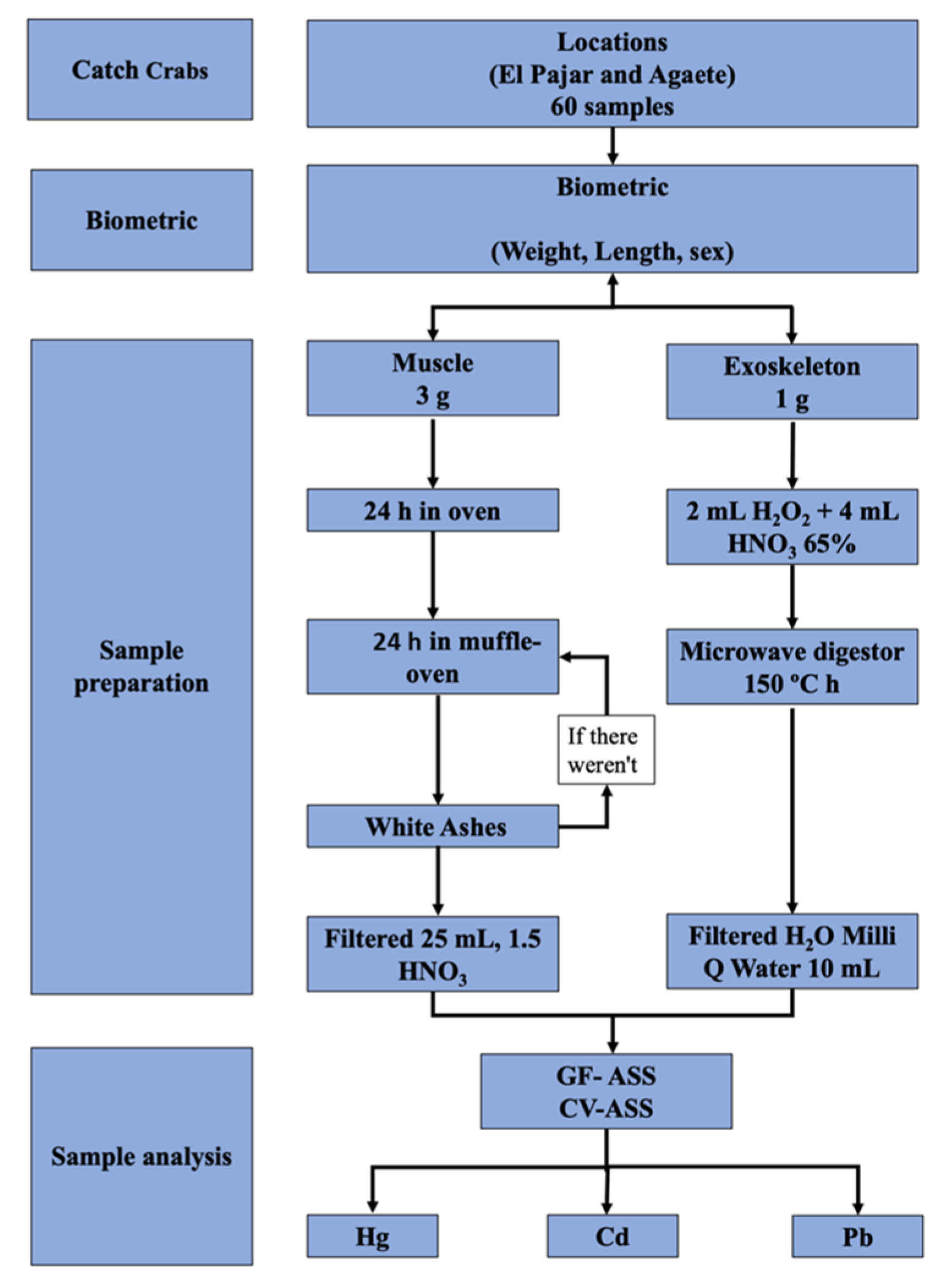
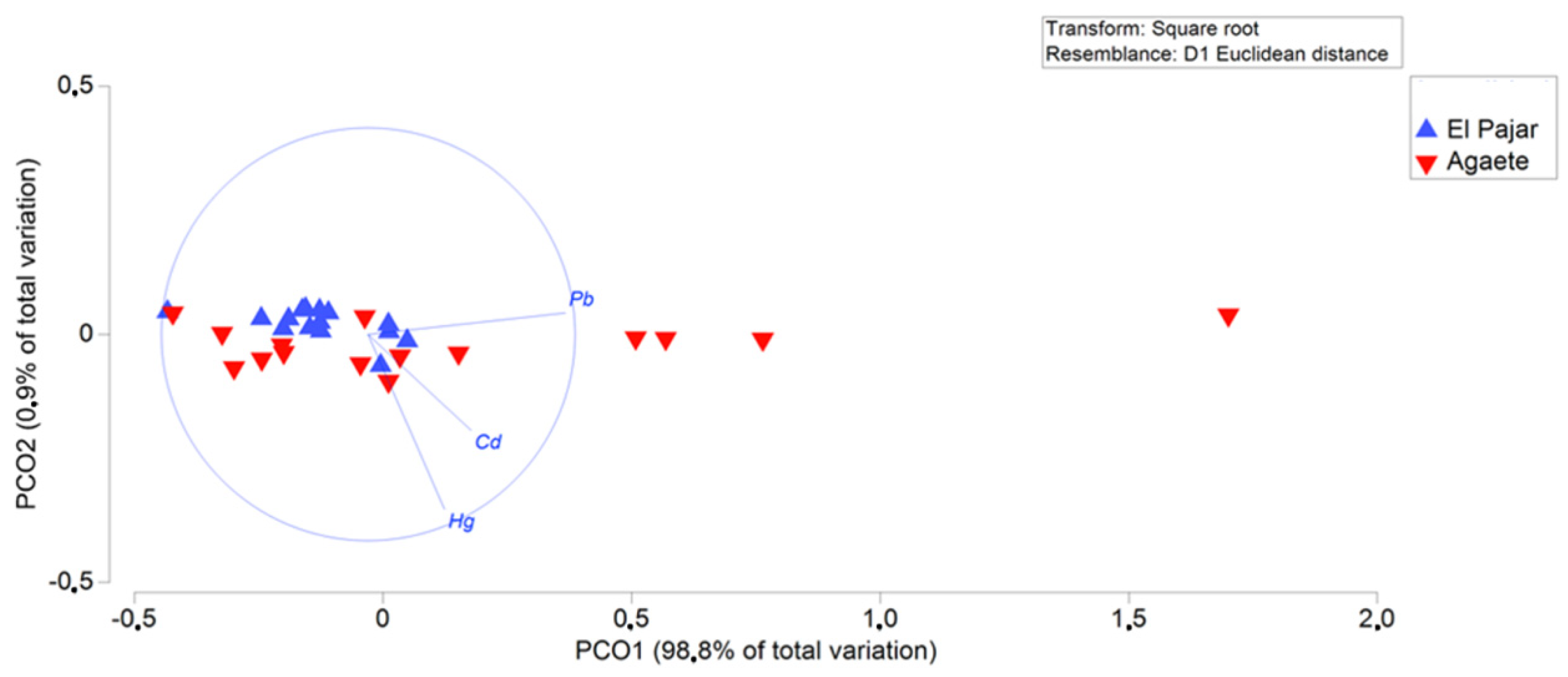
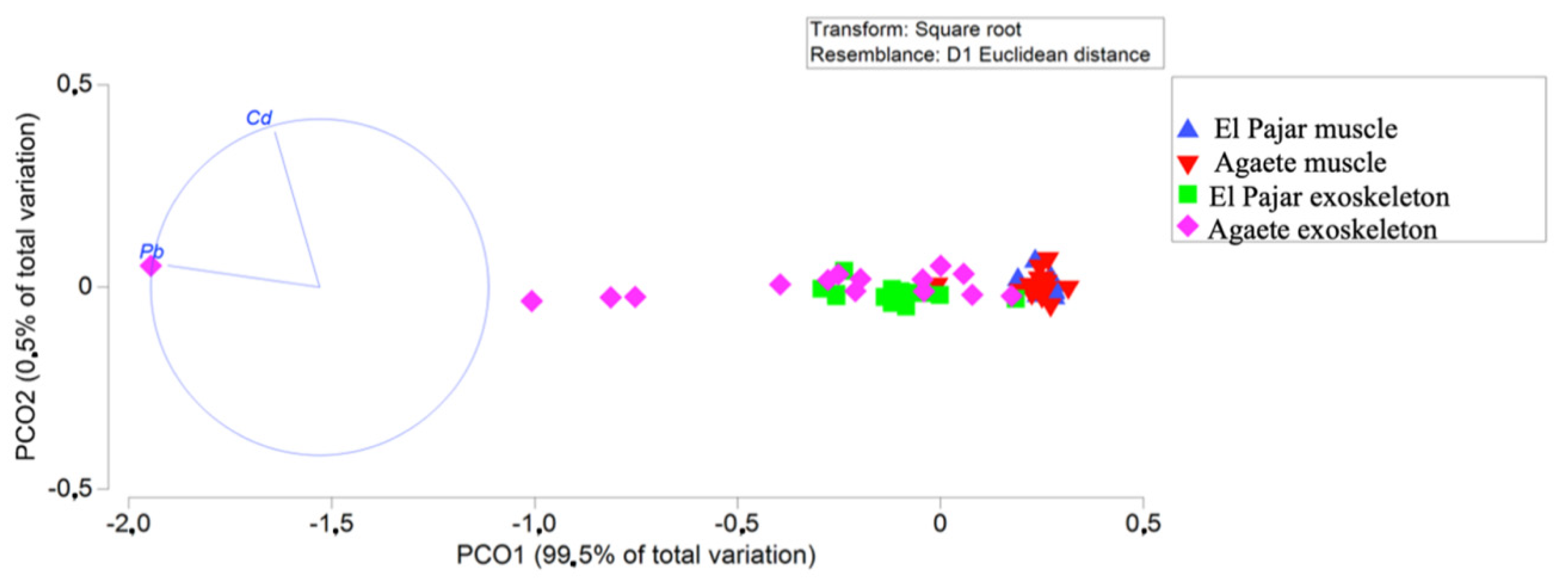
| Location | Sex | Length (Width) | Weight |
|---|---|---|---|
| Agaete | Male | 78.63 ± 9.91 (78.9–83.4) | 135.06 ± 48.11 (119.9–165.1) |
| Female | 76.63 ± 5.84 (69.2–87.4) | 101.66 ± 26.93 (51.2–137.2) | |
| El Pajar | Male | 75.9 ± 7.61 (57.1–84.6) | 112.93 ± 30.25 (46.6–157.3) |
| Female | 68.58 ± 9.22 (77.5–84.2) | 120.05 ± 31.95 (119.5–127.5) | |
| p-value | 0.342 | 0.209 |
| Location | Sample | Mean Concentration (mg/kg) ± SD vs. the Location Factor, and Sample | Sex | Mean Concentration (mg/kg) ± SD vs. the Location Factor, Sample, and Sex | |
|---|---|---|---|---|---|
| Cd | El Pajar | Muscle | 0.003 ± 0.002 | Male | 0.002 ± 0.001 |
| Female | 0.008 ± 0.006 | ||||
| Exoskeleton | 0.001 ± 0.001 | Male | 0.001 ± 0.000 | ||
| Female | 0.005 ± 0.004 | ||||
| Agaete | Muscle | 0.003 ± 0.003 | Male | 0.001 ± 0.001 | |
| Female | 0.004 ± 0.003 | ||||
| Exoskeleton | 0.004 ± 0.004 | Male | 0.001 ± 0.000 | ||
| Female | 0.005 ± 0.004 | ||||
| Pb | El Pajar | Muscle | 0.004 ± 0.003 | Male | 0.004 ± 0.003 |
| Female | 0.005 ± 0.002 | ||||
| Exoskeleton | 0.199 ± 0.096 | Male | 0.178 ± 0.083 | ||
| Female | 0.338 ± 0.045 | ||||
| Agaete | Muscle | 0.012 ± 0.025 | Male | 0.005 ± 0.004 | |
| Female | 0.013 ± 0.027 | ||||
| Exoskeleton | 0.766 ± 1.308 | Male | 0.202 ± 0.105 | ||
| Female | 0.853 ± 1.391 | ||||
| Hg | El Pajar | Exoskeleton | 0.002 ± 0.003 | Male | 0.001 ± 0.001 |
| Female | 0.009 ± 0.004 | ||||
| Agaete | Exoskeleton | 0.009 ± 0.005 | Male | 0.003 ± 0.003 | |
| Female | 0.009 ± 0.005 |
| Metal | Cd | Pb | ||
|---|---|---|---|---|
| Location | El Pajar | Agaete | El Pajar | Agaete |
| Female vs. Male | 0.031 * | 0.174 | 0.549 | 0.633 |
| El Pajar vs. Agaete | 0.477 | 0.15 | ||
| Metal | Cd | Pb | Hg | |||
|---|---|---|---|---|---|---|
| Location | El Pajar | Agaete | El Pajar | Agaete | El Pajar | Agaete |
| Female vs. Male | 0.013 * | 0.373 | 0.072 | 0.52 | 0.011 * | 0.147 |
| El Pajar vs. Agaete | 0.011 * | 0.087 | 0.002 * | |||
| Location | Cd | Pb | ||
|---|---|---|---|---|
| El Pajar | Agaete | El Pajar | Agaete | |
| Muscle vs. Exoskeleton | 0.001 * | 0.015 * | 0.001 * | 0.001 * |
| Species | Sample | Unit. | Cd | Pb | Hg | Study |
|---|---|---|---|---|---|---|
| Cronius ruber | Muscle—El Pajar | mg/kg | 0.003 ± 0.002 | 0.004 ± 0.003 | The present study | |
| Exoskeleton—El Pajar | 0.001 ± 0.001 | 0.199 ± 0.096 | 0.002 ± 0.003 | |||
| Muscle—Agaete | 0.003 ± 0.003 | 0.012 ± 0.025 | ||||
| Exoskeleton—Agaete | 0.004 ± 0.004 | 0.766 ± 1.308 | 0.009 ± 0.005 | |||
| Grapsus adcensionis | Muscle | mg/kg | 1.148 | 1.089 | [67] | |
| Exoskeleton | 4.736 | 13.92 | ||||
| Portunus petagicus | Muscle | μg∙g−1 | 0.08 | 0.008 | [68] | |
| Chasmagnatus granulata | Muscle | μg/g | 10.00–13.20 | |||
| Scylla serrata | Muscle | μg/g | 0.17–0.21 | [72] | ||
| Exoskeleton | 0.15–0.16 | |||||
| Callinectes sapidus | Muscle | mg/kg | 0.24 ± 0.05 | 0.88 ± 0.04 | [75] | |
| Pseudocarcinus gigas | Muscle | mg/g | 0.05 | 0.3 | [74] | |
| Carcinus sp. | Muscle | mg/kg | 0.07 | 5.52 | 0.11 | [73] |
| Metal/Limits | Index | El Pajar | Agaete |
|---|---|---|---|
| Cd 2.5 μg/kg/week | ADI | 0.0001 | 0.0001 |
| MoS | 7.49 × 10−8 | 7.49 × 10−8 | |
| Kg | 734 | 734 | |
| Pb 0.5 μg/kg/day | IDA | 0.087 | 0.025 |
| MoS | 0.001 | 0.02 | |
| Kg | 356 | 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorne-Bazarra, T.; Lozano-Bilbao, E.; Triay-Portella, R.; Hardisson, A.; Paz, S.; Rubio-Armendariz, C.; Martín, V.; Gutiérrez, A.J. Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk. Appl. Sci. 2022, 12, 3217. https://doi.org/10.3390/app12073217
Thorne-Bazarra T, Lozano-Bilbao E, Triay-Portella R, Hardisson A, Paz S, Rubio-Armendariz C, Martín V, Gutiérrez AJ. Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk. Applied Sciences. 2022; 12(7):3217. https://doi.org/10.3390/app12073217
Chicago/Turabian StyleThorne-Bazarra, Thabatha, Enrique Lozano-Bilbao, Raül Triay-Portella, Arturo Hardisson, Soraya Paz, Carmen Rubio-Armendariz, Verónica Martín, and Angel J. Gutiérrez. 2022. "Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk" Applied Sciences 12, no. 7: 3217. https://doi.org/10.3390/app12073217
APA StyleThorne-Bazarra, T., Lozano-Bilbao, E., Triay-Portella, R., Hardisson, A., Paz, S., Rubio-Armendariz, C., Martín, V., & Gutiérrez, A. J. (2022). Metallic Study of the Invasive Species Cronius ruber—Assessment of Toxic Risk. Applied Sciences, 12(7), 3217. https://doi.org/10.3390/app12073217











