Abstract
Gamma rays as a type of ionizing radiation constitute a physical mutagen that induces mutations and could be effectively used in plant breeding. To compare the effects of gamma and ionizing irradiation according to exposure time in common wheat (Keumgang, IT 213100), seeds were exposed to 60Co gamma rays at different dose rates. To evaluate the amount of free radical content, we used electron spin resonance spectroscopy. Significantly more free radicals were generated in the case of long-term compared with short-term gamma-ray exposure at the same dose of radiation. Under short-term exposure, shoot and root lengths were slightly reduced compared with those of the controls, whereas long-term exposure caused severe growth inhibition. The expression of antioxidant-related and DNA-repair-related genes was significantly decreased under long-term gamma-ray exposure. Long-term exposure caused higher radiosensitivity than short-term exposure. The results of this study could help plant breeders select an effective mutagenic induction dose rate in wheat.
1. Introduction
Wheat (Triticum aestivum L.) is one of the most important crops and accounts for approximately 20% of the nutrient calories and proteins consumed by the global population [1]. Wheat is an important source of essential or beneficial nutrients, such as proteins, vitamins (notably several types of vitamin B), dietary fibers, and phytochemicals [2]. As a stable supply of wheat is required for food security due to the increase in the global population and climate change, a need for various genetic resources to support such a supply is emerging. Plant breeders select resources suitable for their purpose by targeting genetic resources with various traits to develop new varieties that can adapt to climate change response and consumption trends. In this process, the most important steps are creating genetic variation in and securing the genetic diversity of breeding materials. To increase genetic diversity, several researchers have used different techniques leading to comprehensive changes in the plant genome, including traditional breeding techniques, such as crossbreeding and mutagenesis, as well as new biotechnologies, such as genetic transformation, genome editing, and introgression [3].
Ionizing radiation is most commonly used to generate useful mutations in plants due to its ease of application and high mutation frequency. Ionizing radiation can cause direct or indirect damage to plants. The direct effect causes damage to the genetic material (DNA or RNA) in the cells of the organism, resulting in structural and functional changes in the DNA molecules [4,5]. Indirect damage generates highly reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide radicals (O2−), and hydroxyl radicals (OH), potentially leading to oxidative stress [6]. The reported effects of ionizing radiation on plant species include effects on the mutation rate, cytogenetic effects, and effects on biological responses [7,8,9]. Among the radiation sources, gamma rays are very important in mutation breeding and in vitro mutagenesis to the development of the required features in plants and increasing the genetic variability [10]. A previous report indicated that gamma rays affect changes in the phytochemical composition of plants [11]. Numerous studies on the use of gamma rays to develop new cultivars with diverse genetic backgrounds reported that gamma rays affected plant characteristics such as grain color, flower color, and plant height [12,13,14,15,16]. Approximately 63% of the mutant varieties registered in the Mutant Variety Database (a joint FAO/IAEA project) are varieties created through gamma irradiation [17]. Gamma irradiation is still considered an attractive tool for mutagenesis.
Most previous research focused on the germination of seeds and physiological and biochemical changes in plants according to the dose of gamma radiation [18,19,20]. This study aimed to investigate the response in wheat according to different gamma radiation doses and exposure times. We irradiated wheat (Keumgang: a Korean wheat variety) seeds with 100, 300, and 500 Gy of gamma rays for 8 h and 14 days (the final dosages were the same, but the dose rates per hour were different after gamma irradiation). To compare the gamma irradiation effect according to the radiation dose and exposure time, we investigated oxidative stress, seed germination percentage, plant growth, chlorophyll content, antioxidant enzyme activity, and gene expression levels. Before gamma irradiation, we examined various doses and exposure times to determine which irradiation conditions could efficiently induce mutations. The results of this study could be used to select the appropriate gamma radiation dose to induce mutations in wheat mutation breeding.
2. Materials and Methods
2.1. Plant Material and Gamma Irradiation
For gamma irradiation, seeds of Keumgang (National Agrobiodiversity Centre, RDA, Suwon, Korea; accession no. IT 213100), a wheat cultivar certified in Korea, were used. For the different gamma irradiation exposure times, we used two gamma irradiation facilities at the Advanced Radiation Technology Institute, an affiliate of the Korean Atomic Energy Research Institute (Jeongeup, Korea). A 60Co gamma phytotron (20 TBq of capacity, Nordion, ON, Canada) and a 60Co gamma irradiator (150 TBq of capacity, Nordion, ON, Canada) were used to irradiate seeds at low- and high-dose rates for 2 weeks and 8 h, respectively. The final exposure doses were set to 100, 300, and 500 Gy. The dose rates were 0.298 Gy/h, 0.893 Gy/h, and 1.488 Gy/h for the low-dose rates and 12.5 Gy/h, 37.5 Gy/h, and 62.5 Gy for the high-dose rates. After irradiation, the seeds were stored at 4 °C until further analysis.
2.2. Germination Assays and Plant Growth
To measure the germination percentage, 100 seeds were placed on a piece of moistened germination paper and then incubated at room temperature under a 16/8 h day/night cycle. The germinated seeds were counted each day for 5 days to determine the final germination percentage. Germination was considered complete when the seeds exhibited a radical emergence size of >2 mm. The germination percentage (GP), germination rate index (GRI), and mean germination time (MGT) were calculated using the following fomula: GP = n/N ∗ 100 (n = total number of seeds germinated; N = total number of seeds) [21]. The germination rate index was calculated according to the equation described by Esechie [22]: GRI = ∑(Ni/i) (Ni = number of seeds germinated on day i). The MGT was calculated based on the formula described by Ellis and Roberts [23]: MGT = ∑(NiTi)/∑Ni (Ni = number of seeds germinated, Ti = number of days from sowing).
For plant growth, the seeds were germinated for 2 days at room temperature and then transferred into plastic containers containing Hoagland’s solution (Sigma, St. Louis, MO, USA). The seedlings were grown in a phytotron room at 23 °C with a photoperiod of 16/8 h (day/night). The Hoagland’s solution was exchanged each day. The shoot and root lengths were measured on day 10 after germination (DAG). DAG10 leaf samples were used for the measurement of chlorophyll content and antioxidant activities and the analysis of gene expression.
2.3. Chlorophyll Content Measurement
The chlorophyll content was measured using a UV spectrophotometer as described by Lichtenthaler and Buschmann [24]. Briefly, wheat leaf samples (0.5 g) were homogenized with 10 mL of 100% acetone and then kept overnight in the dark at 4 °C. The homogenized sample mixtures were centrifuged at 10,000 rpm for 10 min and the supernatant was transferred into a new tube. The supernatant was used for pigment determination. The absorbance was measured using a UV-VIS spectrophotometer (Jenway, Chelmsford, UK) at 470, 644.8, and 661.6 nm. The chlorophyll contents were calculated as described previously [25]. The total chlorophyll, chlorophyll a, and chlorophyll b contents were calculated using the following equations: Ca = 11.24 ∗ A661.6 − 2.04 ∗ A644.8, Cb = 20.13 ∗ A644.8 − 4.19 ∗ A661.6, and Ca+b(total) = 7.05 ∗ A661.6 + 18.09 ∗ A644.8, respectively.
2.4. RNA Extraction and Gene Expression Analysis
Total RNA of DAG10 leaves was extracted using Tri reagent (MRC, Cambridge, UK) according to the manufacturer’s protocol. First-strand cDNA was prepared using a Power cDNA synthesis kit (iNtRON Biotechnology, Seongnam, Korea) according to the manufacturer’s protocol. RT-qPCR was carried out at a reaction volume of 20 microliters with SYBR premix Ex Taq II (Takara, Tokyo, Japan). Quantitative analysis was performed using the Bio-Rad CFX100 (Illumina, San Diego, CA, USA). All RT-qPCR amplification conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 40 cycles of 10 s at 95 °C and 30 s at 65 °C. The gene-specific primers are listed in Table S1. Actin (AB181991) was used as an endogenous control for the RT-qPCR analysis.
2.5. Free Radical Content Measurement
Non-irradiated and irradiated freeze-dried and ground seed samples were transferred to a cylindrical quartz ESR glass tube (diameter, 5 mm). The glass tube was sealed from the open end using Whatman film (GE Healthcare, Buckinghamshire, UK). The glass tube was submitted for ESR measurements using an X band ESR spectrometer (JES-PX2300, JEOL, Tokyo, Japan) equipped with a cylindrical cavity. The operating conditions for the ESR measurements were as follows: power, 0.998 mW; microwave frequency, 9.429 GHz; modulation frequency, 100 kHz; modulation width, 1 mT; magnetic center field, 337.812 mT; sweep time 30 s; time constant, 0.03 s. The ESR signal intensities were assessed using the peak-to-peak amplitude of the first-derivative spectrum.
2.6. Total Phenolic Content
The total phenolic content was measured using the Folin–Ciocalteu method with gallic acid (GA) as a standard [26]. In brief, 0.5 mL of wheat seed extract solution (methanol) was mixed with 2.5 mL of 10% Folin–Ciocalteu reagent. Next, the mixture was supplemented with 0.75 mL of 70% Na2CO3 and incubated for 120 min at room temperature in the dark. After incubation, the optical density was measured at 765 nm using a UV-VIS spectrophotometer (Jenway, Chelmsford, UK). The total phenolic content was calculated as mg/g of gallic acid equivalent (GAE) using the calibration curve of gallic acid.
2.7. DPPH Radical Scavenging Activity
DPPH free radical scavenging capacity measurements were carried out using UV-VIS spectrophotometry [27]. The homogenized wheat seed samples were extracted using methanol for 24 h at 4 °C. The extract solution (0.1 mL) was mixed with 3 mL of DPPH (0.1 mM) methanol solution and the absorbance was measured at 517 nm using a UV-VIS spectrophotometer after reaction for 30 min at room temperature. The DPPH radical scavenging activity was calculated using the following equation:
where Asample is the absorbance of the irradiated samples and Acontrol is the absorbance of the control.
DPPH radical scavenging activity (%) = (1 − Asample/Acontrol) × 100
2.8. Antioxidant Enzyme Assay
To determine the antioxidant enzyme activities, the protein content of the DAG10 leaves was extracted by grinding frozen samples with liquid nitrogen and homogenizing the samples in 1 mL of extraction buffer (0.2 M potassium phosphate buffer (pH 7.0) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA)) at 4 °C. The total protein content was determined using the Bradford method [28]. The ascorbate peroxidase (APX) activity was determined using the Nakano and Asada method [29], and the catalase (CAT) activity was determined using the method of Abei [30]. The POD activity was measured following the method of Kwak [31] using pyrogallol as a substrate. The superoxide dismutase (SOD) activity was analyzed by monitoring the inhibition of the photochemical reduction of Nitro Blue Tetrazolium (NBT) according to the method of Giannopolitis and Ries [32].
2.9. Statistical Analysis
The statistical significance of the differences between the mean values was determined using one-way analysis of variance with Duncan’s multiple range tests. Significant differences were evaluated at a 5% level of significance. All statistical analyses were performed using SPSS version 23 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Free Radical Contents and Plant Growth
To examine the oxidative stress produced by gamma irradiation, we measured the free radical contents in irradiated and control seeds. The mean differences in the free radical levels were significant according to the gamma radiation dose and time (Figure 1). The free radical content increased dose-dependently in the case of both long- and short-term gamma irradiation. Interestingly, we observed that the free radical content was significantly higher in the long-term irradiated seeds compared to their short-term irradiated counterparts. The relative free radical levels significantly increased in the case of all long-term gamma irradiation treatments, showing a more than 5-fold increase compared with the control levels. In the case of the short-term treatment, the free radical content increased slightly (1.1–1.5-fold) compared with the control levels.
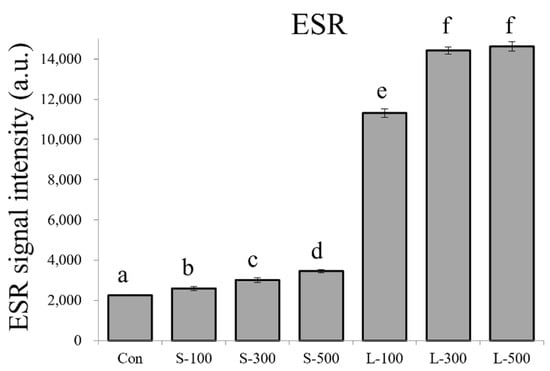
Figure 1.
Measurements of the free radical content in wheat seeds following short- and long-term gamma irradiation using electron spin resonance (ESR). Con, non-irradiated seeds (0 Gy); S, short-term irradiation; L, long-term irradiation; 100, 300, and 500, gamma irradiation doses. Each bar represents the mean ± standard deviation (SD). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
Figure 2 shows the daily wheat seed germination percentage. The germination percentages in DAG1 and DAG2 showed a significant difference between the short-term irradiation, including the control, and the long-term irradiation with gamma rays. The final germination percentage did not show a significant difference between the control and the irradiated seeds. Data analysis revealed that the GRI and MGT were affected by the gamma-ray dose and exposure time (Table 1). The GRI showed a tendency to decrease with prolonged exposure at the same dose of gamma radiation. The MGT was slower in the case of long-term irradiation. The MGT increased with an increase in the gamma-ray dose and exposure time. The shoot and root lengths decreased dose-dependently (Figure 3). The growth of plants treated with short-term gamma irradiation exposure was slightly reduced compared with the non-irradiated samples, whereas exposure to long-term irradiation caused severe growth inhibition. In the case of long-term irradiation, the shoot and root lengths decreased drastically as the radiation doses increased. These results indicate that the free radical content increase according to the radiation dose and exposure time increase is closely related to plant growth. Moreover, our results indicate that the high free radical levels affected the early stage of wheat development by reducing the ability of the seeds to germinate or delaying their development. The RD50 values determined by plant growth characteristics were 158.6 Gy and 206.3 Gy (shoot and root length in the case of short-term irradiation, respectively) and 202.8 Gy and 252.5 Gy (shoot and root length in the case of long-term irradiation, respectively) (Supplementary Figure S1).
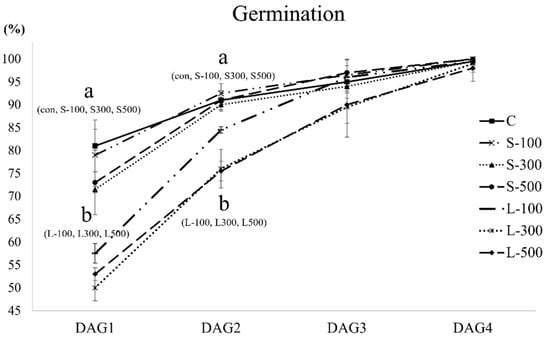
Figure 2.
Gamma radiation dose and exposure time effects on seed germination percentage. The seed germination percentages were determined daily for 4 days. DAG, days after germination. Each bar represents the mean ± SD.

Table 1.
Germination rate index and mean germination time according to the dose of and time of exposure to gamma radiation.

Figure 3.
Gamma irradiation effect on plant growth under different doses and exposure times. (A) Image of plant growth under different irradiation conditions. Scale bar: 2 cm. (B) Shoot and (C) root length 10 days after germination. Each bar represents the mean ± SD (n = 10). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
3.2. Chlorophyll Content Determination
The chlorophyll a content in wheat seedlings increased at 100 Gy (5.84 mg/g FW) and decreased at 300 Gy (5.39 mg/g FW) and 500 Gy (3.96 mg/g FW), and markedly decreased with long-term exposure compared with the control (Figure 4). Compared with the control, 5.45 mg/g FW, the chlorophyll a content decreased by 38% at 500 Gy of short-term gamma irradiation. Furthermore, long-term gamma irradiation of 100 Gy (2.91 mg/g FW), 300 Gy (0.66 mg/g FW), and 500 Gy (0.73 mg/g FW) induced a significant reduction in chlorophyll content (by 47, 88, and 87%, respectively). We confirmed that the chlorophyll b and total chlorophyll contents showed similar patterns to the chlorophyll a content. These results indicate that 500 Gy of short-term gamma irradiation and long-term exposure to beyond 100 Gy of gamma radiation caused an obvious decrease in the chlorophyll a, chlorophyll b, and total chlorophyll contents in Keumgang.
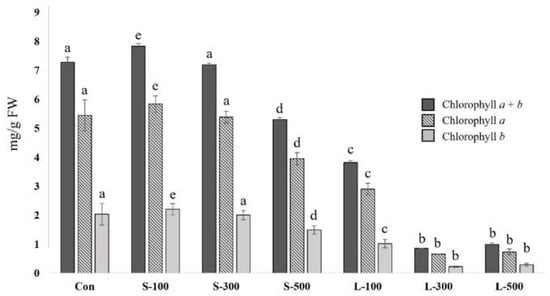
Figure 4.
Determination of total chlorophyll, chlorophyll a, and chlorophyll b contents upon gamma irradiation. Each bar represents the mean ± SD (n = 3). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
3.3. Gamma Radiation Effect on the Expression of DNA-Repair-Related Genes
The effect of gamma irradiation on the expression of DNA-repair-related genes was analyzed using RT-qPCR (Figure 5). After gamma irradiation, most DNA-repair-related gene transcripts showed similar expression patterns, exhibiting a gradual decrease with increasing exposure doses. The expression of X-ray repair cross-complementing protein (XRCC), KU70, and DNA methyltransferase 2 (DnMT2) was slightly upregulated by short-term irradiation at 100 Gy (Figure 5A–C). The MSH6 transcript levels decreased with increasing gamma radiation doses (Figure 5D). The RT-qPCR results show that DNA-repair-related gene transcripts were drastically reduced upon long-term irradiation compared with short-term irradiation. These results indicate that long-term irradiation negatively impacts the DNA repair system in plants compared with short-term irradiation even at equal gamma-ray doses.
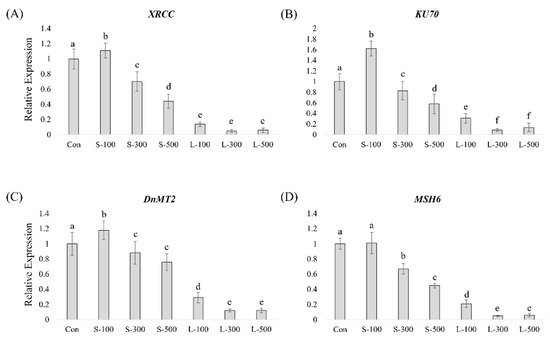
Figure 5.
DNA-repair-related gene expression profiling in wheat seedlings determined by RT-qPCR. (A) XRCC, (B) KU70, (C) DnMT2, and (D) MSH6. RT-qPCR was performed with three biological replicates, and each bar represents the mean ± SD (n = 10). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
3.4. Antioxidant-Related Gene Expression Levels
To investigate how different gamma radiation exposure times affect the expression of antioxidant-related genes, we performed RT-qPCR (Figure 6). APX, CAT, MnSOD, and monodehydroascorbate reductase (MDHAR) showed similar gene expression patterns in plants subject to short-term irradiation (Figure 6A–C,H). CuZnSOD transcript levels decreased at 100 Gy of short-term irradiation and showed a similar level of expression to that of the control at 300 Gy (Figure 6D). In the case of long-term irradiation, CuZnSOD expression decreased with increasing gamma-ray doses. The glutathione reductase (GR) and guaiacol peroxidase (GPX) gene expression levels were the highest when the plants were subject to short-term 100-Gy gamma irradiation, then decreased continuously with increasing doses (Figure 6E,F). Dehydroascorbate reductase (DHAR) transcripts were continuously induced up to 300 Gy, then decreased at 500 Gy of short-term irradiation (Figure 6G). Keumgang subjected to long-term irradiation showed a significant decline in the expression of most antioxidant-related genes. These results indicate that long-term irradiation affects the plants more seriously than short-term irradiation.
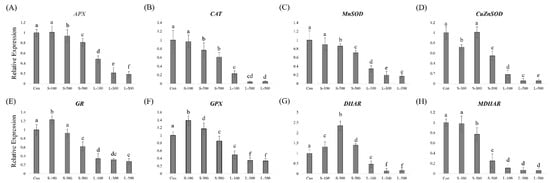
Figure 6.
Antioxidant-related gene expression profiling in wheat seedlings determined by RT-qPCR. (A) APX, (B) CAT, (C) MnSOD, (D) CuZnSOD, (E) GR, (F) GPX, (G) DHAR, and (H) MDHAR. RT-qPCR was performed with three biological replicates, and each bar represents the mean ± SD (n = 10). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
3.5. Antioxidant Activity
To estimate antioxidant enzyme activities upon different gamma radiation exposure times, we measured the APX, CAT, POD, and SOD activities in wheat seedlings (Figure 7). APX activity was the highest at 100 Gy of long-term irradiation, and a lower value could be observed compared with the controls at 300 and 500 Gy of short- and long-term irradiation, respectively (Figure 7A). CAT activity levels were the highest at 300 Gy and the lowest at 500 Gy of short- and long-term irradiation (Figure 7B). SOD activity was significantly higher in the case of short-term irradiation than in the controls, and it decreased in the case of long-term irradiation (Figure 7C). Upon short-term irradiation, the SOD showed the highest activity at 100 Gy and a tendency to decrease with increasing doses. POD activity increased compared with the control after gamma irradiation, and POD activity levels were the highest in the case of long-term irradiation at 500 Gy (Figure 7D).
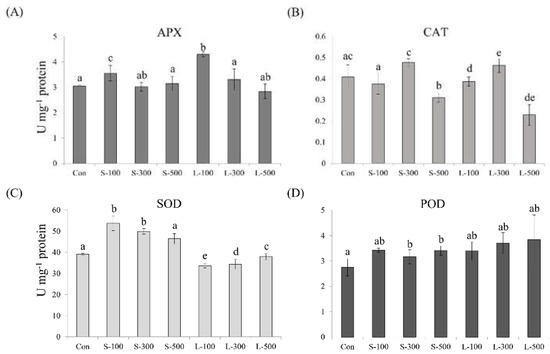
Figure 7.
Short- and long-term gamma irradiation effects on (A) APX, (B) CAT, (C) SOD, and (D) POD antioxidant enzyme activities. Each bar represents the mean ± SD (n = 3). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
3.6. Total Phenolic Content and DPPH Free Radical Scavenging Activity
The total phenolic content was 28.91 mg/g in the control (non-irradiated seeds). The total phenolic content slightly increased to 33.38 and 31.88 mg/g in the case of short-term irradiation with doses of 100 and 500 Gy, respectively (Figure 8). In the case of long-term irradiation, a significant increase in the total phenolic content could be observed after long-term gamma irradiation. The total phenolic content increased by 27.37%, 26.94%, and 23.48% compared with the control at 100-, 300-, and 500-Gy radiation doses, respectively. The DPPH free radical scavenging capacity decreased slightly but non-significantly after gamma irradiation.
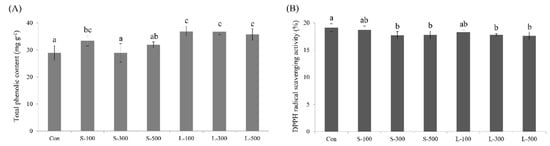
Figure 8.
Different gamma radiation dose and exposure time effects on the (A) total phenolic content and (B) DPPH free radical scavenging activity. Each bar represents the mean ± SD (n = 3). Values with different letters are significantly different using Duncan’s multiple range test (p < 0.05).
4. Discussion
Mutation breeding has been used as a tool to improve the genetic diversity of plants and develop desired traits. Gamma rays are a source of mutagens that can induce mutations in plants. Several breeders use gamma rays to select desired traits by securing genetic diversity. Various studies have shown the effects of gamma irradiation on seed germination, plant growth, chlorophyll content, oxidative stress, and secondary metabolite production [7,33]. In general, high-dose gamma irradiation is known to negatively influence the physiological and biochemical traits of plants [9,34,35]. However, radiosensitivity might reveal different levels of susceptibility depending on the plant variety due to DNA-content-, repair-process-, antioxidant-reaction-, and cell cycle kinetics-related differences [36,37,38,39]. In order to increase the mutation breeding efficiency, the selection of an appropriate radiation dose that could increase the mutation frequency without significantly affecting the plant’s survival and reproduction is important. Most studies focused on the assessment of biological responses to different radiation doses, whereas relatively few studies have examined the different effects of gamma radiation exposure times [9,40,41]. Here, we report the main results of our study on the dose- and time-dependent effects of gamma irradiation, focusing on germination rate, plant growth, chlorophyll content, oxidative stress, gene expression, and antioxidant activity changes.
Long-term exposure to gamma rays had a significantly negative effect on the seed germination percentage. We observed no differences in the final germination percentage between control and irradiated seeds. However, there was a significant difference in the germination pattern between short-term and long-term irradiation in the early germination stages (DAG1 and DAG2) (Figure 2). Germination-related indicators showed a tendency to decrease as the gamma radiation dose and exposure time increased (Table 1). Long-term gamma irradiation significantly reduced the GRI compared with short-term irradiation. We confirmed that the MGT increased as the exposure time increased, even at the same dose of gamma radiation (Table 1). Numerous studies have demonstrated that high gamma radiation doses cause physiological changes, such as a delay in seed germination and a reduction in the survival rate and plant growth, in wheat [19,20,42]. These results are consistent with those of previous researchers who reported that the seed germination potential decreased as the radiation dose increased. In addition, the germination speed was confirmed to be reduced with prolonged exposure to gamma rays even at the same radiation doses. Growth inhibition and retardation are known to be caused by cell cycle arrest in the G2 phase during somatic cell division upon exposure to high-dose gamma radiation [43,44]. Moreover, a gamma-radiation-mediated reduction in auxin and DNA synthesis could lead to a reduced mitotic frequency in the apical meristematic tissues, resulting in reduced plant growth and development [35,36,45]. This study on wheat’s response to gamma radiation showed that the gamma-radiation-related reduction in germination speed and plant growth could be attributed to the cell cycle arrest and changes in plant hormones and DNA synthesis.
We found that the seed germination and seedling growth in wheat might be linked to the gamma radiation dose- and exposure time-related changes in free radical contents (Figure 1, Figure 2 and Figure 3). Free radicals, such as ROS and reactive nitrogen species (RNS), are extremely reactive chemical compounds that potentially cause oxidative stress by damaging cell structures, including lipids, proteins, and DNA [46,47]. Free radicals play crucial roles in radiation sensitivity [48]. Previous studies have shown that high gamma radiation dose rates severely damage plants and induce the formation of more free radicals [49,50,51]. We evaluated the free radical levels according to the radiation dose and exposure time and observed that these levels increased linearly with increasing radiation doses in the case of both short- and long-term gamma irradiation (Figure 1). In particular, the number of free radicals was larger in the case of long-term irradiation than in the case of short-term irradiation. A previous study reported that chronically irradiated colored wheat exhibited lower free radical levels compared with the controls and showed that colored wheat anthocyanins could directly influence free radical scavenging capacity during the early developmental stages of wheat [52]. Unlike the results of previous studies, the results of this study show very high free radical levels in the wheat seeds subjected to long-term exposure to gamma radiation, and plant growth was also significantly reduced. Seeds that contain antioxidants (e.g., anthocyanins), such as those in colored wheat, even when exposed to gamma radiation for a long time, eliminate free radicals via antioxidants, thereby enabling plant growth. However, in the case of non-colored varieties of wheat, such as Keumgang, the antioxidant activity is relatively low; thus, the free radical elimination ability is limited, which seems to negatively affect plant growth (Figure 3). These results suggest that when non-colored wheat seeds are continuously exposed to low doses of gamma radiation, the free radical production increases and this increase in free radicals induces oxidative stress, negatively affecting wheat seed germination and seedling growth.
Chlorophyll content is a useful indicator for evaluating the physiological response to radiation [53]. A previous report indicated that exposure to 100 Gy of gamma radiation increased the content of chlorophyll in wheat [18]. In this study, short-term irradiation at 100 Gy produced the same result (Figure 4). Our results indicate that high gamma radiation doses decreased the chlorophyll content in comparison with the control. In particular, the chlorophyll content decreased significantly in the case of longer gamma radiation exposure times (Figure 4). Numerous studies have reported that high gamma radiation doses reduce photosynthetic activity, ultimately decreasing the chlorophyll content and plant growth [34,41]. This result is consistent with those of previous studies reporting that plants exposed to gamma radiation exhibited lower chlorophyll contents than the controls. We also confirmed that the chlorophyll content in the wheat seeds exposed to gamma radiation significantly decreased in the case of long-term compared with short-term irradiation.
Gamma radiation can affect the integrity of genetic information and impair genomic stability by inducing DNA damage. In contrast to animals, plants are constantly exposed to the threat of DNA damage as they cannot change locations to avoid unfavorable growth conditions. Under adverse conditions, most organisms have mechanisms, such as DNA repair, that prevent mutations caused by damaging ionizing radiation, and the DNA strand breaks induced by ionizing radiation can be repaired with fast kinetics. Ptácek et al. [54] found that the DNA damage induced by 30 Gy of gamma radiation in tobacco was perfectly repaired within 24 h. The DNA-repair-related genes allow for the repair of ionizing-radiation-induced damage. Previous reports indicated that the DNA-repair-related genes, such as PnLIG4, PnKU70, PnXRCC4, PnPCNA, and PnRAD51, from Lombardy poplar (Populus nigra) were upregulated upon gamma irradiation [55]. In addition, the AtRAD51, AtLIG4, and AtXRCC4 transcripts were upregulated upon gamma irradiation treatment [56]. We assessed DNA-repair-related gene expression patterns at various gamma radiation exposure times and doses (Figure 5). We observed that the expression of most DNA-repair-related genes (XRCC, KU70, DnMT2, and MSH6) was downregulated by gamma radiation in a dose- and time-dependent manner except for the case of short-term irradiation at 100 Gy. DNA-repair-related gene transcript levels in the case of long-term irradiation were lower than in the case of short-term irradiation. We demonstrated that long-term exposure to gamma radiation affected wheat growth more seriously than short-term exposure through DNA-repair-related gene expression levels.
To avoid gamma-radiation-induced oxidative stress, plants use antioxidant defense systems comprised of enzymatic antioxidants (such as APX, CAT, SOD, GR, GPX, MDHAR, and DHAR) and non-enzymatic antioxidants (such as ascorbic acid (AA), reduced glutathione (GSH), α-tocopherol, carotenoids, and flavonoids) [47]. The antioxidant defense systems are used to maintain intracellular homeostasis by regulating cellular ROS levels, resulting in the protection of plants against various stress conditions [57]. To determine the antioxidant activity in wheat plants at different gamma radiation doses and exposure times, antioxidant-related gene expressions and antioxidant enzyme activities were measured (Figure 6 and Figure 7). We found that antioxidant-related gene transcripts showed lower expression levels in the case of long-term irradiation compared with the control and the case of short-term-irradiation. The antioxidant enzyme activities did not show the same pattern as the antioxidant-related gene expressions. This result indicates that the gamma-radiation-generated excess ROSs were not eliminated to an appropriate extent due to the reduced gene expression and antioxidant enzyme activity, thereby negatively affecting wheat plant growth.
In this study, the SOD, CAT, and APX gene transcript profiles displayed no direct correlation with the corresponding enzymatic activities, potentially due to the existence of several different antioxidant enzyme isoforms [58]. In this study, no direct relationship appeared to exist between gene expression and antioxidant enzyme activity. However, elucidating the antioxidant-related gene expression patterns could help us to better understand the molecular mechanisms underlying how different gamma radiation doses and exposure times affect wheat.
As most investigations on the effects of gamma irradiation were performed under short-term irradiation conditions, the findings of this study provide direct information on various effects of gamma irradiation on wheat mutagenesis through the comparison of short- and long-term exposure to gamma radiation. Based on previous research, 100 Gy of gamma radiation can be considered an optimal dose for mutagenesis, and the effective dose for generating a high degree of genetic diversity and a small amount of physical damage is 200–400 Gy. Furthermore, 200 Gy of gamma radiation is an appropriate dose to obtain a drought-resistant wheat mutant line [59,60,61]. However, the optimal dose of gamma radiation for mutagenesis differs depending on the cultivar or desired agricultural trait. The results of our study investigating the gamma irradiation conditions-related correlation between plant oxidative stress and plant growth response in non-colored wheat provide useful information for the gamma-radiation-assisted mutation breeding of wheat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12063208/s1, Figure S1: RD50 values in response to gamma-rays for growth characteristics of Keumgang, Table S1: Primers used for gene expression analysis.
Author Contributions
Conceptualization, M.J.H. and J.-B.K.; formal analysis, M.J.H. and D.Y.K.; methodology, M.J.H., S.H.K. and D.Y.K.; data curation, M.J.H. and D.Y.K.; writing—original draft preparation, M.J.H.; writing—review and editing, Y.D.J., H.-I.C., J.-W.A., S.H.K., S.-J.K. and Y.W.S.; supervision, J.-B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Nuclear R&D Program of the Ministry of Science and ICT (MSIT) and the research program of KAERI, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef] [Green Version]
- Esnault, M.A.; Legue, F.; Chenal, C. Ionizing radiation: Advances in plant response. Environ. Exp. Bot. 2010, 68, 231–237. [Google Scholar] [CrossRef]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Kim, J.H.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 2007, 38, 553–564. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effects of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]
- Marcu, D.; Cristea, V.; Daraban, L. Dose-dependent effects of gamma radiation on lettuce (Lactuca sativa var. capitata) seedlings. Int. J. Radiat. Biol. 2013, 89, 219–223. [Google Scholar] [CrossRef]
- Li, F.; Shimizu, A.; Nishio, T.; Tsutsumi, N.; Kato, H. Comparison and characterization of mutations induced by gamma ray and carbon-ion irradiation in rice (Oryza sativa L.) using whole genome resequencing. G3 Genes Genomes Genet. 2019, 9, 3743–3751. [Google Scholar] [CrossRef] [Green Version]
- Han, A.R.; Hong, M.J.; Nam, B.; Kim, B.R.; Park, H.H.; Baek, I.; Kil, Y.S.; Nam, J.W.; Jin, C.H.; Kim, J.B. Comparison of flavonoid profiles in sprouts of radiation breeding wheat lines (Triticum aestivum L.). Agronomy 2020, 10, 1489. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Nam, B.; Ahn, J.W.; Kwon, S.J.; Seo, Y.W.; Kim, J.B. Characterization of novel mutants of hexaploid wheat (Triticum aestivum L.) with various depths of purple grain color and antioxidant capacity. J. Sci. Food Agric. 2019, 99, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, M.M.; Bahrani, A. Influence of gamma irradiation on some physiological characteristics and grain protein in wheat (Triticum aestivum L.). World Appl. Sci. J. 2011, 15, 654–659. [Google Scholar]
- Wu, J.H.; Zhang, J.; Lan, F.; Fan, W.F.; Li, W. Morphological, cytological, and molecular variations induced by gamma rays in ground-grown chrysanthemum ‘Pinkling’. Can. J. Plant Sci. 2019, 100, 68–77. [Google Scholar] [CrossRef]
- Pallavi, B.; Nivas, S.K.; D’Souza, L.; Ganapathi, T.R.; Hegde, S. Gamma rays induced variations in seed germination, growth and phenotypic characteristics of Zinnia elegans var. Dreamland. Adv. Hort. Sci. 2017, 31, 267–273. [Google Scholar]
- Andrew-Peter-Leon, M.T.; Ramchander, S.; Kumar, K.K.; Muthamilarasan, M.; Pillai, M.A. Assessment of efficacy of mutagenesis of gamma irradiation in plant height and days to maturity through expression analysis in rice. PLoS ONE 2021, 16, e0245603. [Google Scholar]
- Hase, Y.; Satoh, K.; Seito, H.; Oono, Y. Genetic consequences of acute/chronic gamma and carbon ion irradiation of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 336. [Google Scholar] [CrossRef] [Green Version]
- Borzouei, A.; Kafi, M.; Khazaei, H.; Naseriyan, B.; Majdabadi, A. Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pak. J. Bot. 2010, 42, 2281–2290. [Google Scholar]
- Melki, M.; Marouani, A. Effects of gamma rays irradiation on seed germination and growth of hard wheat. Environ. Chem. Lett. 2010, 8, 307–310. [Google Scholar] [CrossRef]
- Pane, F.J.D.; Lopez, S.C.; Cantamutto, M.Á.; Domenech, M.B.; Castro-Franco, M. Effect of different gamma irradiation doses on the germination and seedling growth of wheat and triticale cultivars. Aust. J. Crop Sci. 2018, 12, 1921–1926. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). International rules for seed testing. Seed Sci. Technol. 1996, 24, 155–202. [Google Scholar]
- Esechie, H. Interaction of salinity and temperature on the germination of sorghum. J. Agron. Crop Sci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 2, 373–409. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-vis spectroscopy. Curr. Protoc. Food Analyt. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activities. J. Funct. Foods 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H.E. Catalasein Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 273–286. [Google Scholar]
- Kwak, S.S.; Kim, S.K.; Lee, M.S.; Jung, K.H.; Park, I.H.; Liu, J.R. Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry 1995, 39, 981–984. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Aziz, M.A.; Philip, E. Effects of acute gamma irradiation on physiological traits and flavonoid accumulation of Centella asiatica. Molecules 2011, 16, 4994–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, S.; Parween, T.; Siddiqi, T.O. Effects of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Caplin, N.; Willey, N. Ionizing radiation, higher plants, and radioprotection: From acute high doses to chronic low doses. Front. Plant Sci. 2018, 9, 847. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Halawa, M. Cytological effects of gamma radiation and its impact on growth and yield of M1 and M2 plants of cowpea cultivars. Cytologia 2014, 79, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Hakala, K.; Jauhiainen, L.; Koskela, T.; Käyhkö, P.; Vorne, V. Sensitivity of crops to increased ultraviolet radiation in northern growing conditions. J. Agron. Crop Sci. 2002, 188, 8–18. [Google Scholar] [CrossRef]
- Gudkov, I.N.; Grodzinsky, D.M. Cell radiosensitivity variation in synchronously-dividing root meristems of Pisum sativum L. and Zea mays L. during the mitotic cycle. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1982, 41, 401–409. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, J.B.; Yoon, Y.H.; Kim, S.H.; Ahn, J.W.; Jeong, I.Y.; Kang, S.Y.; Seo, Y.W.; Kim, D.S. The effects of chronic gamma irradiation on oxidative stress response and the expression of anthocyanin biosynthesis-related genes in wheat (Triticum aestivum). Int. J. Radiat. Biol. 2014, 90, 1218–1228. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hong, M.J.; Park, C.S.; Seo, Y.W. The effects of chronic radiation of gamma ray on protein expression and oxidative stress in Brachypodium distachyon. Int. J. Radiat. Biol. 2015, 91, 407–419. [Google Scholar] [CrossRef]
- Irfaq, M.; Nawab, K. Effect of gamma irradiation on some morphological characteristics on three wheat (Triticum aestivum L.) cultivars. J. Biol. Sci. 2001, 1, 935–937. [Google Scholar]
- Preuss, S.B.; Britt, A.B. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 2003, 164, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Von Well, E.; Fossey, A.; Booyse, M. Efficiency of energy conversion and growth of gamma irradiated embryos and young seedlings of Triticum monococcum L. cultivar Einkorn. J. Radiat. Res. Appl. Sci. 2018, 11, 75–82. [Google Scholar] [CrossRef]
- Ananthaswamy, H.N.; Vakil, U.K.; Sreenivasan, A. Biochemical changes in gamma-irradiated wheat during germination. Radiat. Bot. 1971, 11, 1–12. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Gu, J.; Zhao, L.; Guo, H.; Xie, Y.; Zhao, S.; Song, X.; Han, L.; Liu, L. Factors affecting the radiosensitivity of hexaploid wheat to γ-irradiation: Radiosensitivity of hexaploidy wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0161700. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Arkhipov, A.; Barylyak, I.; Karachov, I.; Titov, V.; Hohn, B.; Kovalchuk, I. Plants experiencing chronic internal exposure to ionizing radiation exhibit higher frequency of homologous recombination than acutely irradiated plants. Mutat. Res. 2000, 449, 47–56. [Google Scholar] [CrossRef]
- Vandenhove, H.; Vanhoudt, N.; Cuypers, A.; Van Hees, M.; Wannijn, J.; Horemans, N. Life-cycle chronic gamma exposure of Arabidopsis thaliana induces growth effects but no discernable effects on oxidative stress pathways. Plant. Physiol. Biochem. 2010, 48, 778–786. [Google Scholar] [CrossRef]
- Choi, H.I.; Han, S.M.; Jo, Y.D.; Hong, M.J.; Kim, S.H.; Kim, J.B. Effects of acute and chronic gamma irradiation on the cell biology and physiology of rice plants. Plants 2021, 10, 439. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Ahn, J.W.; Kang, S.Y.; Seo, Y.W.; Kim, J.B. Comparison of radiosensitivity response to acute and chronic gamma irradiation in colored wheat. Genet. Mol. Biol. 2018, 41, 611–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Song, M.; Lee, K.J.; Hwang, S.G.; Jang, C.S.; Kim, J.B.; Kim, S.H.; Ha, B.K.; Kang, S.Y.; Kim, D.S. Genome-wide transcriptome profiling of ROS scavenging and signal transduction pathways in rice (Oryza sativa L.) in response to different types of ionizing radiation. Mol. Biol. Rep. 2012, 39, 11231–11248. [Google Scholar] [CrossRef] [PubMed]
- Ptácek, O.; Stavreva, D.A.; Kim, J.K.; Gichner, T. Induction and repair of DNA damage as measured by the comet assay and the yield of somatic mutations in gamma-irradiated tobacco seedlings. Mutat. Res. 2001, 491, 17–23. [Google Scholar] [CrossRef]
- Nishiguchi, M.; Nanjo, T.; Yoshida, K. The effects of gamma irradiation on growth and expression of genes encoding DNA repair-related proteins in Lombardy poplar (Populus nigra var. italica). J. Environ. Radioact. 2012, 109, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mannuss, A.; Trapp, O.; Puchta, H. Gene regulation in response to DNA damage. Biochim. Biophys. Acta 2012, 1819, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Nazarenko, M.; Lykholat, Y.; Grygoryuk, I.; Khromikh, N. Optimal doses and concentrations of mutagens for winter wheat breeding purposes. Part I. Grain productivity. J. Cent. Eur. Agric. 2018, 19, 194–205. [Google Scholar] [CrossRef]
- Dwinanda, P.; Syukur, S.; Suliansyah, I. Induction of mutations with gamma ray radiation to improve the characteristics of wheat (Triticum aestivum L.) genotype IS-Jarissa. IOP Conf. Ser. Earth Environ. Sci. 2020, 497, 012013. [Google Scholar] [CrossRef]
- Sen, Y.; Ozturk, I.; Yaycili, O.; Alikamanoglu, S. Drought tolerance in irradiated wheat mutants studied by genetic and biochemical marker. J. Plant Growth Regul. 2017, 36, 669–679. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).